Abstract
High levels of methylation in the GR gene (nuclear receptor subfamily 3, group C, member 1; NR3C1) have been associated with depression and cardiovascular risk. This study aimed to investigate whether NR3C1 methylation status was associated with the long-term prognosis of acute coronary syndrome (ACS) considering depression and cardiovascular status at the early phase of ACS. A total of 969 patients with recent ACS were recruited at a tertiary university hospital in Korea. Baseline evaluations were made from 2007 to 2012, including DSM-IV depressive disorder, NR3C1 methylation, and various demographic and clinical characteristics such as cardiovascular risk markers. Over a 5~12 year follow-up after the index ACS, time to major adverse cardiac event (MACE) was investigated using Cox regression models. Higher NR3C1 methylation status was associated with depression and several cardiovascular risk markers at baseline. NR3C1 hypermethylation predicted worse long-term prognosis of ACS only in the presence of depressive disorder with significant synergistic interaction terms and independent of potential confounding factors. Synergistic effects of depressive disorder and NR3C1 hypermethylation on long-term cardiac outcomes in ACS were found. NR3C1 methylation status represents a candidate prognostic biomarker for ACS in combination with a diagnosis of depressive disorder. Further research is needed to ascertain the generalisability of these findings.
Similar content being viewed by others
Introduction
Depression is common in patients with acute coronary syndrome (ACS; including myocardial infarction (MI) and unstable angina (UA)), and is associated with poor prognosis including increased morbidity and mortality1. Common biological mechanisms underlying ACS and depression may account for this2. Dysregulation of hypothalamic–pituitary–adrenal (HPA) axis is one candidate, since this is strongly implicated in the pathogenesis of depression3 and also associated with impaired repair after cardiac injury4. The effects of cortisol, the major endogenous glucocorticoid hormone in the HPA axis, are principally mediated by glucocorticoid receptors (GRs), whose expression and sensitivity are modulated by epigenetic modifications5. DNA methylation, one of the widely investigated epigenetic modifications, refers to covalent methylation of the C5 position of cytosine residues followed by guanine residues (CpG dinucleotides)6.
High levels of methylation in the GR gene (nuclear receptor subfamily 3, group C, member 1; NR3C1) have been reported to be correlated with reduced GR expression in animals7, and in humans with depression8, post-traumatic stress disorder9, and suicidality10. With respect to ACS, hypermethylation of NR3C1 has been found to be associated with atherosclerosis and elevated cardiovascular reactivity11,12. Recently, our study group found that higher DNA methylation of NR3C1 was independently associated with depressive disorder at the early phase of ACS13. Based on these findings, it can be postulated that NR3C1 methylation status may be associated with cardiac prognosis of ACS independently of or interactively with depression. However, the role of NR3C1 methylation on prognosis of ACS has not been investigated, despite the clinical importance of depression-ACS comorbidity.
In the present study, we aimed to investigate whether NR3C1 methylation status was correlated with known cardiovascular markers at the early phase of ACS, and whether it was associated with the long-term prognosis of ACS considering the negative impact of depression on these outcomes.
Materials and Methods
Study outline and participants
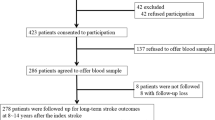
The analyses were performed using data from a large naturalistic cohort study which was set up to investigate the inter-relationships between depression and ACS: the DEPression in ACS (DEPACS) study. The design and main findings have been published14,15. The outline of the recruitment process for the present analysis is presented in online Fig. S1. ACS patients (N = 4809), recently hospitalized at the Department of Cardiology of Chonnam National University Hospital, Gwangju, South Korea, were approached consecutively to participate in the study from 2006 to 2012. With authorization by the Korean Circulation Society, this department has played the role of the central coordinating centre for the Korea Acute Myocardial Infarction Registry (KAMIR)16.
