Abstract
To investigate the antimicrobial activity of a preservative-free 0.6% povidone-iodine eye drop as an antiseptic procedure in decreasing the conjunctival bacterial load in eyes scheduled for intravitreal treatment and to compare its efficacy to the untreated fellow eye used as the control group. Prospective cohort analysis in which 208 patients received preservative-free 0.6% povidone-iodine eye drops three times a day for three days before intravitreal injection. Before and after the prophylactic treatment, a conjunctival swab was collected from both the study eye and the untreated contralateral eye, used as control. The swab was inoculated on different culture media and the colony-forming units were counted. Bacteria and fungi were identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Treatment with 0.6% povidone-iodine eye drops significantly reduced the conjunctival bacterial load from baseline (p < 0.001 for blood agar and p < 0.001 for chocolate agar) with an eradication rate of 80%. The most commonly isolated pathogen at each time-point and in both groups was coagulase-negative Staphylococci, isolated in 84% of the positive cultures. The study provides evidence about the effectiveness of 0.6% povidone-iodine eye drops treatment in reducing the conjunctival bacterial load in eyes scheduled for intravitreal treatment.
Similar content being viewed by others
Introduction
Intravitreal injections (IVI) of antivascular endothelial growth factor (VEGF) agents have become the most performed intraocular procedures in ophthalmology, with numbers that increase dramatically every year1. The use of anti-VEGF injections has proven to be the mainstay of treatment for several retinal diseases, including wet age-related macular degeneration (ARMD), macular edema secondary to diabetes and retinal vein occlusion, myopic choroidal neovascularization (CNV), and proliferative diabetic retinopathy1.
Because of the natural history of these ocular conditions and the short half-life of the drugs delivered into the vitreous, most patients require a series of anti-VEGF IVI to achieve the therapeutic effects.
The retreatment with several IVI per year exposes patients to a greater risk of infectious complications, specifically endophthalmitis2.
Although rare, endophthalmitis is one of the most feared ocular complications of IVI because of its rapid and aggressive evolution, often leading to poor functional prognosis if not promptly and adequately treated3,4. In most cases, the causative agent, most likely originates from the conjunctival bacteria flora, is directly inoculated during the perioperative phase.
Despite the absence of evidence-based data, topical antibiotics prophylaxis is an “accepted practice” related to mandatory use reported in the pivotal clinical trial protocols for IVI and it has long been the standard of care after intravitreal treatment5.
Recently, the real effectiveness of topical antibiotics used in preventing endophthalmitis after IVI has been questioned. Although topical antibiotics administration has been reported to reduce the conjunctival flora, antibiotic prophylaxis does not decrease post-injection endophthalmitis incidence6. Conversely, it may be associated with a greater risk of post-operative infections: the recurrent and short-term administration of antibiotics eye drop may promote antibiotic-resistant bacterial strains with increasing post-injection endophthalmitis rates7.
The use of povidone-iodine (PI), introduced in ophthalmology since the 1990s, is the most reliable form of ocular surface disinfection in the pre-operative site and is the only antiseptic procedure that allows decreasing the risk of postoperative endophthalmitis in the anterior segment surgery8. PI does not induce resistance and is effective against a wide spectrum of microbes8. According to the higher concentration of bactericidal free iodine in 5% than in 10% solution9, the application of 5% PI in the conjunctival sac for at least 3 min before surgery is a mandatory measure to reduce the number of bacteria in the area of the surgical wound10,11. Nevertheless, the PI antisepsis does not allow a complete eradication of conjunctival bacterial load with a residual incidence of endophthalmitis rate after IVI from 0.02 to 0.3% for a single injection, and a cumulative rate up to 1% after a treatment series12.
In the European Society of Cataract and refractive Surgeons guidelines for prevention and treatment of endophthalmitis following cataract surgery, the preservative-free 0.6% PI eye drops was lately proposed as a prophylactic treatment in the days pre-surgery to further reduce the risk of postoperative intraocular infections10.
A recent analysis in patients undergoing anti-VEGF IVI evaluated the efficacy of the preservative-free 0.6% PI eye drops but enrolling the study eye and the control eye from different patients13. However, a variation in conjunctival bacterial flora not only from patient to patient but also over time and from region to region has been demonstrated14,15. To the best of our knowledge, no studies report the direct comparison between treated and control eyes in the same patients, using the fellow eye as the control group.
