Abstract
Methyl bromide (MB), a dominant ozone-depleting substance, is scheduled to be completely phased out for soil fumigation by December 30th 2018, in China. The combined effects of dimethyl disulfide (DMDS) plus metham sodium (MNa) were assessed in controlling soilborne pests for soil fumigation. A study was designed in laboratory for the evaluation of the efficacy of DMDS + MNa to control major soilborne pests. At the same time, two trials were conducted in cucumber field located in Tongzhou (in 2012) and Shunyi (in 2013), respectively, in order to assess the potential of DMDS + MNa in controlling soilborne pests. Laboratory studies disclosed positive synergistic effects of almost all four used combinations on Meloidogyne spp., Fusarium spp., Phytophthora spp., Abutilon theophrasti and Digitaria sanguinalis. Field trials found that DMDS + MNa (30 + 21 g a. i. m−2), both at a 50% reduced dose, effectively suppressed Meloidogyne spp. with a low root galling index (2.1% and 11.7%), significantly reduced the levels of Phytophthora and Fusarium spp. with a low root disease index (7.5% and 15.8%), gave very high cucumber yields (6.75 kg m−2 and 10.03 kg m−2), and increased income for cucumber growers with the highest economic benefits (20.91 ¥ m−2 and 23.58 ¥ m−2). The combination treatment provided similar results as MB standard dose treatment (40 g a. i. m−2) or DMDS standard dose treatment (60 g a. i. m−2) in pest control and yield, but was more effective than MNa standard dose treatment (42 g a. i. m−2). Usage of all chemical treatments gave better significant results than the untreated group of control. Considering the economic benefits, the DMDS plus MNa combination (30 + 21 g a. i. m−2) could be used for soil fumigation in cucumber production in China.
Similar content being viewed by others
Introduction
In northern sites of China, cucumber (Cucumis sativus L.) is a leading and important vegetable. Nematodes, soilborne fungi, oomycetes and weeds have a very great potential to lower yield in the protected cucumber production1,2. Methyl bromide (MB), an important soil fumigant, has widely been used to control soilborne pathogens, nematodes and weeds in areas of northern China. Though, MB must be completely phased out for soil fumigation by 30 December 2018 in China, because of its deleterious effects on stratospheric ozone3. The phasing out of the MB use as a pre-plant soil fumigant has greatly encouraged a deal of research aimed at finding economically acceptable, efficient, and simple alternatives4.
Currently, abamectin, fosthiazate, sulfuryl fluoride2, metham sodium (MNa) and calcium cyanamide are registered as MB chemical alternatives for cucumber in China. Chloropicrin (Pic)5, Dimethyl disulfide (DMDS)6, dazomet (DZ)7, and 1,3-dichloropropene (1,3-D)1,8,9 have been practiced as potential MB alternatives in cucumber. Though, there is no single chemical could replace MB totally10. To enhance the control activities for the pests of soilborne and broaden the action spectrum, the mixtures of promising alternative fumigants alone are becoming the scientific research hotspot in recent years. Several soil fumigant combinations, for example, 1,3-D/DZ7, 1,3-D/Pic5,11, DMDS/DZ12, 1,3-D/DMDS13 and 1,3-D/MNa14 have been tested to control soilborne pests for cucumber production.
Much information could also be retrieved in the literature on combinations of Pic and 1,3-D11,15,16,17,18,19,20,21, Pic and MNa21, 1,3-D and DZ7,22, Pic and DMDS20, 1,3-D and methyl isothiocyanate (MITC)23, Pic and methyl iodide (MeI)24,25 and so on as MB chemical alternatives for other crops production. Combinations of DMDS plus DZ have been reported as an efficient alternative to MB in Europe and China12,22,26. Both DZ and MNa are MITC generators. The combined use of DMDS and MNa to replace MB, therefore, was at least theoretically promising. Recently, the combined effects of DMDS and MNa were reported in muskmelon27 and cut flower28. Little information, however, was reported on combination of DMDS plus MNa in cucumber.
Current study was initiated to assess and confirm the efficacy of the combination of DMDS plus MNa on soilborne fungi and oomycetes (Fusarium and Phytophthora spp.), nematodes (Meloidogyne spp.), and weed seeds (Digitaria sanguinalis and Abutilon theophrasti) under laboratory experiment. Along with, 2 field trials were designed as well, for the determination of the MNa combinations and dimethyl disulfide as an alternative MB for cucumber yield in China at the same time.
Results
Laboratory studies
Root-knot nematodes
All four applied rates of DMDS + MNa combination tested treatments showed a positive synergistic effect on root-knot nematodes (Table 1), by decreasing the Meloidogyne spp. numbers by 90.7%. The maximum practiced dose in our study (80 + 40 mg a.i. kg−1) decreased the number of Meloidogyne spp. in terms of juveniles by 94.2%.
Soilborne fungi and oomycetes
Positive synergistic efficacy of four rates of DMDS + MNa tested was also observed on both Phytophthora and Fusarium spp. (Table 1). The infestation levels through Fusarium and Phytophthora spp. were decreased by 78.5% and 62.0%, respectively. The maximum applied dose in current study (80 + 40 mg a.i. kg−1) decreased Fusarium spp. 93.6% and Phytophthora spp. 74.1%.
