Abstract
Besides anthropometric variables, high-order body representations have been hypothesised to influence postural control. However, this has not been directly tested before. Moreover, some studies indicate that sex moderates the relationship of anthropometry and postural control. Therefore, as a proof of concept we investigated the association of body representations with postural control as well as the influence of participants’ sex/gender. Body image measures were assessed with a figural drawing task. Body schema was tested by a covert and an overt task. Body sway was measured during normal bipedal quiet standing with eyes closed (with/without neck extended). Statistical analysis consisted of hierarchical multiple linear regressions with the following regression steps: (1) sensory condition, (2) sex/gender, (3) age, (4) anthropometry, (5) body schema, (6) body image, (7) sex/gender-interactions. Across 36 subjects (19 females), body schema was significantly associated with body sway variability and open-loop control, in addition to commonly known influencing factors, such as sensory condition, gender, age and anthropometry. While in females, also body image dissatisfaction substantially was associated with postural control, this was not the case in males. Sex differences and possible causes why high-order body representations may influence concurrent sensorimotor control of body sway are discussed.
Similar content being viewed by others
Introduction
A specific domain of motor control, which is constantly and mainly unconsciously involved in daily life is, postural control. By maintaining the center of mass (CoM) within the base of support (BoS), it helps to stay upright and prevents from falling1, e.g. when standing, walking or also when performing sports. Postural control during quiet standing is influenced by several factors, such as age2,3, biological sex4,5,6, anthropometry7,8,9, stance condition or BoS3 and the available sensory channels10. When being involved in a secondary task or a suprapostural task, like often in everyday life, postural control further depends on the type and constraints of a task showing differences e.g. in the positional variability11,12,13,14, thus is task-specific. Often the functional postural control goal is to reduce sway variability to facilitate suprapostural task performance11,12,13,14 However, the postural control goal could also sometimes be to increase sway variability and perform exploratory movements to gain more information about the environment and the orientation of the own body in space15,16,17.
Since body sway during quiet standing is often simulated by a single inverted pendulum model18,19, one important factor influencing human body sway, from biomechanical point of view, is anthropometry, such as body height and body weight/mass7,9, as well as body morphology4,20. Even though the single inverted pendulum discards multi-joint movements compared to the double inverted pendulum model, which is commonly used to capture intra-personal coordination patterns between ankle and hip movements21,22,23,24, the single inverted pendulum model has been argued to functionally correctly capture body sway during quiet standing, using the ankle strategy25, in a simplified and practically acceptable way18.
Moreover, as mentioned before, sex differences have also been reported to affect body sway; whereby, females often show less body sway than males4,6. However, when body sway is normalised by body height or weight, previously observed sex differences disappear9, which indicate anthropometry mediating the influence of gender on body sway. In spite of that, sex differences have also been reported in the relationship of anthropometry and body sway7; thereby some studies have reported a greater influence of anthropometry on body sway in males than in females7, while others have reported the opposite26. The previously reported influencing factors, however, can only explain up to approximately 47% of the inter-individual variability in human body sway3,8,26.
Therefore, other factors might additionally play a role, such as eventually aspects of higher order representations. For example, in the work of Forghieri and colleagues27 when subjects saw a mirror image or their ideal model image during quiet standing, patients with eating disorders, who also showed a higher body dissatisfaction level, had a greater sway increase compared to the control group. Body dissatisfaction addresses one component of body image, which is defined as a conscious, persistent representation of our body size and shape28, and contains different components attributed to the own body shape, such as the physical appearance (e.g. BID: body image distortion) and emotional attitudes (e.g. BIDS: body image dissatisfaction)29. It is influenced by social and cultural expectations30. Moreover, body image dissatisfaction differs in gender29, whereby a greater dissatisfaction is observed among females compared to males. Consequently, disorders involving a distorted body image, are also more prevalent in females29. Thus, gender differences in the influence of anthropometry on body sway might be due to differences in the role of high-order body representations, such as the body image.
Another type of high-order body representations, that has been discussed to influence motor control, is the body schema31. The body schema can be described as an unconscious, dynamic, multi-referential, spatio-temporal and somatotopic real-time representation of our body’s metrics and configuration in space, which is action- and task-relevant28,31. In the context of postural control body schema is often referred to as an internal model32,33,34. Body sway can be generally described by two control mechanisms: the feed-forward and the feedback control35,36. The feed-forward control is often discussed to be based on an internal body representation of geometry, kinetics, verticality and reference frames inducing voluntary and anticipatory movements32. Respectively, the feed-forward component is attributed to the slow dynamics of body sway, accounting for most of the body sway37, whereas the fast dynamics represents the corrective feedback component38.
In everyday life, when physically interacting with the environment, the control system point of view on motor control involves internal representations of the own body and its immediate environment (e.g. the peripersonal space)39 in addition to the integration of diverse sensory modalities. Since our body schema receives continuous feedback from various sensory channels during overt movements, it is continuously updated by motor experience and given a current task (e.g. tool-use40,41); thus, it is discussed to improve with movement experience32, as well as with increased proprioceptive awareness/reliance or self-awareness, such as observed in dancers42,43,44. Moreover, mental simulation of an action has been shown to share central-nervous processes with real actions45, while not including movement-related sensory feedback. Consequently, the body schema is presumably needed to enable performing both overt (actual) and covert (simulations or motor imagery) movements/actions46 and is supposed to merge both motor cognition and motor control31.
A central assumption from an ecological point of view is that humans are “informavores”, that ingest information47. According to this interpretation, which does not assume the existence of internal models, the postural control system actively searches for information by purposefully generating sensory feedback to estimate the equilibrium state of the body. This “active” sensation and perception has been defined by Bajcsy48 as an intelligent sensory data acquisition process. In the domain of postural control, Riccio and Stoffregen49 demonstrated that active perception of body orientation is grounded on the perception of corrective actions required to keep a chosen tilted or listing posture. They suggested that a trade-off between less effortful postural corrections when nearer to the equilibrium point and more reliable interpretation of the forces acting on the body with greater deviation from the equilibrium point governs postural control49. Similarly, Riley et al.16 contrasted body sway dynamics during normal upright standing and forward leaning and concluded that the short-term dynamics of normal standing consists of a greater amount of exploratory behaviour due to the greater distance to the limits of stability. An extension of the ecological point of view was proposed by Stoffregen and co-workers14. They assumed that postural control takes part as a component of a purposeful perception-action coupling, so that body sway is actively modulated to assist in any suprapostural tasks requiring oculomotor or tactile precision50,51,52.
Furthermore, some modeling approaches sucessfully simulate patterns of human behaviour, e.g. sway patterns, by a pure feedback control model53. However, anticipation of situations and movements in the near future are important to reduce time delay54,55 and to create smoother trajectories56. Moreover, the exploratory behaviour described in previous studies15,17, has also been discussed in relation to the feed-forward control of the two-fold control mechanisms underlying postural control16, which was first proposed as open-loop control time-periods by Collins and De Luca35 by performing a stabilogram diffusion analysis on centre of pressure (CoP) measurements.
Since knowledge of the size and weight of the own body and its segments, and especially about its current inertia and postural configuration in space in a given situation are important regarding the control systems’ approach30,31 when moving, body schema is discussed as a factor that influences motor control31 in general, and postural control more specifically33,34. Moreover, body schema and body image have been discussed to interact with each other in a co-constructive manner and reshape one another30.
To better understand inter-individual differences in body sway control, the aim of this study is to investigate the relationship between body representations and postural control and the influence of a participant’s gender on the role of associated factors. We expect body image and body schema to be related to postural control in addition to the commonly reported influencing factors, such as sensory condition, age, gender and anthropometry. Furthermore, gender differences are expected to alter the role of anthropometry and body representations on body sway control.
This study is the first directly testing the association of high-order body representations with postural control. We discuss how high-level representations of the self, both body image and body schema, may be involved in predictive human body sway control and how this relationship may be moderated by gender and biological sex. In order to investigate the relationship of these factors we conduct a hierarchical regression analysis of body sway parameters with measures of known influencing factors and aspects of body representations.
Results
Relationship between body representation and postural control
Body sway variability
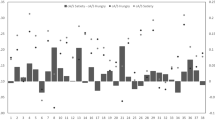
For all 36 subjects (19 females), body schema measures (overt task: inverted Taking-a-Posture task variability (\({TaP}_{\mathrm{v}}\)), covert task: Laterality accuracy (\({LAT}_{\mathrm{a}}\))) significantly increase the goodness of fit (\(R^2\)) of the hierarchical multiple linear regression model (\(\Delta \)F(2,64) = 13.11, \(P\le \)0.01), in addition to commonly influencing factors, such as sensory condition, sex/gender, age and anthropometry (Fig. 1a). Subjects’ gender was equal to their sex. Therefore, we refer to both their sex and gender.
