Abstract
Statin use in end-stage kidney disease (ESKD) patients are not encouraged due to low cardioprotective effects. Although the risk of hepatocellular carcinoma (HCC), a frequently occurring cancer in East Asia, is elevated in ESKD patients, the relationship between statins and HCC is not known despite its possible chemopreventive effect. The relationship between statin use and HCC development in ESKD patients with chronic hepatitis was evaluated. In total, 6165 dialysis patients with chronic hepatitis B or C were selected from a national health insurance database. Patients prescribed with ≥ 28 cumulative defined daily doses of statins during the first 3 months after dialysis commencement were defined as statin users, while those not prescribed with statins were considered as non-users. Primary outcome was the first diagnosis of HCC. Sub-distribution hazard model with inverse probability of treatment weighting was used to estimate HCC risk considering death as competing risk. During a median follow-up of 2.8 years, HCC occurred in 114 (3.2%) statin non-users and 33 (1.2%) statin users. The HCC risk was 41% lower in statin users than in non-users (sub-distribution hazard ratio, 0.59; 95% confidence interval [CI], 0.42–0.81). The weighted incidence rate of HCC was lower in statin users than in statin non-users (incidence rate difference, − 3.7; 95% CI − 5.7 to − 1.7; P < 0.001). Incidence rate ratio (IRR) was also consistent with other analyses (IRR, 0.56; 95% CI, 0.41 to 0.78; P < 0.001). Statin use was associated with a lower risk of incident HCC in dialysis patients with chronic hepatitis B or C infection.
Similar content being viewed by others
Introduction
Although statins, 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase inhibitors, are a mainstay in the primary and secondary prevention of cardiovascular disease in the general population, the benefits of statins in patients undergoing maintenance dialysis are controversial. Large-sized randomized trials have failed to reveal the beneficial effects of statins on cardiovascular outcomes in patients on maintenance dialysis suggesting a relative resistance against statin effect in this population1,2,3. Therefore, current guidelines do not recommend the initiation of statins for patients undergoing dialysis.
The risk of hepatocellular carcinoma (HCC), one of the most frequently occurring cancer types in East Asia4, is relatively high in patients undergoing maintenance dialysis. In an evaluation of 92,348 chronic dialysis patients in Taiwan, the standardized incidence ratio for HCC in patients undergoing dialysis was 1.4, suggesting a significant increase in HCC incidence compared to that in the general population5. Impaired immunity, deoxyribonucleic acid (DNA) repair, and oxidative stress defense due to the accumulation of uremic toxins and chronic inflammatory status have been suggested as possible reasons for the increased cancer risk in this patient group6. In addition, dialysis patients are more prone to chronic hepatitis due to their immunocompromised status and an increased cross-contamination risk7,8. Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are major risk factors for HCC development9.
In addition to their cardiovascular protective effects, statins have been recognized for their pleiotropic properties. Recently, statin use has been proposed to have beneficial effects against multiple cancer types10. In particular, several observational studies and experimental investigations have revealed the preventive and therapeutic potential of statins against HCC. The inhibition of HMG-CoA reductase by statins has been found to control the production of mevalonate and its downstream metabolites, which play pivotal roles in HCC growth and apoptosis11. However, despite the high HCC incidence among dialysis patients, the relationship between statin use and HCC development in this group has not been elucidated.
Therefore, in this study, the association between statin use and the incidence of HCC was examined in patients with chronic HBV or HCV infection in whom dialysis had been newly initiated. This was done by evaluating claims information from a nationwide health insurance database.
Methods
Data source
More than 98% of the Korean population is enrolled in a mandatory Korean National Health Insurance Service (NHIS) program, and the remaining people receive government benefits as they are included in the lowest-income bracket. The Health Insurance Review and Assessment Service (HIRA) is a national organization that reviews and evaluates healthcare costs and quality of care. Healthcare providers in Korea are obligated to participate in this program. Information from the HIRA database, including data on patient demographics, prescriptions, treatments, and diagnoses, were reviewed in this study. The present study was carried out in accordance with the Declaration of Helsinki and was approved by the institutional review board of the Yonsei University Health System (4-2019-0501). The requirement for informed consent was waived because of the retrospective nature of the study. De-identification was performed, and data usage was permitted by the national health information data request review committee of the HIRA.
