Abstract
Wait times are associated with mortality on waiting list for transcatheter aortic valve replacement (TAVR). Whether longer wait times are associated with long term mortality after successful TAVR remains unassessed. Consecutive patients successfully treated with elective TAVR in our center between January 2013 and August 2019 were included. The primary end point was one-year all-cause mortality. TAVR wait times were defined as the interval from referral date for valve replacement to the date of TAVR procedure. A total of 383 patients were included with a mean wait time of 144.2 ± 83.87 days. Death occurred in 55 patients (14.4%) at one year. Increased wait times were independently associated with a relative increase of 1-year mortality by 2% per week after referral (Adjusted Hazard Ratio 1.02 [1.002–1.04]; p = 0.02) for TAVR. Chronic kidney disease, left ventricular ejection fraction ≤ 30%, access site and STS score were other independent correlates of 1-year mortality. Our study shows that wait times are relatively long in routine practice and associated with increased 1-year mortality after successful TAVR. Such findings underscore the need of strategies to minimize delays in access to TAVR.
Similar content being viewed by others
Introduction
Transcatheter aortic valve replacement (TAVR) is an effective and increasingly used procedure for patients with severe aortic stenosis (AS). While it represents the sole or preferred strategy in inoperable and high-risk patients1,2, it also appears as an alternative to surgical aortic valve replacement for intermediate and low risk patients3,4,5,6. This paradigm shift in the approach for patients with severe aortic stenosis has led to a rapid increase of TAVR procedures with > 350,000 procedures performed in > 70 countries in the past 15 years7. A recent projection estimated that approximately 180,000 patients can be considered potential TAVR candidates annually in the European Union and Northern-America8.
This dramatic growth in the demand for TAVR has challenged current capacities which in turn have prolonged wait times9. Longer wait-times are associated with increasing mortality and hospitalizations related to heart failure while on the waiting list9,10. Moreover, patients referred for TAVR—mostly elderly—could experience a decline in their functional status while waiting11. Morbidity associated with wait times may worsen post procedural outcome. The influence of prolonged wait times on late post-TAVR outcomes remains unassessed.
Our study aimed to assess the impact of wait times before TAVR on 1-year mortality in patients successfully treated with TAVR in a real-life cohort.
Methods
Study population
All consecutive patients successfully treated with elective TAVR in Caen University Hospital between January 2013 and August 2019 and enrolled in the nationwide FRANCE-TAVI or FRANCE-2 registries12, were included in our analysis. Urgent TAVR in patients who required a TAVR procedure during a concurrent hospitalization for a cardiovascular event13 were excluded. The registry was approved by the Institutional Review Board of the French Ministry of Higher Education and Research and by the National Commission for Data Protection and Liberties. Patients provided written informed consent before inclusion. All methods were performed in accordance with the relevant guidelines and regulations. The decision to perform TAVR was made by the local heart team after careful individualized evaluation as recommended14. All patients underwent a comprehensive transthoracic echocardiography (iE33, Philips, Amsterdam, Netherlands) before TAVR. The severity of aortic stenosis and left ventricular ejection fraction were evaluated according to guidelines15. Pre-procedural aortic annular sizing and vascular access assessment were performed using multidetector computed tomography (MDCT). Patients received dual antiplatelet therapy with aspirin and/or clopidogrel, or oral anticoagulation as clinically indicated. Wait times were defined as the interval from referral date for valve replacement to the date of the TAVR.
Clinical endpoints
The primary end point was all-cause mortality at 1 year. Other outcomes of interest were: rehospitalization for a cardiac event (heart failure, arrhythmia and acute coronary syndrome), computed tomography-scan or magnetic resonance imaging proven stroke or transient ischemic attack, new cardiac pacemaker, acute kidney injury (increase in serum creatinine of 50% or increase of ≥ 0.3 mg/dL compared to baseline) and bleeding defined according to the Valve Academic Research Consortium 2 criteria16 at 1-year follow-up.
Statistical analysis
Patients were separated into two groups defined by their status dead or alive at 1 year follow-up. Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as numbers of patients and percentages. Baseline characteristics were compared between groups using Student’s t and the Chi2 tests where adequate.