Regression results (entry method) for whole group (a) and for gender groups (b). \(\Delta \) R\(^2\) for each hierarchical regression step. SC = sensory condition (eyes closed (EC); neck extended, eyes closed (NE-EC)); Gender (male; female); Anthr. = anthropometry (height (H); weight (W)); BS = body schema (\({TaP}_{\mathrm{v}}\); \({LAT}_{\mathrm{a}}\)); BI = body image (\(\hbox {BIDS}_{\mathrm{abs}}\); \(\hbox {BID}_{\mathrm{abs}}\)); gender-interactions: Hg, Wg, TaPg, LATg, BIDSg, BIDg. Bold with star indicates significance (\(P\le 0.05\)).
Figure 1 represents the results for the individual regression steps:
-
1.
Sensory condition: EC (eyes closed); NE-EC (neck extended, eyes closed)
-
2.
Gender: male; female
-
3.
Age
-
4.
Anthropometry: body height; body weight
-
5.
Body schema (BS): overt (\({TaP}_{\mathrm{v}}\)); covert (\({LAT}_{\mathrm{a}}\))
-
6.
Body image (BI): absolute body image dissatisfaction (\(\hbox {BIDS}_{\mathrm{abs}}\)); absolute body image distortion (\(\hbox {BID}_{\mathrm{abs}}\))
-
7.
Gender-interactions: gender-height (\(\hbox {H}_{\mathrm{gen}}\)); gender-weight (\(\hbox {W}_{\mathrm{gen}}\)); gender-\({TaP}_{\mathrm{v}}\) (\(\hbox {TaP}_{\mathrm{gen}}\)); gender-\({LAT}_{\mathrm{a}}\) (\(\hbox {LAT}_{\mathrm{gen}}\)); gender-\(\hbox {BIDS}_{\mathrm{abs}}\) (\(\hbox {BIDS}_{\mathrm{gen}}\)); gender-\(\hbox {BID}_{\mathrm{abs}}\) (\(\hbox {BID}_{\mathrm{gen}}\))
Most of the common influencing factors significantly increase the goodness of fit: sensory condition (\(\Delta \)F(1,70) = 9.37, \(P\le 0.01\)), age (\(\Delta \)F(1,68) = 11.57, \(P\le 0.01\)), and anthropometry (\(\Delta \)F(2,66) = 4.34, \(P = 0.02\)). On the other hand, indirect body image measures (\(\hbox {BIDS}_{\mathrm{abs}}\), \(\hbox {BID}_{\mathrm{abs}}\)) do not significantly increase \(R^2\) (\(\Delta \)F(2,62) = 0.27, \(P = 0.77\)). While gender itself does not significantly increase the goodness of fit (\(\Delta \)F(1,69) = 3.83, \(P = 0.06\)), gender-interactions (moderation of the influence of a variable on body sway by gender) with \(\hbox {BIDS}_{\mathrm{abs}}\), \(\hbox {BID}_{\mathrm{abs}}\) significantly increase the goodness of fit (\(\Delta \)F(1,57) = 6.32, \(P = 0.02\); \(\Delta \)F(1,56) = 4.10, \(P = 0.05\), respectively) (Figs. 1b and 2). The best model with minimal number of explanatory variables (explanans/explanantia) (backward model) reveals a goodness of fit of 62.7% (F(7,64) = 15.36, \(P\le 0.01\)) (Fig. 2). A decreased body sway variability is associated with a better overt TaP task performance (less variable = higher inverted \({TaP}_{\mathrm{v}}\)) (\({t}(64) = -4.51\), \(P \le 0.01\), \(pr^2\) = 0.24, \(f^2\) = 0.32, SP = 0.90), and with a more distorted body image (\({t}(64) = -2.03\), P = 0.05, \(pr^2\) = 0.06, \(f^2\) = 0.06, SP = 0.31). On the other hand, an increased body sway variability is associated with a more difficult sensory condition (SC) (NE-EC) (\({t}(64) = 4.50\), \(P\le 0.01\), \(pr^2\) = 0.24, \(f^2\) = 0.32, SP = 0.90), with an increased height, (\({t}(64) = 3.91\), \(P\le 0.01\), \(pr^2\) = 0.19, \(f^2\) = 0.23, SP = 0.80), and with a better covert task (LAT) performance (\({t}(64) = 2.71\), \(P\le 0.01\), \(pr^2\) = 0.10, \(f^2\) = 0.11, SP = 0.49). Finally, the interactions of gender with body image dissatisfaction (females: \({t}(64) = 3.72\), \(P\le 0.01\), \(pr^2\) = 0.18, \(f^2\) = 0.22, SP = 0.77) and body image distortion (males: \({t}(64) = 2.80\), \(P\le 0.01\), \(pr^2\) = 0.11, \(f^2\) = 0.12, SP = 0.53) contribute significantly to the model. In summary, the overt TaP task performance results to be the best explanans, followed by sensory condition, height, the gender-\(\hbox {BIDS}_{\mathrm{abs}}\) interaction, the gender-\(\hbox {BID}_{\mathrm{abs}}\) interaction, the covert LAT task performance and the \(\hbox {BID}_{\mathrm{abs}}\) (see standardised regression weights in Fig. 3a).
Best regression plots with minimal number of predictors. Sway variability (SD CoP): upper row (a–c); Short-term stochastic activity (\(\hbox {D}_{\mathrm{s}}\)): lower row (d–f); whole group (a, d): gender-interactions (\(_{\mathrm{gen}}\)): for males (m), e.g. \(\hbox {BIDS}_{\mathrm{gen}} = 0\); for females (f), e.g. \(\hbox {BID} _{\mathrm{gen}} = 0\); males (b, e); females (c, f).
Short-term stochastic activity
The best regression model (backward model) for the short-term stochastic activity of body sway dynamics, which is represented by the short-term diffusion coefficient \({D}_{s}\), reveals a goodness of fit of 65.8% (F(10,61) = 11.75, \(P\le 0.01\)) (Fig. 2). Due to homoscedascity, parameter estimates are reported as adjusted for robust standard errors. A greater short-term stochastic activity is associated with a more difficult sensory condition (NE-EC) (\({t}_{{a}}(61) = 3.73\), \({P}_{{a}}\le 0.01\), \({p}{\eta } ^{2} = 0.19\), oSP = 0.96), with females compared to males, (\({t}_{{a}}(61) = 3.33\), \({P}_{{a}}\le 0.01\), \({p}{\eta } ^{2} = 0.15\), oSP = 0.91), and with a better covert LAT task performance (ta(61) = 2.73, Pa ≤ 0.01, pη2 = 0.11, oSP = 0.77). Finally, the interactions of gender with the overt TaP task performance (males: \({t}_{{a}}(61) = -3.39\), \({P}_{{a}}\le 0.01\), \({p}\eta ^2 = 0.16\), oSP = 0.92); females: \({t}_{{a}}(61) = -2.09\), \({P}_{{a}} = 0.04\), \({p}\eta ^2 = 0.07\), oSP = 0.54), with height (females: \({t}_{{a}}(61) = 5.92\), \({P}_{{a}}\le 0.01\), \({p}\eta ^2 = 0.37\), oSP = 1.00), with weight (males: \({t}_{{a}}(61) = 3.95\), \({P}_{{a}}\le 0.01\), p \(\eta \) \(^2\) = 0.20, oSP = 0.97), and with body image dissatisfaction (females: \({t}_{{a}}(61) = 3.79\), \({P}_{{a}}\le 0.01\), \({p}\eta ^2 = 0.19\), oSP = 0.96) as well as with the body image distortion (males: \({t}_{{a}}(61) = 4.06\), \({P}_{{a}}\le 0.01\), \({p}\eta ^2 = 0.21\), oSP = 0.98; females: ta(61) = −2.22, Pa = 0.03, pη2 = 0.08, oSP = 0.59) contribute significantly to the model. Standardised regression coefficients calculated based on the unstandardised regression coefficient B corrected by the robust standard error are shown in Fig. 3d).
Gender effect on the relationship between influencing factors and postural control
Body sway variability
As indicated by the previously stated gender-interactions, we observe sex/gender differences when dividing the group into males and females (Figs. 1b and 2). In males (N = 17, age: 20–32), sensory condition (\(\Delta \)F(1,32) = 11.87, P \(\le \) 0.01) and body schema (\(\Delta \)F(2,27) = 6.95, P \(\le \) 0.01) significantly increase the goodness of fit (Fig. 1b). The best model of the CoP variability with minimal number of explanatory variables (backward model) reveals a goodness of fit of 48.4% (F(3,30) = 9.38, P \(\le \) 0.01) (Fig. 2). A decreased body sway variability is associated with a better TaP task performance (\({t}(30) = -3.32\), P \(\le \) 0.01, pr \(^2\) = 0.27, f \(^2\) = 0.37, SP = 0.65). On the other hand, an increased body sway variability is associated with a more difficult sensory condition (\({t}(30) = 3.97\), P \(\le \) 0.01, pr \(^2\) = 0.35, f \(^2\) = 0.54, SP = 0.80) and with an increased height (t(30) = 1.87, P = 0.07, pr \(^2\) = 0.10, f \(^2\) = 0.11, SP = 0.25). In males, sensory condition reveals to be the best explanans, followed by the overt (TaP) task performance, and finally by height (Fig. 3b).