Study population
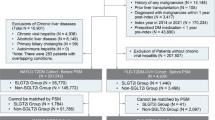
Patients who had initiated maintenance dialysis between January 1, 2009, and December 31, 2017, were initially screened. Dialysis patients in Korea are supported by a copayment assistance policy that covers rare, incurable, malignant, or severe and burdensome diseases through the NHIS. Since the recipients of this program are separately coded, dialysis patients were identifiable in the HIRA database. Maintenance dialysis was defined as the administration of dialysis for at least 3 months. Among the screened patients, those aged ≥ 19 and < 85 years and diagnosed with mono-infection with either HBV or HCV were included. Patients with a history of cancer or organ transplantation prior to dialysis initiation were excluded. A total of 6165 patients undergoing maintenance dialysis with chronic viral hepatitis infection were included in the final analysis (Fig. 1).
Data collection
Baseline demographic data, including data on age and sex, were collected. Comorbidities including diabetes, dyslipidemia, coronary heart disease, congestive heart disease, peripheral vascular disease, cerebral vascular disease, liver cirrhosis, alcoholic liver disease, and fatty liver disease were defined based on the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes and claim records. Data on the presence of chronic hepatitis and the type of viral hepatitis infection were also retrieved. Chronic HBV infection was defined using the codes B16.0, B16.1, B16.2, B16.9, B17.0, B18.0, and B18.1. Chronic HCV infection was defined using the codes B17.1 and B18.2. Medication information was acquired using Anatomical Therapeutic Chemical Classification codes. Detailed operational definitions of comorbidities and medications are provided in Supplementary Table S1.
Statin exposure and outcome measurements
Statin usage was expressed in terms of the defined daily dose (DDD) according to the World Health Organization definition12. Exposure duration was determined by cDDD, which was defined as the sum of the DDDs of any statin during the follow-up period. Atorvastatin, simvastatin, pitavastatin, fluvastatin, rosuvastatin, pravastatin, and lovastatin were identified as statins. Patients who received more than or equal to 28 cDDD of statins in the first 3 months after dialysis commencement were defined as statin users. Statin non-users were defined as those who did not receive statin therapy during this period. The primary outcome was the first diagnosis of HCC, which was identified using the ICD-10 C22.0 code. Data on all-cause mortality events were also collected for competing risk analysis. The observation period ended on December 31, 2018.
Statistical analysis
Continuous variables are presented as the means with standard deviations, and categorical variables are expressed as numbers with percentages. For the main analysis, inverse probability of treatment weighting (IPTW) based on the propensity score was used to balance baseline characteristics between statin users and non-users. Variables included in the propensity score were age; sex; comorbidities of dyslipidemia, diabetes, coronary heart disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, and liver disease (liver cirrhosis, alcoholic liver disease, and fatty liver disease); and the use of aspirin and antiviral agents. Each observation was weighted by the inverse of the probability of patients receiving a statin, which resulted in a pseudo-population wherein exposure was independent of the measured cofounders13. Standardized mean differences were determined to confirm the balance between the groups. Variables were considered to be well balanced when the standardized mean differences after IPTW were < 0.114. To explore the association between statin use and HCC incidence, doubly robust estimation was used, in which the competing risk model was adjusted for covariates included in IPTW estimation15. All-cause death before the primary outcome was considered a competing risk16. Weighted cumulative incidence curve was presented with weighted incidence rate (IR), incidence rate difference (IRD), and incidence rate ratio (IRR) with 95% confidence intervals (CIs). Weighted IR and IRD were presented as per 1000 person-years. Gray’s test for the equivalence of cumulative incidence function was used to compare the weighted cumulative incidence by group17. The sub-distribution hazard ratio (sHR) was assessed with all-cause death as a competing risk to evaluate the association between statin use and HCC incidence. All-cause mortality between the groups was compared using Kaplan–Meier methods. Sensitivity analyses were performed to confirm the main findings.