The relationship between wait time and 1-year mortality, and the calculation of the Hazard Ratio (HR) and its 95% Confidence Interval (CI), was estimated using Cox regression models, unadjusted and adjusted on pre-specified variables (age, sex, body mass index (BMI), Society for Thoracic Surgeons (STS) score, medical history of diabetes, chronic kidney disease (CKD) and severe left ventricular systolic dysfunction defined as left ventricular ejection fraction, LVEF ≤ 30%). Sensitivity analyses were performed: one analysis (I) adjusted on all variables differently distributed between the two groups (Table 1) and considering wait time as a categorical variable (≤ or > 12 weeks17); another analysis adjusted on calendar year periods added to the adjusted model. Additional analysis including only trans-femoral TAVR was added and presented in “Supplementary appendix”. The relationship between wait time and secondary outcomes was assessed using logistic regression models for categorical and continuous variables respectively. A p-value of < 0.05 was considered statistically significant. SAS 9.4 (SAS Institute Inc., Cary. NC. USA) was used for statistical analysis.
Results
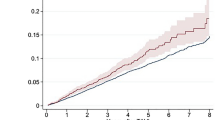
After excluding 35 urgent TAVR, a total of 383 patients were included in our study. Death occurred in 21 (5.5%) patients and 55 (14.4%) patients at 30 days and 1 year, respectively. Baseline demographic and procedural characteristics of the population are detailed in Table 1. The mean wait time was 144.2 ± 83.87 days. Patients who died had higher STS scores (14.7 ± 13.5 vs 11.8 ± 8.4; p = 0.03), more often CKD (67.3% vs 42.7%; p = 0.02), LVEF ≤ 30% (5.5% vs 1.5%; p < 0.01), right ventricular failure (16.4% vs 7.9%; p = 0.03), less trans-femoral approaches (76.4% vs 92.3%; p < 0.01) and longer wait times (168 ± 113 vs 140.2 ± 77.5 days; p = 0.01) compared to those alive at 1 year.
Correlates of 1 year-mortality
In the unadjusted analysis, wait times, CKD, LVEF ≤ 30% and RV failure were associated with increased, while femoral approach was associated with decreased mortality rates at 1 year (Table 2). In the adjusted analysis, wait times (HR 1.02 [1.002–1.04]; p = 0.02), STS score (HR 1.02 [1.001–1.05]; p = 0.03), CKD (HR 3.51 [1.87–6.3]; p < 0.01) and LVEF ≤ 30% (HR 10.05 [3.8–37]; p < 0.01) remained associated with higher and femoral approach with lower mortality rates at 1 year (HR 0.41 [0.2–0.79]; p < 0.01). Sensitivity analyses confirmed significant associations for wait times, CKD and LVEF ≤ 30%. These results were confirmed when analysis was restricted to trans-femoral TAVR (“Supplementary appendix”).
Apart from 1-year mortality, other outcomes were not significantly associated with wait times (Table 3). Echocardiography at 1 year follow-up is described in the “Supplementary appendix”.
Discussion
Mortality at 1 year follow-up remains important after TAVR. The wait time before TAVR was relatively important in our cohort and associated with a 2% per week increase of rates of 1-year mortality independent of other correlates of mortality. CKD, LVEF ≤ 30%, access site and STS score were other independent correlates of mortality.
The expansion of TAVR indications has rapidly challenged current capacities leading to a progressive increase in wait times over the last decade9. The mean wait time in our study (144 days) appears important. This remains poorly described in the literature with a mean time varying between 89 and 132 days9,10,13,18. Such delay in our cohort may be explained by the fact that our center is a tertiary reference center for a mostly rural region.