KAMIR is a web-based registry platform (http://kamir5.kamir.or.kr/) to collect nationwide multicentre data regarding clinical practices and outcomes of patients with acute MI prospectively which enables it to evaluate the prospective associations of a range of exposures or interventions with long-term cardiac outcomes. Treatment of ACS patients was based on international guidelines for the management of ACS17 by cardiologists participating in the present study. Those who met eligibility criteria (detailed in online supplementary material) and agreed to participate (N = 1152) received baseline evaluations as inpatients within 2 weeks (mean 6.3 ± SD 2.4 days) post-ACS. Of these, 969 (84.1%) agreed to phlebotomy and comprised the study sample. All participants were approached for follow-up evaluations for cardiac outcomes at 2017, 5~12 years after the index ACS. Written informed consent was obtained, and the study was conducted in compliance with institutional guidelines and the 1964 Declaration of Helsinki. Moreover, the present study was approved by the Chonnam National University Hospital (CNUH) Institutional Review Board.
Baseline evaluation
Depressive disorders were diagnosed by psychiatrists with the Mini-International Neuropsychiatric Interview (MINI)18, a structured diagnostic interview for psychiatric disorders, according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)19, for defining major or minor depressive disorders.
For NR3C1 methylation status, DNA was extracted from venous blood using standard procedures. The NR3C1 region chosen for methylation analysis was exon 1 F (GeneBank #AY 436590), which includes a binding site for nerve growth factor-inducible protein A (NGFI-A) and is highly expressed in the hippocampus of pups with nurturing mothers20. Methylation status was measured in three CpG sites (Supplementary Fig. 2), located in the CpG-rich region of NR3C1 exon 1 F between −3166 and −3147 (CGGTGGCCCTCTTAACGCCG) relative to the translational start site (+1). These sites correspond to the region investigated in previous studies on adverse life experiences and related disorders including depression21,22,23,24.
Information on the features that could potentially affect cardiac outcomes25 was obtained. Demographic characteristics were collected on age, gender, education, marital status, living alone, housing, and employment status. For depression characteristics, the score from the self-completed Beck Depression Inventory (BDI)26 and previous and family histories of depression were recorded. For cardiac characteristics, ACS diagnosis (MI or UA), previous and family histories of ACS, diagnosed hypertension and diabetes mellitus, and reported current smoking status were ascertained. The following cardiovascular risk markers were measured: echocardiography for left ventricular ejection fraction (LVEF) and wall motion; electrocardiography for heart rate, PR interval, QRS duration, and QTc duration; measurements of body mass index (BMI) and blood pressure; and laboratory tests for troponin I, creatine kinase-MB (CK-MB), tumor necrosis factor-α, Interleukin (IL)-1β, IL-6, IL-18, high sensitivity C-reactive protein (hs-CRP), homocysteine, total- and lower density lipoprotein (LDL) cholesterol, and triglyceride levels.
Long-term cardiac outcomes
Long-term cardiac outcomes were comprehensively evaluated using KAMIR data because detailed electronic data regarding hospital admissions, deaths, recurrent MI, and percutaneous coronary intervention (PCI) was stored in the KAMIR registry. All baseline participants in the present study were followed up for these outcomes. To implement non-hierarchic endpoint analyses, all patients were followed for the evaluation point of interest or until death. The primary endpoint was determined as a major adverse cardiac event (MACE), a defining composite of all-cause mortality, MI and PCI. Secondary endpoints were selected as all-cause mortality, cardiac death (determined as sudden death without other causes, death from arrhythmias or after MI or heart failure, or death caused by heart surgery or endocarditis), MI, and PCI. All potential events were adjudicated by an independent endpoint committee composed of study cardiologists who was blinded to participants’ depression comorbidity.