First, to clarify the potential causal relationship, a dose–response relationship was examined. Patients were categorized as non-users (< 28 cDDD), inconsistent users (< 180 cDDD), and consistent users (≥ 180 cDDD) during the first year after dialysis commencement. Those diagnosed with any type of cancer or who died within 1 year after dialysis initiation were excluded from this analysis. Second, a comparison was performed for patients who had or had not consistently received statin treatment before dialysis initiation. Third, an analysis was performed by excluding patients who had undergone peritoneal dialysis to minimize the effect of the dialysis modality. Fourth, an analysis was performed in patients with and without liver cirrhosis to identify the effect of underlying liver disease on the outcome. Fifth, whether associations with outcomes depended on different types of statins (lipophilic or hydrophilic) were evaluated. Sixth, evaluations were performed without IPTW and without competing risk analysis. Finally, assessments were made in a propensity score-matched cohort in which statin users were matched 1:1 with non-users. All statistical analyses were performed using R (version 3.5.1; http://www.r-project.org; R Foundation for Statistical Computing, Vienna, Austria) and SAS Enterprise Guide (version 6.1; SAS Institute), with P values < 0.05 being considered significant.
Results
Participants
The baseline characteristics of the enrolled patients are shown in Table 1. The mean age was 60.3 ± 12.7 years, and 37.2% of patients were female. Patients with chronic HBV infection comprised 62.0% of the population, while 38.0% of patients were diagnosed with chronic HCV infection. Among the 6165 patients enrolled, 2655 were statin users, and 3510 were statin non-users. Statin users were more likely to have a history of diabetes, dyslipidemia, coronary heart disease, and vascular disease, whereas severe liver diseases (liver cirrhosis, alcoholic liver disease) were less common. There was no difference in the proportion of fatty liver disease between two groups. After IPTW adjustment, the standardized mean differences showed that all variables were well balanced.
Incidence of hepatocellular carcinoma
During a median follow-up of 2.8 years (interquartile range, 1.4–3.4 years), incident HCC occurred in 147 (2.4%) patients, while all-cause death was observed in 1894 (30.7%) patients. Incident HCC was seen in 114 (3.2%) statin non-users and 33 (1.2%) statin users. The weighted IR of HCC was lower in statin users than in statin non-users (IRD, − 3.7; 95% CI, − 5.7 to − 1.7; P < 0.001). Incidence rate ratio (IRR) suggested that statin user was associated with a lower risk for HCC than statin non-user (IRR, 0.56; 95% CI, 0.41–0.78; P < 0.001). The cumulative incidence was significantly higher in statin non-users than in statin users (Gray’s test, P < 0.001, Fig. 2). When the sub-distribution hazard ratio of HCC was assessed, the risk for incident HCC was 41% lower in statin users than in statin non-users (sHR, 0.59; 95% CI, 0.42–0.81; P = 0.001). This relationship remained significant even after adjustments were made for confounding factors (Table 2, Supplementary Table S2). In the weighted Kaplan–Meier analysis, statin use was associated with the lower risk of overall survival (log-rank test P < 0.001) (Supplementary Fig. S1).
Incidence of hepatocellular carcinoma according to the type of viral hepatitis
The incidence of HCC among statin users and non-users was also evaluated separately according to viral hepatitis type. Among those with chronic HBV infection, the weighted IR of HCC was lower in statin users than in statin non-users (IRD, − 4.0; 95% CI, − 6.8 to − 1.2; P = 0.006). IRR suggested that statin user was associated with a lower risk for HCC than statin non-user (IRR, 0.59; 95% CI, 0.40–0.88; P = 0.008). The cumulative incidence was significantly higher in statin non-users than in statin users (Gray’s test, P < 0.001, Fig. 3A). In addition, the risk for incident HCC was significantly lower in statin users than in statin non-users (sHR, 0.62; 95% CI, 0.42–0.91; P = 0.015). A similar finding was observed among patients with chronic HCV infection (Fig. 3B and Table 3).