On the other hand, females (N = 19, age: 18-30) have demonstrated more factors to contribute substantially to the explanation of inter-and intrapersonal variability; age (\(\Delta \)F(1,35) = 20.51, P \(\le \) 0.01) body schema (\(\Delta \)F(2,31) = 5.08, P \(\le \) 0.01) and body image measures (\(\Delta \)F(2,29) = 3.50, P = 0.04) significantly increase the goodness of fit (Fig. 1b). The best model with minimal number of explanatory variables (backward model) reveals a goodness of fit of 68.0% (F(6,31) = 10.98, P \(\le \) 0.01) (Fig. 2). A decreased body sway variability is associated with a better overt (TaP) task performance (\({t}(31) = -3.56\), P \(\le \) 0.01, pr \(^2\) = 0.29, f \(^2\) = 0.41, SP = 0.73), and with a greater body image distortion (\({t}(31) = -1.99\), P = 0.06, pr \(^2\) = 0.11, f \(^2\) = 0.12, SP = 0.29). On the other hand, an increased body sway variability is associated with a more difficult sensory condition (t(31) = 2.21, P = 0.04, pr \(^2\) = 0.14, f \(^2\) = 0.16, SP = 0.37), with an increased height (t(31) = 3.04, P \(\le \) 0.01, pr \(^2\) = 0.23, f\(^2\) = 0.30, SP = 0.59), with a better covert (LAT) task performance (t(31) = 2.37, P = 0.02, pr \(^2\) = 0.15, f \(^2\) = 0.18, SP = 0.39), and with an increased \(\hbox {BIDS}_{\mathrm{abs}}\) (t(31) = 3.46, P \(\le \) 0.01, pr \(^2\) = 0.28, f \(^2\) = 0.39, SP = 0.70) (Fig. 3c). For females the overt (TaP) task performance reveals to be the best explanans, followed by body image dissatisfaction, height, the covert (LAT) task performance, body image distortion, and finally by the sensory condition.
Short-term stochastic activity
When looking at the best model (backward model) of the diffusion coefficient in males, it reveals a goodness of fit of 63.1% (F(5,28) = 9.57, P \(\le \) 0.01) (Fig. 2). A decreased short-term stochastic activity is associated with a better TaP task performance (\({t}(28) = -3.94\), P \(\le \) 0.01, pr \(^2\) = 0.36, f \(^2\) = 0.56, SP = 0.80). On the other hand, an increased short-term stochastic activity is associated with a more difficult sensory condition (t(28) = 3.40, P \(\le \) 0.01, pr \(^2\) = 0.29, f \(^2\) = 0.41, SP = 0.67), with an increased weight (t(28) = 2.31, P = 0.03, pr \(^2\) = 0.16, f \(^2\) = 0.19, SP = 0.38), with a better covert LAT task performance (t(28) = 1.92, P = 0.07, pr \(^2\) = 0.12, f \(^2\) = 0.14, SP = 0.29) and with an increased \(\hbox {BID}_{\mathrm{abs}}\) (t(28) = 2.87, P ≤ 0.01, pr \(^2\) = 0.23, f \(^2\) = 0.30, SP = 0.54). For the short-term stochastic activity the overt TaP task performance reveals to be the best explanans, followed by the body image distortion, the sensory condition, weight, and finally by the covert LAT task performance (Fig. 3e).
In females, the minimal model of short-term stochastic activity reveals a goodness of fit of 65.1% (F(5,32) = 11.96, P \(\le \) 0.01) (Fig. 2). A decreased short-term stochastic activity is associated with an increased overt (TaP) task performance (\({t}(32) = -2.48\), P = 0.02, p \(\eta \) \(^2\) = 0.16, oSP = 0.67), and with an increased body image distortion (\({t}(32) = -2.20\), P = 0.04, p \(\eta \) \(^2\) = 0.13, oSP = 0.57). On the other hand, an increased short-term stochastic activity is associated with a more difficult sensory condition (t(32) = 2.14, P = 0.04, p \(\eta \) \(^2\) = 0.13, oSP = 0.55), with an increased height (t(32) = 6.21, P \(\le \) 0.01, p \(\eta \) \(^2\) = 0.55, oSP = 1.00), and with an increased \(\hbox {BIDS}_{\mathrm{abs}}\) (t(32) = 3.97, P \(\le \) 0.01, p \(\eta \) \(^2\) = 0.33, oSP = 0.97) (Fig. 3f). For females, this time, height reveals to be the best explanans, followed by body image dissatisfaction, body image distortion, by the sensory condition and finally by the overt TaP task performance.
Discussion
This study is the first directly testing the association of body representations, such as body schema and body image, with postural control. For the interpretations it has to be taken in mind that high-order body representations cannot be directly observed but only inferred by indirect measures.
Our study revealed that body schema measures are associated with human postural control in addition to previously reported influencing factors, such as sensory condition, sex/gender, age and anthropometry. Further, the regression models demonstrate that sex/gender moderates the role of high-order body representations in postural control; body image dissatisfaction being related to body sway variability and open-loop control only in females.
Interestingly, when looking at indirect body image measures, we observed that it is rather body image dissatisfaction than body image distortion which seems to play a role in postural control in females. The work of Forghieri et al.27 showed a higher sway increase in female patients with eating disorders compared to controls, when they saw their mirror image or ideal model image during quiet standing. Moreover, patients also showed a greater body dissatisfaction27. This points to a similar relationship between body image and postural control. However, Forghieri et al.27 did not see differences in body sway in patients and controls during eyes closed condition, which was always assessed before the body image trials27. In contrast to their study, in our study, the balance and overt TaP tasks were always performed after the body image task. Thus, it might be that the conscious access of individuals’ body image and dissatisfaction with their body remained active during the balance task in females. Moreover, Williams et al.57 have reviewed the relationship between sensorimotor control and emotions. They proposed that emotional self-awareness can be attributed to interoceptive awareness and that emotions affect internal models of sensorimotor-goal relationships57. Therefore, the interoceptive awareness as part of attention might influence our body image via emotions and consequently the body and sensory dynamics of the internal model potentially involved in postural control. In analogy to the affective-signaling theory by Dignath and colleagues58 in the field of conflict monitoring and cognitive control, which assumes negative affect to be linked to adaptations in cognitive control, similar mechanisms may occur also in postural control.
Even though, body image dissatisfaction resulted to be stronger related to postural control in females (see standardised regression weights in Fig. 3), also body image distortion contributed substantially to the regression model in both females and males; for latter only for short-term stochastic activity. Thereby, an increased absolute body image distortion coincides with a reduced body sway variability and short-term exploratory behaviour in females. This might be explained by an overshooting of torque in relation to the torque needed59, due to controlling for an illusionary increased body mass (overestimated body mass in the internal model). However, effect size was small to medium and power was low.
While on the other hand, even though obese individuals show an increased torque compared to non-obese, they might underestimate the torque needed to stabilise their body (undershooting of torque)60. The observed undershooting of torque in obese patients might also be attributed to a misperception and thus underestimation of their weight status61. Consequently, the increased stability due to especially a sudden weight loss (due to surgery) in obesity62, might thus be related not only to the reduced weight itself, but also to a reduced underestimation of body size, and consequently to a reduced undershooting of torque. Teasdale et al.62 also related increased stability with weight loss in obese males to an increased capacity of the anticipatory system and of the multisensory integration. They argued this to be possibly associated with a better detection of changes with respect to verticality due to a smaller contact area62. Our results observed in the regression of the diffusion coefficient representing the level of short-term exploratory behaviour may support this, as males showed lower body image distortion to be associated with less exploratory behaviour. This indicates less short-term exploratory behaviour and thus potentially a better feed-forward model with a less distorted body image in males. However, there was no substantial relationship observed for sway variability and also power for explaining short-term exploratory behaviour was low. Moreover, the lower relative body image distortion and higher proportion of individuals underestimating their own body size in males compared to women (29.4% vs. 10.5%, respectively) may be a reason for the opposite relationship observed for temporal sway parameters in males and in females. Therefore, it might be possible that also or especially in males body image distortion plays a role in postural control, when it is increased (e.g. increased underestimation).
Gender differences in body image might be attributed to the substantially higher overestimation of body size (see relative BID in Table 2) and the slightly higher drive for thinness (see relative BIDS in Table 2) in females. While the wish to be thinner is attributed to negative emotions27, the wish to be wider or more muscular, which we observed to be higher in males than in females (see DMS in Table 1), might not be affected that much by negative feelings. As previously discussed, negative affect may play a role in adaptations of control mechanisms. Thus, it remains unclear if eventually also in males body image dissatisfaction might be related to postural control when this is attached with more negative emotions (e.g. increased drive for thinness).