This study aimed to investigate the antimicrobial activity of a preservative-free 0.6% PI eye drop as an antiseptic procedure in decreasing the conjunctival bacterial load in eyes scheduled for anti-VEGF intravitreal treatment and compare its efficacy to the untreated contralateral eye.
Results
Among the 280 patients assessed for eligibility, 15 declined to be enrolled in the study, and 36 did not met the inclusion criteria. 21 out of 229 patients admitted were lost to follow-up for inappropriate samples (16 patients) or not completed the visit V1 the day of the IVI (Fig. 1).
Among 208 patients included in the final analysis, 96 (46%) were males and 112 (54%) females. The mean age was 73.4 ± 7.6 years, ranging from 62 to 85 years. The baseline and demographic characteristics of the study cohort are summarized in Table 1.
In the study cohort, 48.6% (101/208) of the study eyes were scheduled to anti-VEGF intravitreal treatment for wet age-related macular degeneration, 30.8% (64/208) for diabetic macular edema, 12% (25/208) for macular edema secondary to retinal vein occlusion, 4.8% (10/208) and 3.8% (8/208) for myopic choroidal neovascularization and proliferative diabetic retinopathy, respectively.
In all 208 blood agar and chocolate agar plates seeded at V0 and V1 a microbial growth was seen. No growth was observed in Mac Conkey agar cultures and Sabouraud dextrose agar cultures with gentamicin at different time point. The CFU results at V0 and V1 on the blood agar and chocolate agar plates are reported in Table 2.
At V0 the mean bacterial growth was similar in the treated and control eyes in the blood agar (p = 0.344) and chocolate agar plates (p = 0.425). The treatment with 0.6% PI eye drops significantly reduced the conjunctival bacterial load in both blood and agar culture media compared to baseline (p < 0.001 blood agar V1 vs. V0, p < 0.001 chocolate agar V1 vs. V0; Table 2). In the control eyes, no statistically significant differences were found between the mean CFU at V0 and V1 (p = 0.286 blood agar V1 vs. V0, p = 0.563 chocolate agar V1 vs. V0).
As previous reported, at V0 in all 208 seeded blood and chocolate agar plates for treated and control eyes a positive culture was found. At V1, the number of positive cultures decreases to 41 (20%) in the study eyes, with a statistically significant decrease compared to V0 (p < 0.001; Table 3), and remains stable in the eyes used as control. The analysis in the study eyes demonstrated an eradication rate of 80%.
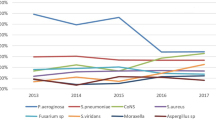
The most commonly isolated pathogen at each time-point and in both groups was coagulase-negative Staphylococci, isolated in 84% of the positive cultures. The treatment with 0.6% PI eyedrops did not significantly affect this ratio after the administration in the study eyes. Other bacteria identified in order of frequency were staphylococci aureus and other gram-positive bacteria, as reported in Table 3. The microbiological analysis at V0 also revealed 3% of gram-negative bacteria in both the study and control eyes. At V1 in the treated eyes, all gram-negative bacteria were eradicated.
None case of traumatic cataract, endophthalmitis, retinal detachment, or vitreous hemorrhage was reported in any of the 208 participants. No systemic adverse reactions related to the topical treatment were detected. 8 (3.9%) out of 208 patients who underwent 0.6% PI eye drops treatment experienced conjunctival hyperemia with light serous conjunctival discharge in 3 eyes (1.4%).
Discussion
This prospective cohort study provides evidence of the effectiveness of 0.6% PI eye drops treatment in reducing the conjunctival bacterial load in eyes scheduled for IVI, compared to contralateral not treated eyes.
Ocular conjunctival flora comprises a wide range of bacteria that do not cause infection in normal conditions but can be a major contamination route during ocular surgery. Since conjunctival microorganism flora differs greatly in individuals based on age, sex, geographical distribution, and related pathologies16, we reported first the direct comparison of the ocular bacteria flora between treated and control eyes in the same patients, detecting a statistically significant efficacy (p < 0.001) of 0.6% PI eye drops in reducing the conjunctival bacterial load.
The IVI is an ever-increasing procedure in ophthalmology, with 20 million estimated IVI in 2016 worldwide17,18. The exponential growth of this treatment highlights the need to reduce possible risks and related complications. Bacteria from the patient’s conjunctiva and eyelids can be a potential source for endophthalmitis occurrence, one of the most feared postoperative complications2,3,4. The risk of endophthalmitis after IVI ranges from 0.029% to 0.057%19.
