Abstract
Marine biomasses capable of fixing carbon dioxide have attracted attention as an alternative to fossil resources for fuel and chemical production. Although a simple co-fermentation of fermentable sugars, such as glucose and galactose, has been reported from marine biomass, no previous report has discussed the fine-control of the galactose-to-glucose consumption ratio in this context. Here, we sought to finely control the galactose-to-glucose consumption ratio in the co-fermentation of these sugars using engineered Escherichia coli strains. Toward this end, we constructed E. coli strains GR2, GR2P, and GR2PZ by knocking out galRS, galRS-pfkA, and galRS-pfkA-zwf, respectively, in parent strain W3110. We found that strains W3110, GR2, GR2P, and GR2PZ achieved 0.03, 0.09, 0.12, and 0.17 galactose-to-glucose consumption ratio (specific galactose consumption rate per specific glucose consumption rate), respectively, during co-fermentation. The ratio was further extended to 0.67 by integration of a brief process optimization for initial sugar ratio using GR2P strain. The strategy reported in this study will be helpful to expand our knowledge on the galactose utilization under glucose conditions.
Similar content being viewed by others
Introduction
Excessive use of fossil fuel has caused global warming, which is one of the biggest problems currently facing humanity. Recently, biobased fuels and chemicals have gained attention as potential alternatives to fossil fuels1,2,3,4,5,6. Some biobased fuels and chemicals can be produced via microbial fermentation from sustainable biomasses, such as wood and seaweed7,8,9,10,11.
In addition to such cellulosic biomasses, marine biomasses have been suggested as promising renewable resources12, as they have the advantages of high biomass yield per unit area, high rates of carbon dioxide fixation, and natural abundance13. Marine macroalgae contain fermentable sugars, such as glucose and galactose whose contents are known about 20% and 23%, respectively14,15,16,17,18. The proper utilization of galactose is important for the production of biobased fuels and chemicals from a marine macroalgal biomass. Also, given that galactose is 10 times more expensive than glucose, proper regulation of the galactose consumption rate is very important in the co-fermentation of glucose and galactose. On the other hand, in our recent study, it is reported that the production of some metabolite, such as hyaluronic acid could also be affected by the modulation of galactose and glucose consumption19. However, the exact determination of galactose and glucose consumption rates remains, in cultures using the metabolically engineered cells, yet.
Most microorganisms use glucose as a primary feedstock in the co-fermentation of glucose and galactose. This preferential consumption of glucose, which occurs through carbon catabolite repression (CCR), makes it difficult for organisms to use glucose and galactose simultaneously. In a recent study, the CCR pathway was found to be completely deregulated when glucose and galactose were co-fermented using an engineered Escherichia coli with knockout of the galactose repressor (galR) and overexpression of the galactose operon (galP, galE, galT, galK and galM) and phosphoglucomutase (pgm)20. The engineered strain simultaneously assimilated galactose and glucose in the co-fermentation of these sugars. However, no previous report has described a strategy for finely controlling the galactose consumption rate during the co-fermentation of glucose and galactose.
In this study, as a proof-of-concept, we tried to finely control the galactose-to-glucose consumption ratio during the co-fermentation of both sugars by engineering the sugar metabolism in E. coli on the basis of our recent study19, followed by a brief process optimization for sugar ratio and concentration.
Results
Galactose-to-glucose consumption ratio in E. coli GR2, which lacked the galactose operon repressors, during glucose consumption
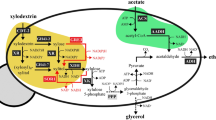
In an effort to finely control the galactose consumption rate in the co-fermentation of glucose and galactose using E. coli, we first constructed the galRS mutant, E. coli strain GR2 (Fig. 1 and Table 1), which was not able to produce the galactose operon repressors encoded from the galRS genes21,22,23,24,25,26,27. We expected that the resulting strain would consume galactose at a higher rate than the parent strain in the co-fermentation of glucose and galactose, although we anticipated that this rate would still be fairly low. To determine the galactose consumption rate of the galRS mutant, we cultured GR2 cells in R/2 medium supplemented with 4 g/L glucose and 4 g/L galactose. Parent strain W3110 was cultured under the same conditions as a control. In the co-fermentation of glucose and galactose, E. coli strain GR2 had completely consumed the glucose at 12 h of culture, yielding a specific glucose consumption rate of 1.3629 g/gDCW/h (Fig. 2a and Table 2). During this period, GR2 consumed 0.36 g/L galactose, for a specific consumption rate of 0.1262 g/gDCW/h. Under the same conditions, parent strain W3110 showed a similar level of glucose consumption (Fig. 2b) but had a lower specific galactose consumption rate at 0.0373 g/gDCW/h (Table 2). Strain GR2 yielded Rgal/glu = 0.09, whereas parent strain W3110 yielded Rgal/glu = 0.03 (Table 2).