Besides gender differences in body image, we also observed differences between males and females in the relevance of the sensory condition and age in explaining intra- and inter-individual variability in body sway. While sensory condition had a stronger relationship with both sway variability and short-term exploratory behaviour in males than in females (see Figs. 1 and 3), age affected body sway substantially only in females (see Fig. 1). The difference in sensory condition can be also observed in Table 2, which shows a substantially higher body sway variability in the neck extended condition in males compared to females. Sex differences in vestibular functioning were also proposed by Nolan and colleagues6 to possibly explain differences in 9-10 year old boys and girls during quiet standing with eyes closed. The lower change in sway variability in females compared to males by extending the neck in the current study suggests that females either rely more on proprioceptive and tactile information or are more sensitive to vestibular disruption. A higher sensitivity to vestibular changes has been also reported in patients with vestibular migraine, who are susceptible to motion sickness63. Moreover, the incidence for motion sickness has been predominantly observed in females5. Thus, females in the current study might have adapted quicker to changes in the neck extended condition than males due to a higher sensitivity, or they might have downgraded the vestibular system due to relying less on vestibular information which is the more plausible explanation, since females did not change the control strategy. Regarding the ecological theory of postural instability and motion sickness64 a generally greater reliance on proprioceptive and tactile information may dynamically constrain postural control during standing on a stable surface, like in our study, while eventually inducing a greater postural instability and thus increase susceptibility to motion sickness when standing on a moving surface.
Furthermore, sex differences in the influence of age (see Fig. 1) might be due to a slightly higher variance in females than in males (see Table 1), as well as due to slightly lower age in females (18–30 years) than in males (20–32 years). This might influence the relationship due to possible growth in the younger adults or other developmental factors. Moreover, it is in line with previous results of age effect2, which showed steepest sway reduction in the age of 7–25 years and then a plateau until the age of 55. However, the age effect disappeared in the final model (see Fig. 3), when also the indirect body schema and body image measures were considered. This indicates a possible mediation effect on the influence of age on body sway via body schema and body image. This is supported by the influence of increased movement experience on body schema32, as well as by a reduced body image dissatisfaction with increased age in females65. In this context, we can also observe a substantial moderate positive correlation of age with the overt body schema measure, and moderate negative correlations with the covert body schema measure and absolute body image dissatisfaction in females (see additional correlation results in Supplementary Table S5). Additional correlation analysis for males and the whole group can be found in the Supplementary Materials (Supplementary Tables S3 and S4).
The second type of hypothetical high-order body representation investigated in this study, the body schema, has been previously discussed as a self-reference which is fed into different control strategies as a reference for motor control31 providing the state estimate34. In postural control, state estimation errors have been proposed to cause the slow dynamics, which represents the majority of body sway and is located within the feedback loop37. The task performance in the overt Taking-a-Posture is associated with human body sway and postural control in both males and females. On the other hand, even though the covert Laterality task contributed substantially in the regression model of sway variability in the whole group without a substantial gender interaction (see Fig. 3a), it did not contribute substantially in males when computed the regression models of sway variability separately for gender (see Fig. 3b). This might be due to a smaller number of participants in males than in females. On the other hand, for the short-term diffusion coefficient the covert task performance contributed only to the model in men. However, effect sizes were again only small to medium and power was. The contribution of both task performances, we observed, is in line with Barrato et al.36, who stated postural control to consist of two components: (1) multisensory integration for estimating the on-line centre of mass (CoM) position (internal model including feedback), and (2) prediction of the CoM displacement based on an internal model to compensate intrinsic sensory delays (feed-forward without feedback).
The higher contribution of the overt body schema task for explaining postural control (see standardised regression weights in Fig. 3a, c) indicates the importance of continuously updating the internal model by sensory feedback, such as from proprioceptive and tactile sensors66, for maintaining postural control. Further, the influence of a potential body schema on body sway might be moderated by coordination- and balance-related sports types, since dancers show better performance in proprioceptive tasks43, an increased anticipatory control in dynamic tasks42 and automated feedback-control in static tasks67, due to higher sensitivity to small changes. Moreover, proprioceptive awareness44 and self-awareness42 have been reported to be higher in e.g. dancers, which would increase the sensory sensitivity to smaller changes and increase their fast dynamics67. Thus, dancers for example might not only have a very accurate internal model42, but they may also quickly adapt to very small deviations67.
However, the positive relationship of the covert Laterality accuracy parameter and body sway in the current study resulted in the opposite than expected; instead of a more accurate Laterality task performance relating to a lower body sway, we observed higher body sway variability and short-term exploratory behaviour. A typical observation made in healthy adults and investigated in the context of body schema is the effect of biomechanical constraints on the accuracy and response time in the Laterality task68,69 as well as the correlation of the response time and the movement time when performing a real hand rotation to match the position of a shown hand68. Thus, it might be possible that even though subjects were instructed to imagine to observe their own limbs, instead of using a motor imagery strategy they might have used an alternative strategy. For example Creem-Regehr et al.70 have reported a higher accuracy in a viewer (perspective transformation) strategy compared to a hand rotation strategy. One explanation, thus could be, that for healthy individuals, like in the current study, a higher accuracy instead of indicating a better body schema might rather indicate e.g. that they might have used an alternative strategy in the biomechanically implausible rotations of the limb instead of a motor imagery strategy69. On the other hand, individuals born with only one hand (congenital one-handers), who are expected to have only a motor representation for the existing hand, have shown a lower accuracy in the Laterality task71 together with a longer response latency compared to controls and individuals with acquired hand loss. Maimon-Mor et al.71 have concluded the current motor control to be a driver in the hand laterality performance. Moreover, Schwoebel et al.46 stated that the task performance in both motor imagery and real movements might partly rely on body schema. On the other hand an increased short-term stochastic activity related to the covert task performance may indicate more exploratory movements15,16,17 to gain more information for improving task performance in both postural control and motor imagery.
Pitron et al.30 have proposed a serial model in which body schema and body image reshape each other. Regarding their hypothesis, body schema is first developed by prior motor experiences and multi-sensory integration. Subsequently, body schema develops and shapes body image together with again multisensory integration as well as other priors, such as cultural and social expectations, which in turn reshape body schema30. Consequently, body image and the covert aspect of body schema (without movement-induced feedback) might influence the internally simulated body and sensor dynamics. Based on an internal model, a state estimate is sent to the controllers, as proposed by Kuo34 and Morasso et al.31. The control strategy used and the weighting of the feed-forward and feedback control might be influenced by the movement strategy and cognitive processing, as factors reported to influence postural control25. The motor commands consequently cause body dynamics34, which can be restricted by biomechanical constraints25,72 and gravity31. The body dynamics causes sensory dynamics, based on which the sensory output will be compared with the predicted sensory output. Furthermore, based on the observations in expert dancers , for example, it is known that also proprioceptive awareness, as part of body schema, can reach an expert level and that dancers rely more on proprioceptive information than non-dancers42,43,44,67. This is also supported by Gallagher33 and Fabre et al.42, who stated that body schema and proprioceptive awareness share same information, and that a higher proprioceptive awareness improves the internal model, respectively. Thus, we suppose that body schema and the sensory proprioceptive awareness influence the sensory noise in overt actions. Consequently, this also might affect the estimator gain, which causes an estimator correction of the internal model34.
Besides these interpretations also other interpretations are possible, which are not necessarily based on internal representations. Thus, for example, it could be that differences between males and females are due to different perception-action couplings50,51,52. However, future studies would be needed to further investigate and discuss the role of body representations, feed-forward and feedback control in postural control.
Limitations and future works
The sample population in the current study consisted of healthy young adults with most of them performing regular sports, at least once per week. Thus, caution has to be taken when generalising the results to the average population. Since this was a first proof-of-concept study about the correlation of high-order body representations and postural control, we can not conclude about a cause-effect relationship and results have to be interpreted with caution. We have not included the type of sports for recruiting and only created groups for different sport types post-hoc based on the responses of the participants (see Supplementary Table S1). In addition to a possible moderation of the effect of the body schema on body sway by balance- or coordination-related sport types, body sway might be further influenced by other types of sports as well, such as strength training and endurance training, since muscle strength especially of the legs influences postural control and the risk of falling20. Future studies should include the type of sports already systematically for recruiting participants. Especially recruiting individuals who participate in coordination- and balance-relevant sports (e.g. expert dancers or martial artists) or strength and endurance sports (e.g. wrestlers or cyclists) might give further insights to when body representations, especially body schema, affect postural control. Moreover, the role of the covert body schema task performance on body sway could be further investigated. However, since the overt body schema measure resulted to be more relevant for explaining postural control, future studies should rather focus on further investigating the influence of the Taking a Posture task on postural control in different population groups, such as mentioned before, but also e.g. in different patient groups, such as stroke patients, but also individuals with body image distortions, such as anorexic, bulimic, obese, or even individuals with a continuous or sudden change of body shape or weight, such as e.g. in pregnant women or after surgery. Finally, future studies including more subjects to increase the power for the individual independent variables, and systematically manipulating indirect high-order body representation variables while maintaining other factors constant are required for a solid cause-effect conclusion.