Topical antibiotics have broadly represented the standard of care of ocular surgery prophylaxis. Still, they have been demonstrated to have a strict correlation with a higher incidence of post-IVI endophthalmitis rate and greater antibiotic resistance6,20,21,22. A systematic review comparing the rates of endophthalmitis in eyes receiving IVI with and without topical antibiotic prophylaxis reported 74 cases of endophthalmitis in 147.203 injections using antibiotic prophylaxis compared with 55 cases in 211.418 injections with no prophylaxis. Menchini et al. concluded that antibiotic prophylaxis does not reduce the rate of endophthalmitis21. Similarly, a recent review detected an estimated incidence of endophthalmitis with topical antibiotic prophylaxis approximated three times the incidence without prophylaxis6.
Furthermore, several studies proved that the risk of endophthalmitis developing after intravitreal injection is not reduced using post-injection topical antibiotic prophylaxis but is rather associated with a trend toward a higher incidence of endophthalmitis7,22. According to these results, perioperative antibiotics cannot be considered the standard of care since there is no evidence of prophylactic effects regarding postoperative endophthalmitis.
Up to now, PI is the most reliable and widely used antiseptic agent for preoperative preparation in ocular surgery, being the 5% concentration recommended by the guidelines of the European Society of Cataract and Refractive Surgeons and American Academy of Ophthalmology10,11. Interestingly, since free iodine is not liberated readily when using high concentration solutions, diluting the solution facilitates its release23. Several in vitro studies and clinical studies evinced that the more diluted 0.6% preparation was more rapidly bactericidal than the 5% PI formulation, probably due to the ready release of iodine from molecular complex and therefore bactericidal activity is enhanced23,24,25.
In our study, 0.6% PI eye drops treatment showed a significant reduction of the CFU value with an eradication rate of 80% of the number of bacterial growth-positive swab cultures. Similarly, Reibaldi et al. detected a significant decrease in bacterial growth from conjunctival swab cultures after a 0.6% PI prophylaxis compared to baseline and placebo prophylaxis, used as a control group13.
Since in our report the control group was represented by the contralateral eye of the same subject, the bias related to a different conjunctival flora between two different subjects included in this study was eliminated.
Additionally, Reibaldi et al. found 13% positive cultures after the treatment than 20% showed in our study. These results are probably due to the more sensitive method used in our investigation to identify the bacteria, the MALDI-ToF–MS, with respect to the broth cultures of the Reibaldi et al. study.
Consistent with the literature13,14,15, the most commonly isolated organism in our analysis was coagulase-negative staphylococcus, which included the most representative Staphylococcus epidermidis, followed by Staphylococci aureus and other gram-positive bacteria. After prophylaxis with 0.6% PI, all gram-negative bacteria were eliminated. The PI in concentrations between 0.1% and 1.0% needs only 15 s to kill bacteria, but unfortunately, the duration of cytotoxic activity is short, and multiple applications are required26,27. Furthermore, high concentrations of PI are responsible for the toxic effect on the corneal epithelium and, therefore, not suitable for continuous administration27. However, in our analysis, the preservative-free 0.6% PI eye drops proved to be well tolerated by the study population.
The main limitation of this study includes the possibility that enrolled patients had not been compliant with the treatment program provided. However, according to the data, all patients proved to be compliant with the assigned treatment schedule during the baseline visit.
In conclusion, this study highlights the role of 0.6% PI eye drops in reducing the conjunctival bacterial load in eyes scheduled for IVI treatment while also showing a good safety profile. The introduction of prophylactic treatment with 0.6% PI eyedrops would limit the emerging phenomenon of antibiotic resistance and reduce the conjunctival bacterial commensal flora further limiting the still existing risk of endophthalmitis.
Materials and methods
Study design
A prospective cohort study was conducted at the University Eye Clinic of Trieste, a tertiary eye care center in the northeast of Italy. The study was performed in conformity with the Declaration of Helsinki and the standards of Good Clinical Practice. The Regional Ethical Committee (Comitato Etico Unico regionale, CEUR) approved the research protocol (ID 245_2019), and written informed consent was obtained from all participants.
All naïve patients affected by CNV in ARMD, diabetic macular edema, macular edema secondary to retinal vein occlusion, myopic CNV or proliferative diabetic retinopathy, and scheduled for the first IVI of anti-VEGF agents were considered eligible to be included in the study. Patients with iodine allergy, use of any eye drops or ointment within six months from enrolment, use of systemic antibiotics or corticosteroids in the last six months, active ocular and periocular infection or inflammation, previous ocular surgery (except for cataract surgery performed in the previous 12 months) were excluded from the study. Patients with cognitive impairment and unable to manage home-assigned treatment were also excluded.