Statistical analyses
Using the sample median value, NR3C1 methylation percentages were classified by a binary variable (‘lower’ or ‘higher’ categories), in line with previous research27. Demographic and clinical characteristics of ACS patients with lower and higher methylation were compared applying t-tests or χ2 tests, as appropriate. Correlations between the methylation percentage and cardiovascular risk markers were examined by the Spearman correlation coefficients, after partial for BDI scores. Factors potentially associated with methylation status (p < 0.05) and other characteristics found to possess significant effects on MACE25,28 were considered as covariates in subsequent adjusted analyses. Kaplan–Meier curves were constructed, and the cumulative proportions of MACE in those with lower vs. higher NR3C1F methylation values were compared using the log-rank tests, and further stratified by the presence or not of depressive disorders at baseline. Time to the first composite and individual MACEs were subsequently compared using Cox proportional hazards models, after adjustment for the potential covariates described above, between the lower vs. higher methylation groups in total participants and then by their depressive disorder comorbidity status. Additional sensitivity analyses were carried out applying the methylation value as a continuous exposure variable (10 percent unit increase) and as tertials (lower, middle, and higher) to re-examine its effect beyond the binary categorical approach. Schoenfeld residuals tests were carried out to test proportional-hazards assumptions in all models. Two-tailed tests were used in all of analyses to determine significance at the 5% level and all statistical analyses were conducted with SPSS 21.0 and STATA 12.0 software.
Results
NR3C1 methylation status and baseline characteristics
Of 969 participants, 378 (39%) ACS patients experienced depressive disorders at the baseline. Median (interquartile rage) and mean (standard deviation) value of average NR3C1 methylation percentages and three individual CpG sites are summarized in online Table S1. Since the individual three CpG sites of NR3C1 methylation percentages were closely correlated (all Spearman’s rho > 0.7, p-value < 0.001), the results regarding average CpG values were robust and similar but less obvious for individual CpG sites. Therefore, the results for average CpG values were solely presented in subsequent analyses. In Table 1, baseline characteristics of ACS patients with lower and higher average NR3C1 methylation values are compared. The mean (standard deviation) and range of lower methylation group were 12.2 (5.2) and 0–20.4% while those of higher group were 31.2 (9.1) and 20.4–66.0%. A higher average methylation value was only significantly associated with higher BDI scores. No significant difference was found between those who did and did not agree to provide blood samples in terms of any baseline characteristic (all p-values > 0.15).
Correlations between NR3C1 methylation percentage and cardiovascular risk markers
NR3C1 average methylation percentage was significantly correlated with longer QTc duration, higher BMI, and higher serum levels of troponin I and CK-MB after partial for BDI scores (Table 2). These characteristics were entered as covariates in the following adjusted analyses. In addition, age, ACS diagnosis, previous ACS, hypertension, diabetes, smoking, LVEF, and/or depressive disorders were also included as covariates because of known association with cardiac outcomes in previous studies25,28.
Effects of NR3C1 methylation percentage on prognosis of ACS
The cardiac outcomes of all participants were followed until 2017 or to their deaths [median; mean (standard deviation) duration of follow-up = 8.4; 8.7 (1.5) years]. 383 participants (39.5%) experienced the primary endpoint (composite MACE). Secondary endpoint numbers were as follows: 178 (18.4%) for all-cause mortality, 98 (10.1%) for cardiac death, 101 (10.4%) for MI and 139 (14.3%) for PCI. The cumulative risk of the composite MACE in subjects with lower and higher average NR3C1 methylation is described in Fig. 1. A significant difference was found in the total sample. However, stratified by depressive disorder status, a significant group difference was found only in those with depressive disorder, and did not appear to be present in those without depressive disorder.
Comparisons of first MACE rates by lower vs. higher methylation values, successively adjusted for potential confounding variables, are summarized in Table 3. In the unadjusted analyses, a higher methylation was significantly associated with higher rates of composite MACE, all-cause mortality, MI, and PCI. The associations were weaker and remained significant only for composite MACE and MI outcomes after adjustment for age, ACS diagnosis, previous ACS, hypertension, diabetes, smoking, LVEF, QTc duration, BMI, and serum levels of troponin I and creatine kinase-MB. The strengths of the associations were substantially weaker after further adjustment for depressive disorder status, and the association was significant only for the composite MACE outcome.