Conclusion
This is the first study that directly links sex/gender differences in the control of body balance to the role of high-order body representations in postural control. We propose two types of measures to assess the body schema: once from motor control side including feedback of the body’s configuration in space generated by movements (overt task), and another from motor cognition side including an internal simulation without receiving movement-generated feedback (covert task: Laterality task (LAT)). To assess the body schema by an overt whole body task, we propose a Taking-a-Posture task (TaP) in which participants are asked to repeatedly take certain postures of different complexities. Body schema as well as subjective evaluation of the own body related to concurrent sensorimotor control of body sway. However, body image dissatisfaction was found to be only associated with postural control in females, but not in males. Finally, we have discussed how high-level representations of the self, both body image and body schema, may be involved in predictive motor/postural control. This hypothesis-generating proof-of-concept study lays the basis for future studies, which should include conditional process analysis and causal inference for a better understanding of the underlying mechanisms.
Methods
Subjects
42 healthy young adults (21 females; 18–35 years) participated in this study (detailed inclusion criteria in Supplementary). Given an anticipated medium to strong effect size (Cohen’s f\(^2\) = 0.32) for the influence of height on root-mean-square CoP medio-lateral (ML)7, and an expected power of 0.9 with a total number of 9 predictors a total sample of at least 36 subjects was required (a-priori, g-power). All participants gave written informed consent. The investigation was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the medical ethical committee of the Technical University of Munich (248/19 S-SR). Demographics and characteristics of included subjects are shown in Tables 1 and 2 as well as in the Supplementary Tables S1 and S2.
Experimental procedure
The experimental procedure is shown in Fig. 4. We first assessed possible exclusion criteria and factors possibly influencing balance or body representations via Psytoolkit73,74 (such as sports experience, mirror usage, dealing with body form, profession75, as well as self-esteem (RSES)76, physical appearance (PACS)77, and drive for muscularity (DMS)78). Furthermore, we assessed the handedness and footedness (preferred leg for single-legged stance, and shooting leg). For the lab visit, participants were asked to wear tight, non-reflective, dark clothes.
During lab visit, participants were asked to approve their previously given answers and adapt them if needed. Informed consent was obtained to publish images in publications. Anthropometric measures were taken without shoes. Body image (BI) was assessed by the Body Image Assessment Score - Body Distortion (BIAS-BD) figural drawing79. To assess body schema (BS), we used an overt and a covert task.
The overt Taking-a-posture (TaP) task, consisted of four target poses with different numbers of extremities actively involved to allow for different level of complexity (Fig. 5a–d) , from one upper limb (non-dominant), via one upper and one lower limb (both non-dominant), and two upper limbs and one lower limb (non-dominant: arm above head) to the most complex posture with all limbs explicitly involved in the posture (non-dominant: arm facing head, foot extended).The hand and foot positions and orientations were inspired by previous works in the field of apraxia and imitation (e.g.80,81). Participants were first shown one of these poses and asked to take the approximate posture, which was then refined by the investigator using a goniometer. Subjects were asked to remember the refined position (reference posture), and press any of the two buttons attached to the index fingers (Fig. 5), as soon as the investigator indicated so (\(\sim \)4s post-correction). In-between trials subjects took a neutral posture (upright quiet standing) for 15s. The task then was to retake the reference posture as quickly and as accurately as possible and press any of the two buttons when having reached the felt correct position, and maintain that for \(\sim \)4s. After a first familiarisation trial, five test trials were conducted. This procedure was repeated for all four postures. Since the non-dominant side performs better in proprioceptive tasks82, the focus of the TaP task was the non-dominant hand and foot (opposite to shooting leg) (Fig. 5).
Taking-a-pasture task: four poses of different complexity (change in numbers of limbs involved) with Vicon full-body plugin-gait marker set and goggles to restrict vision of own body. (a) One upper extremity (non-dominant); (b) one upper and one lower extremity (both non-dominant); (c) two upper extremities and one lower extremity (non-dominant: arm above head); (d) all extremities explicitly involved in posture (non-dominant: arm facing head, foot extended); (e) button placement.
The covert task consisted of the Laterality task (LAT) for hands (HLAT) and feet (FLAT)75. The stimuli for the LAT task contained gender-matched gray-scale pictures83/mirror-pictures68 of the left or right hand/foot. To stimulate motor imagery rather than visuo-spatial rotation of the image84, hand palms and foot soles were presented. 48 trials69 (2 sides x 8 rotations x 3 trials (Fig. 6a) were conducted for each condition (HLAT/FLAT), after a short familiarisation phase (6 randomised trials), respectively. Rotations consisted of 0\(^\circ \), 60\(^\circ \), 120\(^\circ \), 180\(^\circ \), 240\(^\circ \), 300\(^\circ \)69,84, and 90\(^\circ \) and 270\(^\circ \) for comparability with medio-lateral intrinsic joint constraints68 (i.e. future use of data). Stimuli were presented with Matlab using Psychtoolbox (Psychophysics Toolbox Version 3 (PTB-3)). In the FLAT condition (Fig. 6b), participants were asked to press the left or right pedal with their respective foot. For the HLAT condition (Fig. 6c), subjects were asked to press “N” and “M” on the keyboard, respectively, with their index and middle finger of their preferred hand84. To restrict visual input of their own body, participants wore goggles for the HLAT and the TaP task.
Body sway was assessed during quiet standing (35s) in a narrow upright bipedal stance with a 2.5cm inter-foot distance with eyes closed (EC) and arms hanging relaxed on the sides. A narrow foot position (e.g.4,35,85) was chosen for inducing a more challenging stance than hip-wide stance86,87 and simultaneously reduce the contact area between extremities for less cutaneous feedback. We conducted two conditions: with/without neck extended (NE), respectively: NE-EC, EC (Supplementary Fig. S1). Extending the neck was chosen to make the stance condition more difficult without changing the BoS due to reduced sensitivity of the vestibular channel10, which might increase the reliance on proprioceptive and tactile information. Moreover, it has been reported that vestibular disturbances do not alter the internal body representation of verticality88,89. Participants were asked to perform each balance condition six times (1 familiarisation trial, 5 test trials) with a 30s break between trials. For hygiene reasons subjects wore own thin summer socks. Due to the thin material and smooth texture, no substantial affects due to sock texture were expected90.
Measurement devices
For motion analysis, an optoelectronic motion capture (MoCap) system (Vicon Nexus 2.9; 200Hz) with 10 cameras (9 Bonitas/Veros, 1 DV camera) and a force plate are used (Advanced Mechanical Technology, Inc. (AMTI), Watertown, MA; 1000Hz). The buttons used in the TaP task are used for event-triggering within Vicon when the final posture is reached. The MoCap system is calibrated at the beginning of the TaP task by a static and dynamic calibration, and by another static calibration before the balance task. The force plate is calibrated (auto-zeroed) in an unloaded state between conditions in the TaP task and before each balance trial.
Data processing and parameter definition
Body image distortion (BID) and body image dissatisfaction (BIDS) were calculated, based on the differences between the real (R), estimated (A), and ideal BMI (I)79. The absolute values of the mean (\(\mu \)) of the two trials (n) (Eqs. 1, 2) were used for statistical analysis.
For the TaP task, data were first post-processed within Vicon software (10 Hz Woltring Filter). Then, the angle error per joint was calculated, averaged across the total number of trials (\(N_{i}\)), based on which the mean (\(\mu \)) (Eq. 3) and standard deviation (SD \(\sigma \)) (Eq. 4) across the total number of poses (\(N_{p}\)) were computed. \(x_{Imi_i}\) are the joint angles of the imitation trials (i). \(x_{Ref_p}\) are the joint angles of the reference trials of each pose (p). \(N_{c}\) is the total number of Cartesian coordinates (c), here three: in x, y, and z. \(N_{j}\) is the total number of joints j, here 24. To obtain positive values for a better performance, results were inverted at the end.
For the LAT task, we calculated the average across extremities as an overall laterality parameter for response accuracy (LAT accuracy) and response latency (LAT latency), respectively.
Centre of pressure (CoP) data were post-processed by a Matlab-routine in the following steps: (1) 10 Hz low-pass Butterworth-filtered, (2) differentiated one-dimensional (1D) and two-dimensional (2D) parameters and (3) extracted 1D and 2D parameters. Within this work, we focused on the body sway parameter SD CoP as a time-independent sway parameter, previously used in other studies11,12,14 which has also been reported as well reliable parameter for discriminating different groups88. Furthermore, for time-dependent parameter we used the stabilogram diffusion analysis (SDA)35, which describes the CoP trajectories as fractional Brownian motion. Collins and De Luca35 have proposed the SDA to provide information about both the feedforward and feedback control as the two control strategies. On the one hand, the short-term intervals (\(\Delta \)t < 1s) are discussed to be dominated by open-loop control due to predominantly positively correlated CoP trajectories and thus a persistent behaviour (Hurst exponent \(\hbox {H}_{\mathrm{s}} > 0.5\)). On the other hand, the long-term intervals (1 < \(\Delta \)t < 10 s) represent the feedback control due to predominantly negatively correlated CoP trajectories and thus an anti-persistent behaviour (\(\hbox {H}_{\mathrm{l}} < 0.5\)). However, the SDA has been criticised to underestimate long-term correlations91 due to fractional Brownian motions being unbounded, and other approaches to estimate the long-term Hurst exponent have been proposed, such as e.g. the Detrended Fluctuation Analysis. In this study we focus on the analysis of the short-term region extracted by the SDA to connect body representations measures with feed-forward processes during body sway. While the scaling exponent H describes the correlation of consecutive CoP displacements, the diffusion coefficient (\(\hbox {D}_{\mathrm{s}}\)) represents the degree of stochastic activity, a higher value indicating a higher stochastic activity and thus a drift away from the equilibrium. A higher stochastic activity also indicates more exploratory behaviour16. Finally, the transition point (TP; coordinates [\({TP}_{{t}}\), \({TP}_{{d}}\)]) represents the switching point between feed-forward (open-loop/short-term intervals) and feedback (closed-loop/long-term intervals) control, whereby an exceeding of the feedback threshold at lower time intervals indicates an earlier switch to feedback-control in terms of time and a lower threshold indicates a tighter controlled sway area. Since the diffusion coefficients have previously reported to have a higher inter-individual variability than the Hurst exponent, and was able to discriminate well different groups and sensory conditions92,93,94, \({D}_{{s}}\) was used for further statistical analysis, while all SDA parameters are reported in the descriptive results.