Previous studies have reported that 2–14% of patients referred for TAVR die while on the waiting list10,18,19. Recently, a study focused on the impact of wait times on early post-TAVR outcomes and found a somewhat unexpected higher hazard associated with short wait times13. This relationship was entirely mediated by the emergency status with urgent patients having worse outcomes. After adjusting on this point, there was no longer a relationship between wait times and 30-day mortality. Our study confirms the lack of association between wait times and early mortality in elective TAVR patients. However, we found a significant relationship between wait times and 1-year mortality with a relative increase of 2% per week after referral. Sensitivity analyses confirmed this result taking into account other covariables, the possible technical improvements over the study period or the preferential use of transfemoral access. Our data are consistent with a prior study showing dramatically higher 1-year mortality in patients who undergo TAVR after an initial refusal20.
Our findings may be explained by a decline of the patients’ functional status while on the waiting list. Decreased cognition, alteration of mobility and renal function are known factors of poor outcomes and recovery after TAVR21. Previous studies have reported that frailty scores, which are known to be associated with 1-year mortality22, worsened in elderly patients while waiting for TAVR11. Additionally, myocardial overload may get worse leading to heart failure and reduced ventricular function which represents a prognostic turning point in AS23,24.
Previous studies and our results highlight the need for strategies to minimize delays in access to TAVR and identifying high-risk patients who require a faster processing. Knowing the need of multiple disciplines to evaluate these patients25, optimal coordination of care may reduce wait times. The simplification of the procedure performed under local anesthesia which are faster and consume less medical resources, represent a valuable option26,27. Individualized risk stratification to consider the urgency of the TAVR is important. The recent position paper of the Canadian Cardiovascular Society recommends performing TAVR within 2 weeks for urgent cases and within 12 weeks for elective cases17. Several conditions highlighted in our study and others, such as CKD, STS score, left ventricular systolic dysfunction18,19,21,28 may help determining the ideal timing of TAVR.
Limitations
Given the observational nature of the study, different known or unknown correlates of outcomes may have not been considered in the analyses. The time from referral to TAVR may underestimate the magnitude of effect of longer wait times on outcomes as compared to the time symptom onset (unknown in our cohort). However, our definition of wait times before TAVR remains the most accurately assessable and widely used10,13. Finally, our center is the reference center of a semi-rural region and our results may not apply to all regions.
Conclusion
Patients awaiting TAVR represent a growing population. Our study shows that wait times remain important in daily practice and are associated with a 2% per week increase of 1-year mortality after referral. Our findings underscore the need for physicians and health system administrators to minimize such delays in order to improve prognosis.
References
Adams, D. H. et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 370, 1790–1798. https://doi.org/10.1056/NEJMoa1400590 (2014).
Smith, C. R. et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364, 2187–2198. https://doi.org/10.1056/NEJMoa1103510 (2011).
Leon, M. B. et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 374, 1609–1620. https://doi.org/10.1056/NEJMoa1514616 (2016).
Mack, M. J. et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 380, 1695–1705. https://doi.org/10.1056/NEJMoa1814052 (2019).
Popma, J. J. et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 380, 1706–1715. https://doi.org/10.1056/NEJMoa1816885 (2019).
Reardon, M. J. et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 376, 1321–1331. https://doi.org/10.1056/NEJMoa1700456 (2017).
Barbanti, M., Webb, J. G., Gilard, M., Capodanno, D. & Tamburino, C. Transcatheter aortic valve implantation in 2017: State of the art. EuroIntervention 13, AA11–AA21 (2017).
Durko, A. P. et al. Annual number of candidates for transcatheter aortic valve implantation per country: Current estimates and future projections. Eur. Heart J. 39, 2635–2642. https://doi.org/10.1093/eurheartj/ehy107 (2018).
Albassam, O. et al. Increasing wait-time mortality for severe aortic stenosis: A population-level study of the transition in practice from surgical aortic valve replacement to transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 13, e009297. https://doi.org/10.1161/circinterventions.120.009297 (2020).
Elbaz-Greener, G. et al. Temporal trends and clinical consequences of wait times for transcatheter aortic valve replacement: A population-based study. Circulation 138, 483–493. https://doi.org/10.1161/circulationaha.117.033432 (2018).
Forman, J. M., Currie, L. M., Lauck, S. B. & Baumbusch, J. Exploring changes in functional status while waiting for transcatheter aortic valve implantation. Eur. J. Cardiovasc. Nurs. 14, 560–569. https://doi.org/10.1177/1474515114553907 (2015).