All patients received, only in the eye to be injected (study eye), the preservative-free 0.6% PI eye drops (IODIM® Medivis, Catania, Italy) three times a day for three days before IVI. Patients were instructed to close their eyes for 5 min after each application. On the injection day, all study eyes received one IODIM® drop one hour before the intravitreal treatment. No treatment was administered in the fellow eye (control eye).
Before starting the IODIM® treatment (V0) and before the IVI (V1), all patients underwent a complete ophthalmologic examination, including the best-corrected visual acuity evaluation using Early Treatment Diabetic Retinopathy Study acuity charts, slit-lamp biomicroscopy, IOP measurement with Goldmann tonometer, and fundus examination. At V1 also any systemic or ocular adverse events were recorded.
Conjunctival sample collection
At V0 and V1 visits, a conjunctival swab with ESwab™ system (Liquid Amies Elution Swab, Copan, Brescia, Italy) was collected from the study eye and the untreated contralateral one, used as control.
The ESwab™ included sterile nylon flocked swab combined with 1 mL of Liquid Amies transport medium. While the patient looked upward, the specimen collection was performed exposing the inferior fornix and twirling the mini tip flocked nylon applicator into the inferotemporal conjunctiva. Extreme attention was paid to no getting in contact with eyelids and lashes. If accidental contamination with these structures occurred, the patient was excluded from further analysis. At the end of the collection, the swab was immediately placed in the Liquid Amies transport medium and transported within two hours to the laboratory, where it was refrigerated at 4 °C and processed within 24 h.
Microbiological analysis
The microbiologists involved in the study worked in a masked mode. In strict sterile condition at the lab, the swab was inoculated on different culture media: blood agar plate incubated at 35 °C for 72 h, chocolate agar plate incubated at 35 °C in CO2 enriched atmosphere for 72 h for fastidious bacteria, Mac Conkey agar plate at 35 °C for 72 h for gram-negative bacteria like pseudomonas, and Sabouraud dextrose agar plate with gentamicin at 25 °C for five days selective for fungi. If the culture was positive, the colony-forming units (CFUs) were counted in the different culture media.
Bacteria and fungi were identified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-ToF–MS, VITEK® MS Plus)28.
Statistical analysis
Statistical analyses were performed using SPSS software 11.0 (SPSS Inc, Chicago, IL). Descriptive statistics are used to report the demographic and ocular characteristics of the study population, expressed as means and standard deviation for quantitative variables, and summarized as frequency and percentage for qualitative variables. The comparison between the study and control group was performed using the non-parametric Wilcoxon paired test. Statistical significance was set at p < 0.05.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Data availability
All data that supports the findings of this study are available from the corresponding author, M. R. P., upon reasonable request.
References
Grzybowski, A. et al. Update on intravitreal injections: Euretina expert consensus recommendations. Ophthalmologica 239, 181–193 (2018).
Benoist d’Azy, C., Pereira, B., Naughton, G., Chiambaretta, F. & Dutheil, F. Antibioprophylaxis in prevention of endophthalmitis in intravitreal injection: A systematic review and meta-analysis. PLoS ONE 11(6), e0156431 (2016).
Sigford, D. K., Reddy, S., Mollineaux, C. & Schaal, S. Global reported endophthalmitis risk following injections of anti-VEGF: A literature review and analysis. Clin. Ophthalmol. 9, 773–781 (2015).
Wykoff, C. C., Flynn, H. W. Jr. & Rosenfeld, P. J. Prophylaxis for endophthalmitis following intravitreal injection: Antisepsis and antibiotics. Am. J. Ophthalmol. 152, 717–719 (2011).
Hunyor, A. P. et al. Topical antibiotics and intravitreal injections. Acta Ophthalmol. 96, 435–441 (2018).
Reibaldi, M. et al. Pooled estimates of incidence of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents with and without topical antibiotic prophylaxis. Retina 38, 1–11 (2018).
Milder, E., Vander, J., Shah, C. & Garg, S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology 119, 1420–1424 (2012).
Wass, S., Albrektsen, G., Ødegård, M. T., Sand, M. & Austeng, D. Antiseptic effect of low-concentration povidone-iodine applied with a depot device in the conjunctiva before cataract surgery. Eye (Lond). 32, 1900–1907 (2018).