Statistical analysis
Due to missing data in two subjects, one univariate outlier (>3SD) in body weight, and three subjects being multivariate outliers (Cook’s Distance (D)> 3*\(\mu \); \(\mu \) = mean D), descriptive and statistical analysis were computed for 36 subjects (19 females).
Hierarchical multiple linear regressions were computed for body sway (SD CoP) with six steps: (1) sensory condition (SC: EC; NE-EC), (2) gender (male; female), (3) age, (4) anthropometry (Anthr.: height; weight), (5) body schema (BS: TaPv; LATa), (6) body image (BI: BIDSabs; BIDabs). In the whole group further gender-interactions were added in separate steps for height (Hgen), weight (Wgen), TaPv (TaPgen), LATa (LATgen), BIDSabs (BIDSgen) and BIDabs (BIDgen). For this, gender was dummy coded once coding females as 1 and males as 0 (f), and once coding males as 1 and females as 0 (m). The interaction variables were then computed once for males and once for females by the product of the dummy variable and the other interaction variable; e.g. in the regression step of BIDS both I-BIDS(m) and I-BIDS(f) were added. The order of the steps has been chosen from low-level to high-level control and from intra-individual to inter-individual factors. Gender and age are demographic influencing factors. Since we expected age to influence inter-individual variability less in our sample, gender was added first. Demographics was added before anthropometry, since latter can vary within a certain gender or age group.
In a next step we computed backward multiple linear regressions (exclusion if P > 0.1) using the overall models to find the best regression models with minimal number of explanatory variables. Normal distribution was given for all regression models (Shapiro-Wilk of residuals: P > 0.05; and visual inspection of normal P-P-plot). Explanatory variables (explanans/explanantia) were independent of each other so that multicollinearity was not observed (r<0.7; variable inflation factor (VIF)<10; Tolerance > 0.1). Homoscedascity was also given in all regression models for SD CoP (visual inspection of scatter plots of standardised predicted values and standardised residuals; Breusch Pagan test: P > 0.05). If homoscedascity was not given, the regression model was corrected by using robust standard errors and corrected t- and p-values are reported (ta and \({P}_{{a}}\), respectively). Standardised regression coefficients were then calculated by multiplying the unstandardised regression coefficient B corrected by the robust standard error with the division of the standard deviation of the independent variable by the standard deviation of the dependent variable. Due to exploratory nature and comparability reasons across gender and the whole group, variables were also included, even though they did not show a significant linear relationship with body sway. The amount of the variance explained by an individual variable when controlling for the others is reported by the squared partial correlation (pr \(^2\)). Effect sizes are reported as Cohen’s f\(^2\) (small: f\(^2\) = 0.02, medium: f\(^2\) = 0.15, large: f\(^2\) = 0.35)95. Finally, post-hoc statistical power was calculated with G-power96 for each independent variable and is reported as SP. pr \(^2\), f\(^2\) and SP are only reported for the best (backward) models. In case that homoscedascity was violated, effect sizes are reported as partial squared eta p\(\eta \)\(^2\) (small: p\(\eta \)\(^2\) = 0.01, medium: p\(\eta \)\(^2\) = 0.06, large: p\(\eta \)\(^2\) = 0.14) and observed statistical power oSP. A statistical power of 0.8 was considered as good.
Ethics declaration
This study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the medical ethical committee of the Technical University of Munich (248/19 S-SR). All participants gave written informed consent.
Availability of materials and data
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
References
Winter, D. A. Human balance and posture control during standing and walking. Gait Posture 3, 193–214. https://doi.org/10.1016/0966-6362(96)82849-9 (1995).
Hegeman, J., Shapkova, E., Honegger, F. & Allum, J. Effect of age and height on trunk sway during stance and gait. J. Vestib. Res. Equilib. Orientat. 17, 75–87 (2007).
Riemann, B. L., Lininger, M., Kirkland, M. K. & Petrizzo, J. Age related changes in balance performance during self-selected and narrow stance testing. Arch. Gerontol. Geriatr. 75, 65–69. https://doi.org/10.1016/j.archger.2017.11.012 (2018).
Farenc, I., Rougier, P. & Berger, L. The influence of gender and body characteristics on upright stance. Ann. Hum. Biol. 30, 279–94. https://doi.org/10.1080/0301446031000068842 (2003).
Koslucher, F., Haaland, E. & Stoffregen, T. Sex differences in visual performance and postural sway precede sex differences in visually induced motion sickness. Exp. Brain Res. 234, 313–322. https://doi.org/10.1007/s00221-015-4462-y (2016).
Nolan, L., Grigorenko, A. & Thorstensson, A. Balance control: Sex and age differences in 9 to 16 year olds. Dev. Med. Child Neurol. 47, 449–54. https://doi.org/10.1111/j.1469-8749.2005.tb01170.x (2005).
Chiari, L., Rocchi, L. & Cappello, A. Stabilometric parameters are affected by anthropometry and foot placement. Clin. Biomech. 17, 666–677. https://doi.org/10.1016/S0268-0033(02)00107-9 (2002).
Kejonen, P., Kauranen, K. & Vanharanta, H. The relationship between anthropometric factors and body-balancing movements in postural balance. Arch. Phys. Med. Rehabil. 84, 17–22. https://doi.org/10.1053/apmr.2003.50058 (2003).
Oliveira, J. Statokinesigram normalization method. Behav. Res. 49, 310–317. https://doi.org/10.3758/s13428-016-0706-4 (2016).
Paloski, W. et al. Destabilization of human balance control by static and dynamic head tilts. Gait Posture 23, 315–323. https://doi.org/10.1016/j.gaitpost.2005.04.009 (2006).
Chen, F.-C., Tsai, C.-L., Stoffregen, T., Chang, C.-H. & Wade, M. Postural adaptations to a suprapostural memory task among children with and without developmental coordination disorder. Dev. Med. Child Neurol. 54, 155–9. https://doi.org/10.1111/j.1469-8749.2011.04092.x (2011).
Haddad, J., Emmerik, R., Wheat, J. & Hamill, J. Developmental changes in the dynamical structure of postural sway during a precision fitting task. Exp. Brain Res. 190, 431–41. https://doi.org/10.1007/s00221-008-1483-9 (2008).
Riley, M., Baker, A., Schmit, J. & Weaver, E. Effects of visual and auditory short-term memory tasks on the spatiotemporal dynamics and variability of postural sway. J. Motor Behav. 37, 311–24. https://doi.org/10.3200/JMBR.37.4.311-324 (2005).
Stoffregen, T. A., Pagulayan, R. J., Bardy, B. G. & Hettinger, L. J. Modulating postural control to facilitate visual performance. Hum. Mov. Sci. 19, 203–220. https://doi.org/10.1016/S0167-9457(00)00009-9 (2000).
Carpenter, M., Murnaghan, C. & Inglis, J. Shifting the balance: Evidence of an exploratory role for postural sway. Neuroscience 171, 196–204. https://doi.org/10.1016/j.neuroscience.2010.08.030 (2010).
Riley, M., Mitra, S., Stoffregen, T. & Turvey, M. Influences of body lean and vision on unperturbed postural sway. Motor Control 1, 229–246. https://doi.org/10.1123/mcj.1.3.229 (1997).
Stoffregen, T. & Riccio, G. An ecological theory of orientation and the vestibular system. Psychol. Rev. 95, 3–14. https://doi.org/10.1037//0033-295X.95.1.3 (1988).
Morasso, P., Cherif, A. & Zenzeri, J. Quiet standing: The single inverted pendulum model is not so bad after all. PLoS One 14, e0213870. https://doi.org/10.1371/journal.pone.0213870 (2019).
Winter, D. A. Biomechanics and Motor Control of Human Movement (Wiley, 2009).
Lee, J. et al. Relationship between obesity and balance in the community-dwelling elderly population: A cross-sectional analysis. Am. J. Phys. Med. Rehabil. 99, 65–70. https://doi.org/10.1097/PHM.0000000000001292 (2020).