Control of the galactose-to-glucose consumption ratio in the co-fermentation of both sugars using the engineered E. coli. ‘X’ indicates gene knockout. The relevant genes and their encoded enzymes are as follows: galR, DNA-binding transcriptional repressor; galS, DNA-binding transcriptional isorepressor; galK, galactokinase; galT, galactose-1-phosphate uridylyltransferase; pfkA, 6-phosphofructokinase I; pfkB, 6-phosphofructokinase II; zwf, glucose-6-phosphate dehydrogenase. Abbreviations: Gal1P, galactose-1-phosphate; G1P, glucose-1-phosphate; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; FBP, fructose-1,6-phosphate; PYR, pyruvate; 6PG, 6-phosphate-gluconate; Ru5P, ribulose-5-phosphate; X5P, xylulose-5-phosphate; E4P, erythorse-4-phosphate; KDPG, 2-keto-3-deoxy-phosphogluconate.
Galactose-to-glucose consumption ratio in E. coli GR2P, which lacked the galactose operon repressors and 6-phosphofructokinase, during glucose consumption
Embden–Meyerhof–Parnas pathway (EMPP) was chosen as the next target to be engineered, because a metabolic intermediate glucose-6-phosphate is formed from both glucose and galactose. However, glucose-6-phosphate formation from both sugars are quietly differ from each other. To yield glucose-6-phosphate, glucose is catabolized by one-step reaction, while galactose is metabolized via Leior pathway28. Thus, it was expected that glucose and galactose consumption could be individually affected by modulation of EMPP. To further modulate Rgal/glu, rather than overexpressing the galactose operon, we next knocked out the pfkA gene, which encodes ATP-dependent 6-phosphofructokinase, in E. coli strain GR2. As 6-phosphofructokinase is a key enzyme in the EMPP, we expected that the specific glucose consumption rate could be negatively affected by the lack of 6-phosphofructokinase in the resulting GR2P cells under the co-fermentation of glucose and galactose. In flask cultures, GR2P showed a maximum specific growth rate (µmax) of 0.2993/h with a short lag time, which was rather lower than the 0.5653/h and 0.4469/h obtained from cultures of W3110 and GR2, respectively (Fig. 3 and Table 2). This indicates that the additional knockout of the pfkA gene negatively affected cell growth. E. coli GR2P had completely consumed the glucose at 24 h of culture, yielding a specific glucose consumption rate of 0.4714 g/gDCW/h (Fig. 3 and Table 2). Meanwhile, the specific galactose consumption rate was 0.0552 g/gDCW/h, yielding an Rgal/glu of 0.12 (Table 2). Thus, our results indicate that the specific glucose consumption rate was negatively influenced by regulation of the EMPP in the engineered E. coli, whereas the specific galactose consumption rate was less sensitive to this manipulation.
Galactose-to-glucose consumption ratio in E. coli GR2PZ, which lacked the galactose operon repressors, 6-phosphofructokinase, and glucose-6-phosphate dehydrogenase, during glucose consumption
A previous report found that zwf-mutant E. coli, which lacked glucose-6-phosphate dehydrogenase, showed carbon flow through the oxidative pentose phosphate pathway (OPPP)29. To additionally modulate the glucose consumption rate of our engineered strain, we knocked out the zwf gene in E. coli GR2P. As glucose-6-phosphate dehydrogenase is involved in converting glucose-6-phosphate to 6-phosphogluconate in the OPPP, we expected that the glucose consumption rate would be further reduced in the resulting GR2PZ strain, compared to the parent strain, GR2P. In the co-fermentation of glucose and galactose, GR2PZ showed a maximum specific growth rate of 0.2076 /h with a lag time, and thus had the lowest µmax among the strains used in this study (Fig. 4 and Table 2). Furthermore, the sudden drop in cell mass was also observed at the very early stage (Fig. 4), which might be caused from the simultaneous knockout of the pfkA and zwf genes in E. coli. GR2PZ had completely consumed the glucose at 36 h of culture, yielding a specific glucose consumption rate of 0.4419 g/gDCW/h (Fig. 4 and Table 2). The specific galactose consumption rate was 0.0737 g/gDCW/h, for an Rgal/glu of 0.17.