Auffret, V. et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 70, 42–55. https://doi.org/10.1016/j.jacc.2017.04.053 (2017).
Elbaz-Greener, G. et al. Association between wait time for transcatheter aortic valve replacement and early postprocedural outcomes. J. Am. Heart Assoc. 8, e010407. https://doi.org/10.1161/jaha.118.010407 (2019).
Vahanian, A. et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 29, 1463–1470. https://doi.org/10.1093/eurheartj/ehn183 (2008).
Vahanian, A. et al. Guidelines on the management of valvular heart disease (version 2012). Eur. Heart J. 33, 2451–2496. https://doi.org/10.1093/eurheartj/ehs109 (2012).
Kappetein, A. P. et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 33, 2403–2418. https://doi.org/10.1093/eurheartj/ehs255 (2012).
Asgar, A. W. et al. 2019 Canadian Cardiovascular Society Position Statement for transcatheter aortic valve implantation. Can. J. Cardiol. 35, 1437–1448. https://doi.org/10.1016/j.cjca.2019.08.011 (2019).
González Saldivar, H. et al. Prognosis of patients with severe aortic stenosis after the decision to perform an intervention. Rev. Esp. Cardiol. (Engl. Ed.) 72, 392–397. https://doi.org/10.1016/j.rec.2018.03.023 (2019).
Nuis, R. J. et al. Patients with aortic stenosis referred for TAVI: Treatment decision, in-hospital outcome and determinants of survival. Neth. Heart J. 20, 16–23. https://doi.org/10.1007/s12471-011-0224-z (2012).
Shimura, T. et al. Patients refusing transcatheter aortic valve replacement even once have poorer clinical outcomes. J. Am. Heart Assoc. 7, e009195. https://doi.org/10.1161/jaha.118.009195 (2018).
Arnold, S. V. et al. Predictors of poor outcomes after transcatheter aortic valve replacement: Results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation 129, 2682–2690. https://doi.org/10.1161/circulationaha.113.007477 (2014).
Kiani, S. et al. The effect and relationship of frailty indices on survival after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 13, 219–231. https://doi.org/10.1016/j.jcin.2019.08.015 (2020).
Kang, D. et al. Mortality predictors in patients referred for but not undergoing transcatheter aortic valve replacement. Am. J. Cardiol. 116, 919–924. https://doi.org/10.1016/j.amjcard.2015.06.014 (2015).
Makkar, R. R. et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 366, 1696–1704. https://doi.org/10.1056/NEJMoa1202277 (2012).
Généreux, P. et al. Transcatheter aortic valve implantation 10-year anniversary: Review of current evidence and clinical implications. Eur. Heart J. 33, 2388–2398. https://doi.org/10.1093/eurheartj/ehs220 (2012).
Husser, O. et al. Conscious sedation versus general anesthesia in transcatheter aortic valve replacement: The German Aortic Valve Registry. JACC Cardiovasc. Interv. 11, 567–578. https://doi.org/10.1016/j.jcin.2017.12.019 (2018).
Thiele, H. et al. General versus local anesthesia with conscious sedation in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Circulation 142, 1437–1447. https://doi.org/10.1161/circulationaha.120.046451 (2020).
Furer, A. et al. Effect of baseline left ventricular ejection fraction on 2-year outcomes after transcatheter aortic valve replacement: Analysis of the PARTNER 2 trials. Circ. Heart Fail. 12, e005809. https://doi.org/10.1161/circheartfailure.118.005809 (2019).
Author information
Authors and Affiliations
Contributions
V.R. and I.R.: formal analysis, writing—original draft; A.L., K.B., M.B., C.B. and R.S.: investigation, data curation, validation; F.B.: writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roule, V., Rebouh, I., Lemaitre, A. et al. Impact of wait times on late postprocedural mortality after successful transcatheter aortic valve replacement. Sci Rep 12, 5967 (2022). https://doi.org/10.1038/s41598-022-09995-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09995-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.