Comparisons of first MACE rates by lower vs. higher methylation values stratified by baseline depressive disorder after adjustment are summarized in Table 4. Higher methylation was significantly associated with higher rates of composite MACE, all-cause mortality, cardiac death, and PCI only in those with depressive disorder after adjustment for age, ACS diagnosis, previous ACS, hypertension, diabetes, smoking, LVEF, QTc duration, BMI, and serum levels of troponin I and creatine kinase-MB. The NR3C1 methylation x depressive disorder multiplicative interaction terms were statistically significant for the composite MACE, all-cause mortality, and cardiac death outcomes. Results of additional sensitivity analysis using the methylation percentages as a continuous variable and as tertials (lower, middle, and higher) are displayed in online Table S2 and online Table S3 respectively: in summary, the strengths of the associations were not substantially changed using continuous variables while the strength of the associations were generally lost but the significance of the association with MACE was remained in those with depressive disorder after adjustment. Model assumptions were all met (Schoenfeld p-values > 0.30).
Discussion
The principal findings from this cohort study were that higher NR3C1 methylation status was correlated with several cardiovascular risk markers at the early phase of ACS and predicted worse long-term prognosis. The longitudinal associations were significant only in the presence of depressive disorder with significant synergistic interaction terms, and were independent of a range of potential confounding factors.
In these patients with ACS, a higher NR3C1 methylation value was significantly correlated with worse profiles of several cardiovascular risk markers including QTc duration, BMI, and serum troponin I and CK-MB. These findings are in line with recent studies reporting that hypermethylation of NR3C1 is associated with atherosclerosis11, and with heightened cardiovascular reactivity12. Although functional levels including GR mRNA or cortisol levels were not measured in the present study, hypermethylation of the NR3C1 has been found to be related with reduced GR mRNA level8 and abnormal cortisol response22, which might affect glucocorticoid receptor sensitivities in turn cause endothelial dysfunctions, atherosclerosis and impaired metabolic signaling29,30. Based on these, it can be postulated that the association between NR3C1 hypermethylation and cardiovascular risk markers may underlie its deleterious effects on long-term prognosis of ACS. However, in the present study, the longitudinal associations were explained partially but not fully by these variables.
These prognostic effects were more strongly explained by depressive disorder status, in that they were significant only in the presence of depressive disorder, independent of cardiovascular risk markers. Several explanations are possible for the observed synergistic effects. With respect to biological mechanisms, NR3C1 hypermethylation is associated with adverse profiles of cardiovascular risk markers as stated above. Added to this, depression itself is associated with alterations in pro-inflammatory cytokines, and with autonomic and platelet dysfunction, which also have adverse effects on cardiac outcomes31,32; the synergy may therefore reflect multiple risk pathway involvement. Considering behavioural aspects, NR3C1 methylation has functional influences affecting GR expression and finally dysregulating stress responses via HPA axis7,8,9, which may result in a reduced ability to cope with stressful situations such as unexpected ACS itself, financial and occupational problems, disabilities and other difficulties faced by patients with ACS. That is, ACS patients with NR3C1 hyper methylation, which was associated with cardiovascular risk markers, are more likely to also have depression. Moreover, depression has been associated with unhealthy behaviors such as sedentary lifestyle, and irregular hospital visits and medication taking, which themselves have negative effects on cardiac outcomes33. Summing up, ACS patients with both NR3C1 hypermethylation and depressive disorder may be liable to poor prognosis through a combination of biological and behavioural factors.
As the first evaluation of these questions, this study has several strengths. Participants were consecutively recruited from all eligible patients with a recent ACS, which in turn contributed to decrease the risk of error originating from heterogeneous examination times and to increase the sample homogeneity. A depressive disorder was determined with a structured diagnostic interview, and psychiatric and cardiovascular characteristics were ascertained using well validated measurements. Vast amounts of covariates potentially affecting cardiac outcomes as well as methylation status were included in the present analyses. A single site recruitment was performed, which potentially results in a limitation of generalization of the present findings but possesses advantages in terms of consistency of evaluation and treatment. One important limitation of the present study is that only one CpG island in the NR3C1 gene was examined, although this area has been repeatedly investigated in previous epigenetic studies pertaining to both depression and adverse life events22,24,34. Since this region was evaluated in relation to cardiac outcomes for the first time in our study, replication is needed. Attrition in the recruitment process can be another source of limitation because methylation analysis was available only in 84% of the baseline samples. However, no difference in the baseline demographic and clinical characteristics was found between patients with or without methylation analyses. Furthermore, long-term follow-up data on MACE have been completely collected for the primary analysis. Finial limitation was lack of functional measurement such as GR mRNA or cortisol levels. Although previous studies suggested that hypermethylation of NR3C1 methylation was associated with lower GR mRNA as well as abnormal cortisol response8,22, further investigations for underlying biological mechanisms between NR3C1 methylation and cardiac outcomes with interaction of depression.