Galactose-to-glucose consumption ratio was further modulated by integration of a brief process optimization for initial sugar ratio
We demonstrated the control of glucose-to-galactose consumption ratio during the co-fermentation of glucose and galactose by engineering central sugar metabolism. Next, one of the engineered E. coli strains was applied to the process optimization for initial sugar ratio and concentrations, to see if the galactose-to-glucose consumption ratio could be further modulated by different initial sugar ratio and concentration. Among the engineered three strains, GR2P was randomly selected, and cultured in R/2 medium supplemented with sugars at the different initial ratio and concentration, such as 4 g/L glucose and 2 g/L galactose (4glu-2gal); and 4 g/L glucose and 8 g/L galactose (4glu-8gal) (Fig. 5). The culture parameters using GR2P in R/2 medium supplemented 4 g/L glucose and 4 g/L galactose (4glu-4gal) were obtained from Fig. 3. All parameters obtained from the culture conditions, such as 4glu-2gal, 4glu-4gal, and 4glu-8gal were listed in Table 3. E. coli GR2P achieved an Rgal/glu of 0.12, 0.12, and 0.67 under the condition of 4glu-2gal, 4glu-4gal, and 4glu-8gal, respectively (Table 3). Taken together, our results indicate that galactose-to-glucose consumption ratio was further modulated by integration of a brief process optimization together with the modulation of sugar metabolism.
Discussion
Galactose produced by a marine biomass is a promising fermentable sugar for the production of biofuels and biochemicals. A recent report described an engineered E. coli strain capable of consuming galactose at the same rate as glucose20. In the same study, an Rgal/glu of nearly 1.0 was achieved by overexpressing the galactose operon in a mutant E. coli lacking the galactose operon repressor20. However, the fine-tuned control of galactose consumption by E. coli has not previously been reported in a co-fermentation with glucose. In this study, we demonstrate that the galactose consumption rate may be finely controlled during co-fermentation with glucose by comparing our engineered E. coli strains and their parent W3110. Strains W3110, GR2, GR2P, and GR2PZ achieved Rgal/glu values of 0.03, 0.09, 0.12, and 0.17, respectively, during the co-fermentation of these sugars. Meanwhile, Rgal/glu of 0.67 was achieved by a brief process optimization using GR2P strain.
We found that the galactose-to-glucose consumption ratio could be slightly modulated by disrupting the galactose operon repressors encoded by the galRS genes, and further modulated by regulating the central metabolic pathway. The galRS-mutant E. coli strain, GR2, had an Rgal/glu ratio of 0.09, compared to the 0.03 ratio of parent strain W3110. Products of the galRS genes have been reported to negatively regulate galactose metabolism by binding upstream of the galactose operon19,30,31,32. Thus, the inactivation of galactose operon repressors could logically increase the specific galactose consumption rate in E. coli while leaving the specific glucose consumption rate unchanged. Indeed, in the co-fermentation of glucose and galactose, GR2 and W3110 showed specific galactose consumption rates of 0.1262 g/gDCW/h and 0.0373 g/gDCW/h, respectively, while yielding specific glucose consumption rates of 1.3629 g/gDCW/h and 1.3706 g/gDCW/h (Fig. 2, Table 2).