Sensitivity analyses
When patients were re-classified as non-users (< 28 cumulative defined daily dose [cDDD]), inconsistent users (≥ 28, < 180 cDDD), and consistent users (≥ 180 cDDD), consistent users were associated with a lower risk of incident HCC (adjusted sHR, 0.51; 95% CI, 0.31–0.83; P = 0.007) than non-users. However, such a relationship was not found with inconsistent users (adjusted sHR, 0.95; 95% CI, 0.60–1.50; P = 0.823; Supplementary Table S3). When the effect of consistent statin use was considered, consistent statin use after dialysis commencement was related to a decreased risk of HCC development (Supplementary Table S4). In addition, evaluation of hemodialysis patients revealed findings similar to the main results, suggesting that the relationship between statin use and HCC incidence is independent of the dialysis modality (adjusted sHR, 0.53; 95% CI, 0.37–0.77; P < 0.001; Supplementary Table S4). However, in an evaluation based on the presence of liver cirrhosis, the lower risk of incident HCC in statin users was lost among those with underlying liver cirrhosis (adjusted sHR, 0.82; 95% CI, 0.50 to 1.35; P = 0.434; Supplementary Table S5). When considering the type of statin, incident HCC risk was significantly reduced in both lipophilic (adjusted sHR, 0.58; 95% CI, 0.41–0.82; P = 0.004) and hydrophilic statin (adjusted sHR, 0.47; 95% CI, 0.27–0.82; P = 0.015) users compared to non-users (Supplementary Table S6). Assessments performed without IPTW (adjusted sHR, 0.50; 95% CI, 0.33–0.77; P = 0.001) or without considering the competing risk (adjusted HR, 0.52; 95% CI, 0.37–0.72; P < 0.001) also revealed that the incident HCC risk was lower in statin users (Supplementary Tables S7and S8). Lastly, additional assessments using a 1:1 propensity score matched cohort were consistent with the main analysis (adjusted sHR 0.42; 95% CI, 0.27–0.68; P = < 0.001) (Supplementary Tables S9 and S10).
Discussion
Statin use was associated with a reduced risk of HCC incidence in a nationwide evaluation of 6165 dialysis patients with chronic viral hepatitis, a high-risk group for HCC. During a median follow-up duration of 2.8 years after dialysis commencement, the risk of HCC was 41% lower in statin users than in statin non-users. This relationship was independent of viral hepatitis type, comorbidities, or the prescription of concomitant medications known to influence HCC risk, such as aspirin and antiviral agents.
The reduction in incident HCC risk found in this study has been previously recognized in several patient populations. Statin use was associated with a decreased risk of HCC and HCC-related death in high-risk groups such as chronic HBV and HCV carriers18,19,20,21,22,23,24, patients with non-alcoholic liver disease25, or diabetes mellitus26,27. In a recent nested case–control study, statin use was demonstrated to lower the risk of HCC development in the general population28. CKD patients aged over 50 who have not yet initiated dialysis are recommended to use statin for cardiovascular disease prevention in current guideline. Considering the high frequency of risk factors for HCC in CKD patients, statin use might have a protective effect on HCC in patients with CKD regardless of viral hepatitis infection. Meanwhile, dialysis patients have been considered “statin resistant” based on clinical studies that have failed to recapture the efficacy of statins against cardiovascular disease noticed among the non-dialysis population. In large-scale prospective trials, such as the Die Deutsche Diabetes Dialyse (4D) study, the rate of mortality from all cardiac causes did not differ between the statin and placebo groups, despite the significant decline in low-density lipoprotein levels in the statin group3. Complex lipid abnormalities such as highly oxidized or carbamylated lipoproteins found in uremic patients have been suspected as a cause of this discrepancy in the statin effect between patients undergoing and not undergoing dialysis29. In addition, the increase in intracellular cholesterol synthesis, which is not fully inhibited by statin use, under chronic inflammatory conditions such as chronic dialysis has also been postulated to play a role in statin resistance30. The fact that the risk of HCC development was significantly lower among statin users in this study suggests that patients undergoing dialysis are not resistant to the antineoplastic effect of statins found in those without kidney disease. Considering that the risk of HCC development is increased among dialysis patients compared to that in the general population and that the initiation of statin therapy is discouraged by current guidelines, the results of the current study may open the possibility of statin use being suggested in dialysis patients with high HCC risk.