In conclusion, higher NR3C1 methylation status at early phase of ACS predicted worse long-term prognosis of ACS in the presence of depressive disorder, independent of potential confounders including important cardiovascular risk markers. Similarly, associations of depression with worse long-term cardiac outcomes were stronger in ACS patients with higher NR3C1 methylation status. NR3C1 methylation tests might have clinical implications in screening for epigenetic susceptibility to identify high risk groups of poor ACS prognosis; therefore, this status serves as a promising prognostic biomarker in these patients particularly with the diagnosis of depressive disorders. Future studies investigating the effects of drugs capable of regulating NR3C1 methylation on prognosis of ACS are anticipated, and the present study may serve as a foundation for future research.
References
Lichtman, J. H. et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 129, 1350–1369 (2014).
Granville Smith, I., Parker, G., Rourke, P., Cvejic, E. & Vollmer-Conna, U. Acute coronary syndrome and depression: A review of shared pathophysiological pathways. Aust. N. Z. J. Psychiatry 49, 994–1005 (2015).
Pariante, C. M. & Lightman, S. L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 31, 464–468 (2008).
Brown, E. S., Varghese, F. P. & McEwen, B. S. Association of depression with medical illness: does cortisol play a role? Biol. Psychiatry 55, 1–9 (2004).
Farrell, C. & O’Keane, V. Epigenetics and the glucocorticoid receptor: A review of the implications in depression. Psychiatry Res. 242, 349–356 (2016).
Wolffe, A. P. & Matzke, M. A. Epigenetics: regulation through repression. Science 286, 481–486 (1999).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 (2004).
Roy, B., Shelton, R. C. & Dwivedi, Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J. Psychiatr. Res. 89, 115–124 (2017).
Labonté, B., Azoulay, N., Yerko, V., Turecki, G. & Brunet, A. Epigeneticmodulation of glucocorticoid receptors in posttraumatic stress disorder. Transl. Psychiatry 4, e368 (2014).
McGowan, P. O. et al. Epigenetic regulation of the glucocorticoid receptor inhuman brain associates with childhood abuse. Nat. Neurosci. 12, 342–348 (2009).
Zhao, J., An, Q., Goldberg, J., Quyyumi, A. A. & Vaccarino, V. Promoter methylation of glucocorticoid receptor gene is associated with subclinical atherosclerosis: A monozygotic twin study. Atherosclerosis 242, 71–76 (2015).
Li-Tempel, T. et al. The cardiovascular and hypothalamus-pituitary-adrenal axis response to stress is controlled by glucocorticoid receptor sequence variants and promoter methylation. Clin. Epigenetics 8, 12 (2016).
Kang, H. J. et al. Methylation of the glucocorticoid receptor gene associated with depression in patients with acute coronary syndrome. Psychoneuroendocrinology 101, 42–49 (2019).
Kim, J.-M. et al. Effect of Escitalopram vs Placebo Treatment for Depression on Long-term Cardiac Outcomes in Patients with Acute Coronary Syndrome: the DEPACS Randomized Clinical Trial. JAMA. 320, 350–357 (2018).
Kim, J.-M. et al. Serotonin transporter gene association between anxiety and long-term cardiac outcomes in acute coronary syndrome. J. Am. Coll. Cardiol. 71, 2706–2707 (2018).
Lee, K. H. et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J. Am. Coll. Cardiol. 58, 1664–1671 (2011).