In the co-fermentation of glucose and galactose using strains GR2P and GR2PZ, in which the central metabolic pathway was further modified by knockout of the pfkA and pfkA-zwf genes, respectively, we obtained Rgal/glu ratios of 0.12 and 0.17, respectively. For GR2P and GR2PZ, the specific consumption rates for both sugars were decreased compared to that of parent strain GR2. Glucose consumption was more sensitive than galactose consumption to modulation of the central metabolic pathway: The specific glucose consumption rate decreased from 1.3629 g/gDCW/h (GR2) to 0.4714 g/gDCW/h (GR2P; 65% decrease) and 0.4419 g/gDCW/h (GR2PZ; 68% decrease), while the specific galactose consumption rate decreased from 0.1262 g/gDCW/h (GR2) to 0.0552 g/gDCW/h (GR2P; 56%) and 0.0737 g/gDCW/h (GR2PZ; 41%). Rgal/glu ratios of 0.12 and 0.17 were obtained for the co-fermentation of glucose and galactose by strains GR2P and GR2PZ, respectively (Fig. 4, Table 2). We do not yet know why glucose consumption was more sensitive than galactose consumption to the pfkA and pfkA-zwf mutations. However, this may relate to a previous report that deletion of the zwf gene altered the metabolism of the mutant cells by changing the redox balance33,34,35.
The engineered GR2P and GR2PZ strains utilized both sugars simultaneously in the presence of the major CCR. Under the galRS double-knockout condition, it seems that the repression of galactose metabolism by the CCR was slightly leaky, which was supported by Table 2 and Fig. 2. The galRS were knocked-out in GR2P and GR2PZ strains also, which means that two strains were leaky in CCR, too. Thus, we believe that the simultaneous utilization of both sugars was not resulted from the complete deregulation of CCR. However, the simultaneous utilization of both sugars in GR2P and GR2PZ strains could be explained by dramatic decrease of specific glucose consumption rate compared to the parent GR2 strain (Table 2), which was more sensitive compared to the decrease of specific galactose consumption.
In conclusion, we herein report the regulation of the galactose-to-glucose consumption ratio by engineering E. coli strains for the co-fermentation of these sugars. We achieved Rgal/glu values of 0.03, 0.09, 0.12, and 0.17 in the co-fermentation of glucose and galactose using E. coli strains W3110, GR2, GR2P, and GR2PZ, respectively. The Rgal/glu value of 0.67 was achieved by a brief process optimization for initial sugar ratio using GR2P strain. In the engineered E. coli strains, the galactose-to-glucose consumption ratio could be changed by activation of the galactose operon and modulation of central metabolic pathways (EMPP and OPPP) in the co-fermentation of both sugars. The strategy reported in this study can help regulate the galactose-to-glucose consumption ratio in a microbial co-fermentation of both sugars from galactose-rich biomass. Furthermore, the repression of carbohydrate metabolism might be applicable to enhance acetyl-CoA pool from another carbon source which is uncommon to E. coli, such as polyethylene.
Materials and methods
Microbial strains and plasmids
Details on the utilized engineered E. coli strains and their parent strain, K-12 W3110, are listed in Table 1. Plasmid pCW611, which harbored the genes encoding lambda red recombinase and Cre-recombinase, was used for gene knockout in E. coli36. Plasmid pMtrc9, which contained the lox66-cat-lox77 cassette, was used as a PCR template to amplify the DNA fragment for target gene knockout37. Details on the utilized plasmids are presented in Table 1.
Media and culture conditions
Escherichia coli cells were cultured in R/2 medium supplemented with the indicated carbon sources. R/2 medium (pH 6.8) contained 2 g (NH4)2HPO4, 6.75 g KH2PO4, 0.7 g MgSO4·7H2O, 0.85 g citric acid, and 5 mL trace metal solution per liter38. The trace metal solution contained (per liter): 0.1 M HCl, 10 g FeSO4·7H2O, 2.25 g ZnSO4·7H2O, 0.58 g MnSO4·5H2O, 1 g CuSO4·5H2O, 0.1 g (NH4)6Mo7O24·4H2O, 0.02 g Na2B4O7·10H2O, and 2 g CaCl2·2H2O38.
To prepare the seed culture, a colony grown on an LB agar plate was inoculated into 25-mL test tubes containing 5 mL R/2 medium. The test tubes were incubated in a shaking incubator (IST-4075, JEIOTECH, Korea) at 37 °C and 200 rpm. The next day, 2.2 mL of seed culture was transferred to each 500-mL flask containing 220 mL R/2 medium. For co-fermentation experiments, 3 g/L glucose and 3 g/L galactose were supplemented in the medium as carbon sources.