Bonnet, V. et al. A structurally optimal control model for predicting and analyzing human postural coordination. J. Biomech. 44, 2123–2128. https://doi.org/10.1016/j.jbiomech.2011.05.027 (2011).
Gueugnon, M. et al. Postural coordination during socio-motor improvisation. Front. Psychol. 7, 1168. https://doi.org/10.3389/fpsyg.2016.01168 (2016).
Varoqui, D., Froger, J., Lagarde, J., Pélissier, J.-Y. & Bardy, B. G. Changes in preferred postural patterns following stroke during intentional ankle/hip coordination. Gait Posture 32, 34–38. https://doi.org/10.1016/j.gaitpost.2010.03.004 (2010).
Varoqui, D., Froger, J., Pélissier, J.-Y. & Bardy, B. G. Effect of coordination biofeedback on (re)learning preferred postural patterns in post-stroke patients. Motor Control 15, 187–205. https://doi.org/10.1123/mcj.15.2.187 (2011).
Horak, F. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls?. Age Ageing 35(Suppl 2), ii7–ii11. https://doi.org/10.1093/ageing/afl077 (2006).
Kim, J. et al. Relationship between body factors and postural sway during natural standing. Int. J. Precis. Eng. Manuf. 13, 963–968. https://doi.org/10.1007/s12541-012-0125-0 (2012).
Forghieri, M. et al. Posturographic destabilization in eating disorders in female patients exposed to body image related phobic stimuli. Neurosci. Lett. 629, 155–159. https://doi.org/10.1016/j.neulet.2016.07.002 (2016).
Longo, M. R. Body representations and the sense of self. In The Subject’s Matter: Self-consciousness and the Body (eds De Vignemont, F. & Alsmith, A. J. T.) 75–95 (MIT Press, 2017).
Thaler, A. et al. Visual perception and evaluation of photo-realistic self-avatars from 3d body scans in males and females. Front. ICT 5, 18. https://doi.org/10.3389/fict.2018.00018 (2018).
Pitron, V., Alsmith, A. & de Vignemont, F. How do the body schema and the body image interact?. Conscious. Cogn. 65, 352–358.https://doi.org/10.1016/j.concog.2018.08.007 (2018).
Morasso, P., Casadio, M., Mohan, V., Rea, F. & Zenzeri, J. Revisiting the body-schema concept in the context of whole-body postural-focal dynamics. Front. Hum. Neurosci. 9, 83. https://doi.org/10.3389/fnhum.2015.00083 (2015).
Assaiante, C., Barlaam, F., Cignetti, F. & Vaugoyeau, M. Body schema building during childhood and adolescence: A neurosensory approach. Neurophysiol. Clin. 44, 3–12.https://doi.org/10.1016/j.neucli.2013.10.125 (2013).
Gallagher, S. How the Body Shapes the Mind (Oxford University Press, 2005).
Kuo, A. An optimal state estimation model of sensory integration in human postural balance. J. Neural Eng. 2, S235-49. https://doi.org/10.1088/1741-2560/2/3/S07 (2005).
Collins, J. & De Luca, C. Open-loop and closed-loop control of posture: A random-walk analysis of center-of-pressure trajectories. Exp. Brain Res. 95, 308–18. https://doi.org/10.1007/BF00229788 (1993).
Baratto, L., Morasso, P., Re, C. & Spada, G. A new look at posturographic analysis in the clinical context: Sway-density vs. other parameterization techniques. Motor Control 6, 246–270. https://doi.org/10.1123/mcj.6.3.246 (2002).
Kiemel, T., Oie, K. & Jeka, J. Slow dynamics of postural sway are in the feedback loop. J. Neurophysiol. 95, 1410–1418. https://doi.org/10.1152/jn.01144.2004 (2006).
Chiba, R., Takakusaki, K., Ota, J., Yozu, A. & Haga, N. Human upright posture control models based on multisensory inputs; in fast and slow dynamics. Neurosci. Res. 104, 96–104. https://doi.org/10.1016/j.neures.2015.12.002 (2016).
Holmes, N. & Spence, C. The body schema and multisensory representation(s) of peripersonal space. Cogn. Process. 5, 94–105. https://doi.org/10.1007/s10339-004-0013-3 (2004).
Baccarini, M. et al. Tool use imagery triggers tool incorporation in the body schema. Front. Psychol. 5, 492. https://doi.org/10.3389/fpsyg.2014.00492 (2014).
Martel, M., Cardinali, L., Roy, A. C. & Farne, A. Tool-use: An open window into body representation and its plasticity. Cogn. Neuropsychol. 33, 82–101. https://doi.org/10.1080/02643294.2016.1167678 (2016).
Fabre, M., Blouin, J. & Mouchnino, L. Enhancing the internal representation of the body through sensorimotor training in sports and dance improves balance control. Res. Investig. Sports Med. 6, 469–473. https://doi.org/10.31031/RISM.2020.06.000629 (2020).
Jola, C., Davis, A. & Haggard, P. Proprioceptive integration and body representation: Insights into dancers’ expertise. Exp. Brain Res. 213, 257–65. https://doi.org/10.1007/s00221-011-2743-7 (2011).
Ramsay, J. R. & Riddoch, M. J. Position-matching in the upper limb: Professional ballet dancers perform with outstanding accuracy. Clin. Rehabil. 15, 324–330. https://doi.org/10.1191/026921501666288152 (2001).
Grèzes, J. & Decety, J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Hum. Brain Map. 12, 1–19. https://doi.org/10.1002/1097-0193(200101)12:1<1::aid-hbm10>3.0.co;2-v (2001).
Schwoebel, J. & Coslett, H. Evidence for multiple, distinct representations of the human body. J. Cogn. Neurosci. 17, 543–53. https://doi.org/10.1162/0898929053467587 (2005).
Dennett, D. Kinds of Minds: Toward an Understanding of Consciousness. Science Masters (Basic Books, 1997).
Bajcsy, R. Active perception. Proc. IEEE 76, 966–1005. https://doi.org/10.1109/5.5968 (1988).
Riccio, G. E. & Stoffregen, T. A. Affordances as constraints on the control of stance. Hum. Mov. Sci. 7, 265–300. https://doi.org/10.1016/0167-9457(88)90014-0 (1988).
Riley, M. A., Stoffregen, T. A., Grocki, M. J. & Turvey, M. Postural stabilization for the control of touching. Hum. Mov. Sci. 18, 795–817. https://doi.org/10.1016/S0167-9457(99)00041-X (1999).
Stoffregen, T., Bardy, B., Bonnet, C., Hove, P. & Oullier, O. Postural sway and the frequency of horizontal eye movements. Motor Control 11, 86–102 (2007).
Stoffregen, T., Bardy, B. G., Bonnet, C. & Pagulayan, R. Postural stabilization of visually guided eye movements. Ecol. Psychol. 18, 191–222.https://doi.org/10.1207/s15326969eco1803_3 (2006).
Peterka, R. Postural control model interpretation of stabilogram diffusion analysis. Biol. Cybern. 82, 335–43. https://doi.org/10.1007/s004220050587 (2000).
Massion, J. Movement, posture and equilibrium: Interaction and coordination. Prog. Neurobiol. 38, 35–56. https://doi.org/10.1016/0301-0082(92)90034-C (1992).
Wolpert, D. M. & Flanagan, J. R. Motor prediction. Curr. Biol. 11, R729–R732. https://doi.org/10.1016/S0960-9822(01)00432-8 (2001).
Tuhkanen, S., Pekkanen, J., Wilkie, R. M. & Lappi, O. Visual anticipation of the future path: Predictive gaze and steering. J. Vis. 21, 25. https://doi.org/10.1167/jov.21.8.25 (2021).
Williams, J. H. et al. A sensorimotor control framework for understanding emotional communication and regulation. Neurosci. Biobehav. Rev. 112, 503–518. https://doi.org/10.1016/j.neubiorev.2020.02.014 (2020).
Dignath, D., Eder, A., Steinhauser, M. & Kiesel, A. Conflict monitoring and the affective signaling hypothesis—An integrative review. Psychon. Bull. Rev. 27, 193–216. https://doi.org/10.3758/s13423-019-01668-9 (2020).
Fitzpatrick, R., Taylor, J. & McCloskey, D. Ankle stiffness of standing humans in response to imperceptible perturbation: Reflex and task-dependent components. J. Physiol. 454, 533–47. https://doi.org/10.1113/jphysiol.1992.sp01927 (1992).
Corbeil, P., Simoneau, M., Rancourt, D., Tremblay, A. & Teasdale, N. Increased risk for falling associated with obesity: Mathematical modeling of postural control. IEEE Trans. Neural Syst. Rehabil. Eng. 9, 126–36. https://doi.org/10.1109/7333.928572 (2001).
Robinson, E. Overweight but unseen: A review of the underestimation of weight status and a visual normalization theory: Weight status misperceptions. Obes. Rev. 18, 1200–1209. https://doi.org/10.1111/obr.12570 (2017).