Anderson, J. L. et al. 2012 ACCF/AHA Focused Update Incorporated Into the ACCF/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 61, e179–347 (2013).
Sheehan, D. V. et al. The mini-international neuropsychiatric interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(suppl), 22–33 (1998).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press Inc.: Washington, DC, 1994.
McCormick, J. A. et al. 5′-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Mol. Endocrinol. 14, 506–517 (2000).
Perroud, N. et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl. Psychiatry 1, e59 (2011).
Perroud, N. et al. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J. Biol. Psychiatry 15, 334–345 (2014).
Martin-Blanco, A. et al. Association between methylation of the glucocorticoid receptor gene, childhood maltreatment, and clinical severity in borderline personality disorder. J. Psychiatr. Res. 57, 34–40 (2014).
Bustamante, A. C. et al. Glucocorticoid receptor DNA methylation, childhood maltreatment and major depression. J. Affect. Disord. 206, 181–188 (2016).
Jaffe, A. S. et al. Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Trial Investigators. Prediction of medical morbidity and mortality after acute myocardial infarction in patients at increased psychosocial risk in the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) study. Am. Heart J. 152, 126–135 (2006).
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571 (1961).
Breitling, L. P., Salzmann, K., Rothenbacher, D., Burwinkel, B. & Brenner, H. Smoking, F2RL3 methylation, and prognosis in stable coronary heart disease. Eur. Heart J. 33, 2841–2848 (2012).
Panteghini, M. Role and importance of biochemical markers in clinical cardiology. Eur. Heart J. 25, 1187–1196 (2004).
Walker, B. R. Glucocorticoids and cardiovascular disease. Eur. J. Endocrinol. 157, 545–59 (2017).
Rosmond, R. & Bjorntorp, P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J. Intern. Med. 247, 188–197 (2000).
Maes, M., Ruckoanich, P., Chang, Y. S., Mahanonda, N. & Berk, M. Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO & NS) pathways explain the co-association of depression and cardiovasucular disorder (CVD), and increased risk for CVD and due to mortality in depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 769–783 (2011).
Hu, M. X., Lamers, F., de Geus, E. J. & Penninx, B. W. Differential Autonomic Nervous System Reactivity in Depression and Anxiety During Stress Depending on Type of Stressor. Psychosom. Med. 78, 562–572 (2016).
Whooley, M. A. et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 300, 2379–2388 (2008).
Daskalakis, N. P. & Yehuda, R. Site-specific methylation changes in the glucocorticoid receptor exon 1F promoter in relation to life adversity: systematic review of contributing factors. Front. Neurosci. 8, 369 (2014).
Acknowledgements
The study was funded by a grant of National Research Foundation of Korea Grant (NRF-2015M3C7A1028899 [to JMK]) and was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2016R1C1B2006793 [to HJK]). RS is part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Author information
Authors and Affiliations
Contributions
The study concept, design and interpretation of data were constructed by J.-M.K., M.H.J., and J.-S.Y. Statistical analysis was performed by H.-J.K. and J.-M.K. Supervision was conducted by J.-M.K. and R.S. The data acquisition and analysis was conducted by H.-J.K., J.-W.K., S.-W.K., I.-S.S., M.-C.K., Y.J.H., Y.A., and M.-G.S. Drafting of the manuscript was made by Prof. J.-M.K. and H.-J.K. Critical revision of the manuscript for important intellectual content was conducted by R.S., J.-W.K., S.-W.K., I.-S.S., M.-G.S., M.-C.K., Y.J.H., Y.A.M.H.J. and J.-S.Y. All authors approved the final version of manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, HJ., Stewart, R., Kim, JW. et al. Synergistic effects of depression and NR3C1 methylation on prognosis of acute coronary syndrome. Sci Rep 10, 5519 (2020). https://doi.org/10.1038/s41598-020-62449-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62449-2
This article is cited by
-
Transcription Factor Motifs Associated with Anterior Insula Gene Expression Underlying Mood Disorder Phenotypes
Molecular Neurobiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.