Construction of knockout mutants
Wild type E. coli strain K-12 W3110 was used for the construction of all studied mutants. The quadruple gene knockout mutant, GR2PZ (described here as an example) was constructed using the modified one-step inactivation method36 to knock out the galR, galS, pfkA, and zwf genes. The primers listed in Table S1 were designed from the sequences of the target genes and plasmid pMtrc9, which contained the lox66-cat-lox71 cassette. To construct a linear DNA fragment for knockout of the galR, galS, pfkA and zwf genes, we used primer pairs galR-KO-F1 and galR-KO-R1, galS-KO-F1 and galS-KO-R1, pfkA-KO-F1 and pfkA-KO-R1, and zwf-KO-F1 and zwf-KO-R1, respectively. The resulting DNA fragments were individually PCR amplified using the corresponding primer pairs: galR-KO-F2 and galR-KO-R2, galS-KO-F2 and galS-KO-R2, pfkA-KO-F1 and pfkA-KO-R1, and zwf-KO-F2 and zwf-KO-R2, respectively. The final fragments were individually electroporated into E. coli cells harboring the pCW611 helper plasmid, using a BTX ECM 630 electroporator (BTX, San Diego, CA, USA; 1.8 kV, 25 μF, 200Ω, 1-mm electroporation cuvettes). Using pCW611, which contains both lambda red recombinase and the Cre recombinase, target gene knockout in E. coli was conducted as previously described36. Mutation of the target genes, galR, galS, pfkA, and zwf, was validated by colony PCR using primer pairs galR-KO-F1 and galR-KO-R1, galS-KO-F1 and galS-KO-R1, pfkA-KO-F1 and pfkA-KO-R1, and zwf-KO-F1 and zwgal-KO-R1, respectively. Finally, plasmid pCW611 was removed from the mutant E. coli cells by incubation at 42℃. Double and triple gene knockout mutants, GR2 and GR2P were also constructed by knockout combination of the corresponding genes in the same manner.
Determination of the galactose-to-glucose consumption ratio
To determine the galactose-to-glucose consumption ratio during glucose consumption, E. coli cells were cultured in 500-mL flasks containing R/2 medium supplemented with 4 g/L glucose and 4 g/L galactose, at 37 °C and 200 rpm. One milliliter of culture broth was taken at 0, 3, 6, 9, 12, 16, 24, 28, 32, 36, and 48 h and used to determine the residual glucose and galactose concentrations (see below for analytical methods). Another 1 mL of broth was taken at the same time and used to determine the dry cell weight. From these analyses, the specific glucose consumption rate (Rglu) was determined, while the specific galactose consumption rate (Rgal) was determined during glucose consumption. The galactose-to-glucose consumption ratio, Rgal/glu, was determined. All flask fermentations were independently performed in triplicate.
Analytic methods
Cell growth was monitored by determining the absorbance at 600 nm using a spectrophotometer (HIACHI, JP/U-1900, Tokyo, Japan). For determination of the glucose and galactose concentrations, culture broth supernatants were prepared by centrifugation followed by filtration using a 0.22-μm pore syringe filter. Samples were applied to high performance liquid chromatography (HPLC; 1100 series, Agilent Technologies, Santa Clara, CA, USA) equipped with a refractive index detector (RID; RI-101, Shodex, Klokkerfaldet, Denmark)39,40,41,42. A MetaCarb 87H column (Agilent Technologies) was used with 0.01 N H2SO4 as a mobile phase at 25 °C, with a flow rate of 0.5 mL/min.
References
Banerjee, S., Mishra, G. & Roy, A. Metabolic engineering of bacteria for renewable bioethanol production from cellulosic biomass. Biotechnol. Bioproc. Eng. 1–21 (2019).
Chum, H. L. & Overend, R. P. Biomass and renewable fuels. Fuel Process. Technol. 71, 187–195 (2001).
Liu, C.-L. et al. Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 11, 285 (2018).
Woo, J. E., Lee, S. Y. & Jang, Y.-S. Effects of nutritional enrichment on acid production from degenerated (non-solventogenic) Clostridium acetobutylicum strain M5. Appl. Biol. Chem. 61, 469–472 (2018).
Song, M. K. et al. Biological synthesis and anti-inflammatory activity of arylalkylamine. Appl. Biol. Chem. 60, 597–602 (2017).
Choo, H. J. et al. Microbial synthesis of hydroxytyrosol and hydroxysalidroside. Appl. Biol. Chem. 61, 295–301 (2018).