Statin use was not associated with HCC risk reduction in the subgroups with underlying liver cirrhosis. In contrast to this finding, several previous evaluations in the non-dialysis population have shown that the preventive effect of statins against HCC was maintained in patients with liver cirrhosis. A recent nested case–control study of 1642 HCC patients revealed that statin use was significantly associated with a reduction in HCC incidence in patients with liver cirrhosis as well as in those without liver cirrhosis28. The relatively small number of statin users with liver cirrhosis in this study could have played a role in producing a statistically insignificant relationship between statin use and HCC incidence. However, the probability that statin treatment alone may not be sufficient to prevent HCC development in high-risk patients, such as those with liver cirrhosis under uremic conditions, should also be considered.
In this study, only 39.8% of statin users had dyslipidemia. Since patients with kidney disease are a high-risk group for cardiovascular diseases, statins would have been also prescribed for cardio-protective purposes, in addition to the goal of managing dyslipidemia. The fact that 37.1% of the participants were reported to have a medical history of cardiovascular disease (coronary heart disease, congestive heart failure, peripheral vascular disease, or cerebral vascular disease) in this study supports this possibility.
The preventive effects of statins on HCC development could be attributed to several mechanisms. Simvastatin, fluvastatin, and lovastatin have been shown to induce a selective apoptotic effect in human HCC cell lines31,32. Statins, including atorvastatin, have been found to block MYC phosphorylation, resulting in tumor-suppressive effects33,34. Among patients with HBV, the transcriptional activation of HBV protein X alters the expression of growth control genes such as Ras, Raf, MAPK, and ERK35. By inhibiting the mevalonate pathway, statins have been demonstrated to effectively prevent the detrimental consequences of the signaling proteins encoded by these genes11. HCV is known to stimulate nuclear factor κB, resulting in chronic inflammation, a neoplastic-prone state36. HCV infection also promotes cell growth by downregulating members of the growth arrest and DNA damage (Gadd45) gene family37. Statins have been recognized to effectively counter these effects, which could lead to anti-tumor properties.
This study has several strengths. First, this study evaluated an unbiased selection from a nationwide dialysis population. Due to the obligatory copayment assistance policy of the Korean NHIS, dialysis patients and those diagnosed with cancerous disease are coded separately in the HIRA database. This allowed the detection of an entire population of patients undergoing chronic dialysis as well as those diagnosed with HCC during the study period. Second, double robust estimation, adjusting for covariates after IPTW application, was used. Since statin treatment is dependent on underlying metabolic abnormalities and comorbidities that may affect HCC development, such an evaluation strategy would further strengthen the possible independent association between statin use and incident HCC.
However, the findings of this study should be interpreted in light of the following limitations. First, limitations due to the observational nature of the study should be considered. Although a significant association between statin use and HCC development was found, the cause-effect relationship should be further assessed in future prospective evaluations. Second, due to the nature of health insurance claim data, the possibility of missing diagnostic codes for comorbidities including HBV or HCV infection, diabetes, dyslipidemia, and liver disease cannot be excluded. While claims database analysis has advantage of utilizing a large sized data, this analysis is potentially susceptible to errors from inaccuracies. However, screening for viral hepatitis (both B and C) is routinely performed before starting dialysis in real-world practice, and HIRA periodically performs a nationwide obligatory quality assessment which includes viral serologic tests, lowering the possibility of HBV or HCV carriers not being detected. In addition, specific malignant disease insurance code that were issued by the Korea NHIS (V193) was utilized for accurate HCC diagnosis although tumor staging data were not available. Third, as the evaluation used claims data from a national insurance service database, potential confounding variables including lifestyle factors; anthropometric factors; and laboratory information including hepatitis viral copy number, liver function, tumor markers, and lipid abnormalities could not be examined. Further analyses considering laboratory information associated with liver function would be needed to reduce the possibility of selection bias. Fourth, this study was conducted in a single nation, with a predominately Asian population, in which HBV and HCV infection rates are relatively higher than those in Western countries. Therefore, to generalize these findings, assessments including other populations wherein viral hepatitis is not the predominant cause of HCC should be performed.