Teasdale, N. et al. Reducing weight increases postural stability in obese and morbid obese men. Int. J. Obes. 2005(31), 153–60. https://doi.org/10.1038/sj.ijo.0803360 (2007).
King, S., Priesol, A., Davidi, S., Merfeld, D. & Lewis, R. Self-motion perception is sensitized in vestibular migraine: Pathophysiologic and clinical implications. Sci. Rep. 9, 14323. https://doi.org/10.1038/s41598-019-50803-y (2019).
Riccio, G. & Stoffregen, T. An ecological theory of motion sickness and postural instability. Ecol. Psychol. 3, 195–240. https://doi.org/10.1207/s15326969eco0303_2 (1991).
Karazsia, B., Murnen, S. & Tylka, T. Is body dissatisfaction changing across time? A cross-temporal meta-analysis. Psychol. Bull. 143, 293–320. https://doi.org/10.1037/bul0000081 (2016).
Nelson, R. J. The Somatosensory System—Deciphering the Brain’s Own Body Image (CRC Press LLC, 2002).
Michalska, J. et al. Effect of expertise in ballet dance on static and functional balance. Gait Posture 64, 68–74. https://doi.org/10.1016/j.gaitpost.2018.05.034 (2018).
Parsons, L. Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J. Exp. Psychol. Hum. Percept. Perform. 20, 709–730. https://doi.org/10.1037//0096-1523.20.4.709 (1994).
Rosser, C., David, P. T. & Ryan, C. Left/right limb judgement task performance following total knee replacement. J. Back Musculoskelet. Rehabil. 32(1), 77–84. https://doi.org/10.3233/BMR-171104 (2019).
Creem-Regehr, S., Neil, J. A. & Yeh, H. Neural correlates of two imagined egocentric transformations. NeuroImage 35, 916–927. https://doi.org/10.1016/j.neuroimage.2006.11.057 (2007).
Maimon-Mor, R. O., Schone, H. R., Moran, R., Brugger, P. & Makin, T. R. Motor control drives visual bodily judgements. Cognition 196, 104120. https://doi.org/10.1016/j.cognition.2019.104120 (2020).
Park, S., Horak, F. & Kuo, A. Postural feedback responses scale with biomechanical constraints in human standing. Exp. Brain Res. 154, 417–27. https://doi.org/10.1007/s00221-003-1674-3 (2004).
Stoet, G. Psytoolkit: A software package for programming psychological experiments using Linux. Behav. Res. Methods 42, 1096–1104 https://doi.org/10.3758/BRM.42.4.1096 (2010).
Stoet, G. Psytoolkit: A novel web-based method for running online questionnaires and reaction-time experiments. Teach. Psychol. 44, 24–31. https://doi.org/10.1177/0098628316677643 (2017).
Edwards, L., Causby, R., Stewart, H. & Stanton, T. Differential influence of habitual third-person vision of a body part on mental rotation of images of hands and feet. Exp. Brain Res. 237, 1325–1337. https://doi.org/10.1007/s00221-019-05512-3 (2019).
Ferring, D. & Filipp, S.-H. Messung des selbstwertgefuehls: Befunde zu reliabilitaet, validitaet und stabilitaet der rosenberg-skala. diagnostica. Diagnostica 42, 284–292 (1996).
Behrens, S., Hautzinger, M., Karnath, H.-O., Zipfel, S. & Giel, K. Validierung der deutschsprachigen version der physical appearance comparison scale (pacs): Psychometrische eigenschaften und zusammenhang mit essverhalten, koerperbild und selbstwert. PPmP - Psychotherapie - Psychosomatik - Medizinische Psychologie 67, 91–97. https://doi.org/10.1055/s-0042-123842 (2017).
Waldorf, M., Cordes, M., Vocks, S. & McCreary, D. Die deutschsprachige drive for muscularity scale (DMS). Zusammenstellung sozialwissenschaftlicher Items und Skalen (ZIS). https://doi.org/10.6102/zis246 (2016).
Gardner, R., Jappe, L. & Gardner, L. Development and validation of a new figural drawing scale for body-image assessment: The bias-BD. J. Clin. Psychol. 65, 113–22. https://doi.org/10.1002/jclp.20526 (2009).
Ambrosoni, E., Sala, S. D., Motto, C., Oddo, S. & Spinnler, H. Gesture imitation with lower limbs following left hemisphere stroke. Arch. Clin. Neuropsychol. 21, 349–358. https://doi.org/10.1016/j.acn.2006.05.001 (2006).
Goldenberg, G. Imitating gestures and manipulating a mannikin—The representation of the human body in ideomotor apraxia. Neuropsychologia 33, 63–72. https://doi.org/10.1016/0028-3932(94)00104-W (1995).
Han, J., Anson, J., Waddington, G. & Adams, R. Proprioceptive performance of bilateral upper and lower limb joints: Side-general and site-specific effects. Exp. Brain Res. 226, 313–323.https://doi.org/10.1007/s00221-013-3437-0 (2013).
Ferri, F., Frassinetti, F., Costantini, M. & Gallese, V. Motor simulation and the bodily self. PLoS One 6, 1–6. https://doi.org/10.1371/journal.pone.0017927 (2011).
Mellet, E., Mazoyer, B., Leroux, G., Joliot, M. & Tzourio-Mazoyer, N. Cortical asymmetries during hand laterality task vary with hand laterality: A fMRI study in 295 participants. Front. Hum. Neurosci. 10, 628. https://doi.org/10.3389/fnhum.2016.00628 (2016).
Wall, C., Weinberg, M., Schmidt, P. & Krebs, D. Balance prosthesis based on micromechanical sensors using vibrotactile feedback of tilt. IEEE Trans. Bio-med. Eng. 48, 1153–1161. https://doi.org/10.1109/10.951518 (2001).
Day, B. L., Steiger, M. J., Thompson, P. D. & Marsden, C. D. Effect of vision and stance width on human body motion when standing: Implications for afferent control of lateral sway. J. Physiol. 469, 479–499. https://doi.org/10.1113/jphysiol.1993.sp019824 (1993).
Stoffregen, T., Yoshida, K., Villard, S., Scibora, L. & Bardy, B. Stance width influences postural stability and motion sickness. Ecol. Psychol. 22, 169–191. https://doi.org/10.1080/10407413.2010.496645 (2010).
Paillard, T. & Noe, F. Techniques and methods for testing the postural function in healthy and pathological subjects. BioMed Res. Int. 2015, 891390. https://doi.org/10.1155/2015/891390 (2015).
Wardman, D., Taylor, J. & Fitzpatrick, R. Effects of galvanic vestibular stimulation on human posture and perception while standing. J. Physiol. 551, 1033–42. https://doi.org/10.1113/jphysiol.2003.045971 (2003).
Tsai, Y.-J. & Lin, S.-I. Older adults adopted more cautious gait patterns when walking in socks than barefoot. Gait Posture 37, 88–92. https://doi.org/10.1016/j.gaitpost.2012.06.034 (2013).
Delignières, D., Deschamps, T., Legros, A. & Caillou, N. A methodological note on nonlinear time series analysis: Is the open-and closed-loop model of Collins and de Luca (1993) a statistical artifact?. J. Motor Behav. 35, 86–97. https://doi.org/10.1080/00222890309602124 (2003).
Meyer, P., Oddsson, L. & De Luca, C. The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 156, 505–12. https://doi.org/10.1007/s00221-003-1804-y (2004).
Norris, J. A., Marsh, A. P., Smith, I. J., Kohut, R. I. & Miller, M. E. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. J. Biomech. 38, 1263–1272. https://doi.org/10.1016/j.jbiomech.2004.06.014 (2005).
Wuehr, M. et al. Inadequate interaction between open- and closed-loop postural control in phobic postural vertigo. J. Neurol. 260, 1314–1323. https://doi.org/10.1007/s00415-012-6797-7 (2013).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn. (Routledge, 1988).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A.-G. Statistical power analyses using g*power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. https://doi.org/10.3758/BRM.41.4.1149 (2009).
Acknowledgements
The authors acknowledge the Prevention Center of the SG department of TUM for lab access, providing the measurement devices and supporting regarding setup preparation. Moreover, the authors thank Dr. Rick M. Gardner for providing the BIAS-BD figural drawings. Finally the authors acknowledge David Paulius Ramos for proofreading.
Funding
Open Access funding enabled and organized by Projekt DEAL. This project was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - SPP 2134, and the HCR department of TUM.
Author information
Authors and Affiliations
Contributions
K.S., L.J. and D.L. have designed and planned the study. K.S. has collected the data. K.S. and Y.M. have processed the data. K.S., L.J. and D.L. have analysed the data. K.S., L.J., Y.M. and D.L. have prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schulleri, K.H., Johannsen, L., Michel, Y. et al. Sex differences in the association of postural control with indirect measures of body representations. Sci Rep 12, 4556 (2022). https://doi.org/10.1038/s41598-022-07738-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07738-8
This article is cited by
-
Gender differences in the effect of a 0.11% breath alcohol concentration on forward and backward gait
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.