John, R. P., Anisha, G. S., Nampoothiri, K. M. & Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 102, 186–193. https://doi.org/10.1016/j.biortech.2010.06.139 (2011).
Dang, N. M. & Lee, K. Utilization of organic liquid fertilizer in microalgae cultivation for biodiesel production. Biotechnol. Bioprocess. Eng. 23, 405–414 (2018).
Noh, H. J., Woo, J. E., Lee, S. Y. & Jang, Y.-S. Metabolic engineering of Clostridium acetobutylicum for the production of butyl butyrate. Appl. Microbiol. Biotechnol. 102, 8319–8327 (2018).
Noh, H. J., Lee, S. Y. & Jang, Y.-S. Microbial production of butyl butyrate, a flavor and fragrance compound. Appl. Microbiol. Biotechnol. 103, 2079–2086 (2019).
Jeon, H.-S., Park, S. E., Ahn, B. & Kim, Y.-K. Enhancement of biodiesel production in Chlorella vulgaris cultivation using silica nanoparticles. Biotechnol. Bioprocess. Eng. 22, 136–141 (2017).
Noreen, A., Zia, K. M., Zuber, M., Ali, M. & Mujahid, M. A critical review of algal biomass: A versatile platform of bio-based polyesters from renewable resources. Int. J. Biol. Macromol. 86, 937–949 (2016).
Packer, M. Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 37, 3428–3437 (2009).
Wei, N., Quarterman, J. & Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 31, 70–77 (2013).
Wi, S. G., Kim, H. J., Mahadevan, S. A., Yang, D.-J. & Bae, H.-J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour. Technol. 100, 6658–6660 (2009).
Jeong, G.-T. & Park, D.-H. Production of sugars and levulinic acid from marine biomass Gelidium amansii. Appl. Biochem. Biotechnol. 161, 41–52 (2010).
Chi, W.-J., Chang, Y.-K. & Hong, S.-K. Agar degradation by microorganisms and agar-degrading enzymes. Appl. Microbiol. Biotechnol. 94, 917–930 (2012).
Kim, P.-H. et al. Simulated moving bed separation of agarose-hydrolyzate components for biofuel production from marine biomass. J. Chromatogr. A 1406, 231–243 (2015).
Woo, J. E., Seong, H. J., Lee, S. Y. & Jang, Y.-S. Metabolic engineering of Escherichia coli for the production of hyaluronic acid from glucose and galactose. Front. Bioeng. Biotechnol. 7, 351 (2019).
Lim, H. G., Seo, S. W. & Jung, G. Y. Engineered Escherichia coli for simultaneous utilization of galactose and glucose. Bioresour. Technol. 135, 564–567 (2013).
Majumdar, A. & Adhya, S. Demonstration of two operator elements in gal: In vitro repressor binding studies. Proc. Natl. Acad. Sci. 81, 6100–6104 (1984).
Majumdar, A. & Adhya, S. Probing the structure of gal operator-repressor complexes. Conformation change in DNA. J. Biol. Chem. 262, 13258–13262 (1987).
Saedler, H., Gullon, A., Fiethen, L. & Starlinger, P. J. M. Negative control of the galactose operon in E. coli. Mol. Gen. Genet. 102, 79–88 (1968).
Hogg, R. W., Voelker, C. & Von Carlowitz, I. Nucleotide sequence and analysis of the mgl operon of Escherichia coli K12. Mol. Gen. Genet. 229, 453–459 (1991).
Lengeler, J., Hermann, K. O., UnsÖLD, H. J. & Boos, W. The regulation of the β-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur. J. Biochem. 19, 457–470 (1971).
Weickert, M. J. & Adhya, S. Isorepressor of the gal regulon in Escherichia coli. J. Mol. Biol. 226, 69–83 (1992).
Weickert, M. J. & Adhya, S. Control of transcription of gal repressor and isorepressor genes in Escherichia coli. J. Bacteriol. 175, 251–258 (1993).
Sellick, C. A., Campbell, R. N. & Reece, R. J. Galactose metabolism in yeast—structure and regulation of the Leloir pathway enzymes and the genes encoding them. Int. Rev. Cell Mol. Biol. 269, 111–150 (2008).
Hollinshead, W. D. et al. Examining Escherichia coli glycolytic pathways, catabolite repression, and metabolite channeling using Δ pfk mutants. Biotechnol. Biofuels 9, 212 (2016).