In conclusion, this nationwide observational study showed that statin use was associated with a reduced risk of incident HCC in chronic dialysis patients with HBV or HCV infection. The decrease in risk was independent of comorbidities and was evident regardless of the viral hepatitis type. Further prospective trials are needed to verify the protective effects of statins against HCC in this patient group.
Data availability
The data underlying this article are available through the Health Insurance Review and Assessment Service (HIRA) (https://opendata.hira.or.kr). Data usage was permitted by the national health information data request review committee of the HIRA.
References
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377, 2181–2192. https://doi.org/10.1016/s0140-6736(11)60739-3 (2011).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353, 238–248. https://doi.org/10.1056/NEJMoa043545 (2005).
Fellström, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360, 1395–1407. https://doi.org/10.1056/NEJMoa0810177 (2009).
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Lin, H. F. et al. Increased risk of cancer in chronic dialysis patients: A population-based cohort study in Taiwan. Nephrol. Dial. Transplant. 27, 1585–1590. https://doi.org/10.1093/ndt/gfr464 (2012).
Schupp, N., Heidland, A. & Stopper, H. Genomic damage in endstage renal disease-contribution of uremic toxins. Toxins (Basel) 2, 2340–2358. https://doi.org/10.3390/toxins2102340 (2010).
Ribot, S., Rothstein, M., Goldblat, M. & Grasso, M. Duration of hepatitis B surface antigenemia (HBs Ag) in hemodialysis patients. Arch. Intern. Med. 139, 178–180 (1979).
Froio, N. et al. Contamination by hepatitis B and C viruses in the dialysis setting. Am. J. Kidney Dis. 42, 546–550. https://doi.org/10.1016/s0272-6386(03)00787-x (2003).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462. https://doi.org/10.1056/NEJMra1713263 (2019).
Boudreau, D. M., Yu, O. & Johnson, J. Statin use and cancer risk: A comprehensive review. Expert Opin. Drug Saf. 9, 603–621. https://doi.org/10.1517/14740331003662620 (2010).
Mullen, P. J., Yu, R., Longo, J., Archer, M. C. & Penn, L. Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 16, 718–731. https://doi.org/10.1038/nrc.2016.76 (2016).
WHO Collaborating Centre for Drug Statistics Methodology: ATC classification index with DDDs and Guidelines for ATC classification and DDD assignment (2021).
Austin, P. C. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat. Med. 29, 2137–2148. https://doi.org/10.1002/sim.3854 (2010).
Austin, P. C. & Stuart, E. A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 34, 3661–3679. https://doi.org/10.1002/sim.6607 (2015).
Funk, M. J. et al. Doubly robust estimation of causal effects. Am. J. Epidemiol. 173, 761–767. https://doi.org/10.1093/aje/kwq439 (2011).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999).
Gray, R. J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 16, 1141–1154 (1988).
Tsan, Y. T., Lee, C. H., Wang, J. D. & Chen, P. C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 30, 623–630. https://doi.org/10.1200/jco.2011.36.0917 (2012).
Tsan, Y. T. et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J. Clin. Oncol. 31, 1514–1521. https://doi.org/10.1200/jco.2012.44.6831 (2013).
Hsiang, J. C. et al. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J. Hepatol. 63, 1190–1197. https://doi.org/10.1016/j.jhep.2015.07.009 (2015).
Mohanty, A., Tate, J. P. & Garcia-Tsao, G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology 150, 430-440.e431. https://doi.org/10.1053/j.gastro.2015.10.007 (2016).