Ta, C. N., Singh, K., Egbert, P. R. & de Kaspar, H. M. Prospective comparative evaluation of povidone-iodine (10% for 5 minutes versus 5% for 1 minute) as prophylaxis for ophthalmic surgery. J. Cataract. Refract. Surg. 34, 171–172 (2008).
Barry, P., Cordovès, L. & Gardner, S. ESCRS Guidelines for prevention and treatment of endophthalmitis following cataract surgery: Data dilemmas and conclusion. Eur. Soc. Cataract Refract. Surg. 2, 2 (2013).
Olson, R. J. et al. Cataract in the adult eye preferred practice pattern. Ophthalmology 124, 1–119 (2017).
Rosenfeld, P. J. et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1419–1431 (2006).
Reibaldi, M. et al. The effectiveness of 0.6% povidone iodine eye drops in reducing the conjunctival bacterial load and needle contamination in patients undergoing anti-vegf intravitreal injection: A prospective, randomized study. J. Clin. Med. 8(7), 1031 (2019).
Matsuura, K. et al. Conjunctival bacterial flora and antimicrobial susceptibility in bacterial pathogens isolated prior to cataract surgery. Jpn. J. Ophthalmol. 64, 423–428 (2020).
Suto, C., Morinaga, M., Yagi, T., Tsuji, C. & Toshida, H. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect. Drug Resist. 5, 37–41 (2012).
Grzybowski, A., Brona, P. & Kim, S. J. Microbial flora and resistance in ophthalmology: A review. Graefes Arch. Clin. Exp. Ophthalmol. 255, 851–862 (2017).
Berkowitz, S. T., Sternberg, P. Jr., Feng, X., Chen, Q. & Patel, S. Analysis of anti-vascular endothelial growth factor injection claims data in US medicare part B beneficiaries from 2012 to 2015. JAMA Ophthalmol. 137, 921–928 (2019).
Martin, D. F. Evolution of intravitreal therapy for retinal diseases from CMV to CNV: The LXXIV edward jackson memorial lecture. Am. J. Ophthalmol. 191, 1–8 (2018).
Cheung, C. S. et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology 119, 1609–1614 (2012).
Storey, P. et al. The effect of prophylactic topical antibiotics on bacterial resistance patterns in endophthalmitis following intravitreal injection. Graefes Arch. Clin. Exp. Ophthalmol. 254, 235–242 (2016).
Menchini, F. et al. Antibiotic prophylaxis for preventing endophthalmitis after intravitreal injection: A systematic review. Eye (lond). 32, 1423–1431 (2018).
Storey, P. et al. Post-Injection Endophthalmitis Study Team. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology 121, 283–289 (2014).
Zamora, J. L. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am. J. Surg. 151, 400–406 (1986).
Musumeci, R., Bandello, F., Martinelli, M., Calaresu, E. & Cocuzza, C. E. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur. J. Ophthalmol. 29, 673–677 (2019).
Pinna, A. et al. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM®). Acta Ophthalmol. 98, 178–180 (2020).
Ahmed, Y., Scott, I. U., Pathengay, A., Bawdekar, A. & Flynn, H. W. Povidone-iodine for endophthalmitis prophylaxis. Am. J. Ophthalmol. 157, 503–504 (2014).
Peden, M. C., Hammer, M. E. & Suñer, I. J. Dilute Povidone-Iodine prophylaxis maintains safety while improving patient comfort after intravitreal injections. Retina 39, 2219–2224 (2019).
Jang, K. S. & Kim, Y. H. Rapid and robust MALDI-TOF MS techniques for microbial identification: A brief overview of their diverse applications. J. Microbiol. 56, 209–216 (2018).
Funding
No specific grant or funding from any source or agency was received for this work.
Author information
Authors and Affiliations
Contributions
D.T., M.R.P. and G.C. conceived and designed the experiments; M.R.P., L.B., R.M., and G.C. performed the experiments; M.B. and G.B. analyzed the data; M.R.P. and A.L.V. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tognetto, D., Pastore, M.R., Belfanti, L. et al. In vivo antimicrobial activity of 0.6% povidone-iodine eye drops in patients undergoing intravitreal injections: a prospective study. Sci Rep 11, 23271 (2021). https://doi.org/10.1038/s41598-021-02831-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02831-w
This article is cited by
-
Prophylaxis of Ocular Infection in the Setting of Intraocular Surgery: Implications for Clinical Practice and Risk Management
Ophthalmology and Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.