Geanacopoulos, M. & Adhya, S. Functional characterization of roles of GalR and GalS as regulators of the gal regulon. J. Bacteriol. 179, 228–234. https://doi.org/10.1128/jb.179.1.228-234.1997 (1997).
Geanacopoulos, M., Vasmatzis, G., Zhurkin, V. B. & Adhya, S. Gal repressosome contains an antiparallel DNA loop. Nat. Struct. Mol. Biol. 8, 432 (2001).
Semsey, S., Krishna, S., Sneppen, K. & Adhya, S. Signal integration in the galactose network of Escherichia coli. Mol. Microbiol. 65, 465–476 (2007).
Hua, Q., Yang, C., Baba, T., Mori, H. & Shimizu, K. Responses of the central metabolism in Escherichia coli to phosphoglucose isomerase and glucose-6-phosphate dehydrogenase knockouts. J. Bacteriol. 185, 7053–7067. https://doi.org/10.1128/jb.185.24.7053-7067.2003 (2003).
Zhao, J., Baba, T., Mori, H. & Shimizu, K. Global metabolic response of Escherichia coli to gnd or zwf gene-knockout, based on 13C-labeling experiments and the measurement of enzyme activities. Appl. Microbiol. Biotechnol. 64, 91–98. https://doi.org/10.1007/s00253-003-1458-5 (2004).
Zhao, J., Baba, T., Mori, H. & Shimizu, K. Effect of zwf gene knockout on the metabolism of Escherichia coli grown on glucose or acetate. Metab. Eng. 6, 164–174. https://doi.org/10.1016/j.ymben.2004.02.004 (2004).
Song, C. W. & Lee, S. Y. Rapid one-step inactivation of single or multiple genes in Escherichia coli. Biotechnol. J. 8, 776–784 (2013).
Kim, J. M., Lee, K. H. & Lee, S. Y. Development of a markerless gene knock-out system for Mannheimia succiniciproducens using a temperature-sensitive plasmid. FEMS Microbiol. Lett. 278, 78–85 (2008).
Lee, S. Y. & Chang, H. N. High cell density cultivation of Escherichia coli W using sucrose as a carbon source. Biotechnol. Lett. 15, 971–974 (1993).
Kim, D.-S. et al. Comparison of the retention rates of thiamin, riboflavin, and niacin between normal and high-oleic peanuts after roasting. Appl. Biol. Chem. 61, 449–458 (2018).
Lee, J. et al. Validation protocol for whole-body dosimetry in an agricultural exposure study. Appl. Biol. Chem. 61, 125–130 (2018).
Mirahmadi, F., Mizani, M., Sadeghi, R. & Givianrad, M. H. Chemical composition and thermal properties of Pistacia atlantica subsp. Kurdica gum. Appl. Biol. Chem. 62, 4 (2019).
Arora, R., Behera, S., Sharma, N. K. & Kumar, S. Evaluating the pathway for co-fermentation of glucose and xylose for enhanced bioethanol production using flux balance analysis. Biotechnol. Bioprocess. Eng. 24, 924–933. https://doi.org/10.1007/s12257-019-0026-5 (2019).
Acknowledgements
This work was supported by a grant from the Ministry of Science and ICT (MSIT) through the National Research Foundation (NRF) of Korea (NRF-2019R1A4A1029125). This work was further supported by Cooperative Research Program for Agricultural Science & Technology Development (Project No. PJ01492601).
Author information
Authors and Affiliations
Contributions
Y.S.J. conceived the project. H.J.S. and J.E.W. performed experiments. H.J.S. and Y.S.J. were involved in analysis and interpretation of experimental data. H.J.S. and Y.S.J. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seong, H.J., Woo, J.E. & Jang, YS. Control of the galactose-to-glucose consumption ratio in co-fermentation using engineered Escherichia coli strains. Sci Rep 10, 12132 (2020). https://doi.org/10.1038/s41598-020-69143-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69143-3
This article is cited by
-
HyfF subunit of hydrogenase 4 is crucial for regulating FOF1 dependent proton/potassium fluxes during fermentation of various concentrations of glucose
Journal of Bioenergetics and Biomembranes (2022)
-
Effect of deregulation of repressor-specific carbon catabolite repression on carbon source consumption in Escherichia coli
Applied Biological Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.