Simon, T. G. et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: Results from a nationwide Swedish population. Ann. Intern. Med. 171, 318–327. https://doi.org/10.7326/m18-2753 (2019).
Goh, M. J. et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic hepatitis B. Hepatology 71, 2023–2032. https://doi.org/10.1002/hep.30973 (2020).
Papatheodoridis, G. V., Chan, H. L., Hansen, B. E., Janssen, H. L. & Lampertico, P. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. J. Hepatol. 62, 956–967. https://doi.org/10.1016/j.jhep.2015.01.002 (2015).
German, M. N., Lutz, M. K., Pickhardt, P. J., Bruce, R. J. & Said, A. Statin use is protective against hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A case-control study. J. Clin. Gastroenterol. 54, 733–740. https://doi.org/10.1097/mcg.0000000000001260 (2020).
El-Serag, H. B., Johnson, M. L., Hachem, C. & Morgana, R. O. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 136, 1601–1608. https://doi.org/10.1053/j.gastro.2009.01.053 (2009).
Kim, G. et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. Int. J. Cancer 140, 798–806. https://doi.org/10.1002/ijc.30506 (2017).
Kim, G., Jang, S. Y., Nam, C. M. & Kang, E. S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J. Hepatol. 68, 476–484. https://doi.org/10.1016/j.jhep.2017.10.018 (2018).
Kassimatis, T. I. & Goldsmith, D. J. Statins in chronic kidney disease and kidney transplantation. Pharmacol. Res. 88, 62–73. https://doi.org/10.1016/j.phrs.2014.06.011 (2014).
Chen, Y. et al. Inflammatory stress induces statin resistance by disrupting 3-hydroxy-3-methylglutaryl-CoA reductase feedback regulation. Arterioscler. Thromb. Vasc. Biol. 34, 365–376. https://doi.org/10.1161/atvbaha.113.301301 (2014).
Relja, B. et al. Simvastatin inhibits cell growth and induces apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int. J. Mol. Med. 26, 735–741. https://doi.org/10.3892/ijmm_00000520 (2010).
Kah, J. et al. Selective induction of apoptosis by HMG-CoA reductase inhibitors in hepatoma cells and dependence on p53 expression. Oncol. Rep. 28, 1077–1083. https://doi.org/10.3892/or.2012.1860 (2012).
Cao, Z. et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297. https://doi.org/10.1158/0008-5472.Can-10-3367 (2011).
Shachaf, C. M. et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 431, 1112–1117. https://doi.org/10.1038/nature03043 (2004).
Arbuthnot, P., Capovilla, A. & Kew, M. Putative role of hepatitis B virus X protein in hepatocarcinogenesis: Effects on apoptosis, DNA repair, mitogen-activated protein kinase and JAK/STAT pathways. J. Gastroenterol. Hepatol. 15, 357–368. https://doi.org/10.1046/j.1440-1746.2000.02069.x (2000).
Elsharkawy, A. M. & Mann, D. A. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 46, 590–597. https://doi.org/10.1002/hep.21802 (2007).
Higgs, M. R., Lerat, H. & Pawlotsky, J. M. Downregulation of Gadd45beta expression by hepatitis C virus leads to defective cell cycle arrest. Cancer Res. 70, 4901–4911. https://doi.org/10.1158/0008-5472.Can-09-4554 (2010).
Author information
Authors and Affiliations
Contributions
J.T.P. contributed to the concept and design of the study, supervised the study, and revised drafts of article; H.W.K. contributed to the concept and design of the study, analyzed, performed the statistical analysis, interpreted the data, wrote the first draft, and revised the article; Y.S.J., S.C.K. and H.K. performed the statistical analysis, and interpreted the data; S.H.H., T-H.Y. and S-W.K. were involved in scientific discussion. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, H., Joo, Y., Kang, S. et al. Association of statin treatment with hepatocellular carcinoma risk in end-stage kidney disease patients with chronic viral hepatitis. Sci Rep 12, 10807 (2022). https://doi.org/10.1038/s41598-022-14713-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14713-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.