Weed control
The four combinations of DMDS + MNa completely controlled the seeds of Abutilon theophrasti. However, the combination with the lower rate of MNa only showed synergistic efficacy (i.e. DMDS + MNa at 40 + 20 and 80 + 20 mg kg−1); while the combination with the maximum MNa rate did not exhibit synergistic efficacy (i.e. DMDS + MNa at 40 + 40 and 80 + 40 mg kg−1) (Table 1). The four practiced rates of DMDS + MNa were all shown synergistic effects on Digitaria sanguinalis. The combination with the higher rate of DMDS delivered 100% Digitaria sanguinalis control (i.e. DMDS + MNa at 80 + 20 and 80 + 40 mg kg−1); the combination with the lower DMDS rate (i.e. DMDS + MNa at 40 + 20 and 40 + 40 mg kg−1) provided 70.8% and 95.9% control of Digitaria sanguinalis, respectively (Table 1).
Field trials
Root-knot nematodes
The control groups at trial one (in 2012) and trial two (in 2013) were found seriously occupied by Meloidogyne spp. (Table 2). The significantly higher levels of Meloidogyne spp. were observed in the control group than to all treatments of fumigant (P = 0.05).
The chemical treatments, except for MNa treatment, decreased the nematode infestation levels in trial one and trial two by 86.1% and 88.5%, respectively. The combination treatment of DMDS + MNa suppressed nematode levels by 86.1% and 89.7% in trial one and trial two, respectively; and both results statistically were not found different from MB or DMDS treatment, but higher significantly than MNa treatment at trial one (P = 0.05).
Soilborne fungi and oomycetes
The groups of control were seriously infested by both Phytophthora and Fusarium spp. in both trials (Table 2). The levels of infestation with Fusarium and Phytophthora spp. were observed maximally significant in the control untreated group than to all fumigant treatments (P = 0.05) (Table 2).
The lowest Fusarium spp. levels were both provided by MB treatment at both trials, followed by the combination DMDS + MNa treatment, which decreased 93.6% Fusarium spp. and 80.0% at trial one and two. (Table 2).
Similarly, MB treatment also had the lowest Phytophthora spp. level, followed by the DMDS + MNa treatment, and reduced 86.2% and 81.1% Phytophthora spp. in trial one and two, respectively (P = 0.05) (Table 2). While no statistically change was observed between the MNa + DMDS treatments and MB treatment (P = 0.05) (Table 2).
The first cucumber fruit yield
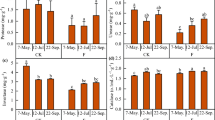
The 1st production of cucumber fruit was varied with fumigation treatments (Table S1). Cucumbers that were grown in the untreated control plots provided the minimum yield of first fruiting (0.02 and 0.12 kg m−2, respectively) at both field trials (Table S1). No significant change was shown between the chemical treatments at both trials. And the first fruit yield in all chemical treatments were significantly higher than the untreated group of control except for MNa treatment at trial one, 2012 (P = 0.05) (Table S1).
Cucumber plant height, root galling index and root disease index
The height of cucumber fruit plant at 4 WAT varied with fumigation treatments at both field trials (Table 3). Throughout the chemical treatments at both trials, significantly higher plant height was observed compared to the untreated group of control. And no significant change was shown between the chemical treatments apart from the DMDS treatment at trial one, 2012 (P = 0.05) (Table 3).
By the end of all trials, the nematode infestation was calculated through index of root galling. Root galling index was significantly affected after fumigant treatments (P = 0.05). The maximum root galling indexes were both noticed in the untreated plots (61.4% and 78.3% at trials one and two, respectively). At trial one, the lowest index of root galling was provided by MB treatment, which was not different statistically from DMDS + MNa or DMDS alone, but observed lower significantly than MNa alone. At trial two, the minimum root galling index was also gained in MB treated plots, which did not change significantly from DMDS + MNa treatment but was lower significantly than DMDS alone or MNa alone (P = 0.05) (Table 3).
While comparing with the untreated control groups, chemical treatments affected root disease index significantly (P = 0.05). The maximum index for root disease (50.0% and 67.5%) were provided by the untreated control plots at two trials. At trial one, the lowest root disease index (3.3%) was provided by MB treatment, which was not different statistically from DMDS + MNa (7.5%), but was lower significant than DMDS or MNa alone. At trial two, the lowest root disease index (4.2%) was observed in treated MB plots, which was not significantly different from DMDS + MNa treatment or DMDS alone, but was observed lower than MNa alone, significantly (P = 0.05) (Table 3).
Cucumber yield, income and economic benefits
The yield of cucumber fruit was differed with chemical treatment (Table 4). Compared with the chemical treatments, yield in control plots was the lowest (3.03 and 5.66 kg m−2, respectively, at trials one and two). At trial one, treated plots with DMDS + MNa had the maximum production (6.75 kg m−2, 122.8% increase), which was not significantly different from the plots treated with MB (6.66 kg m−2, 119.8% increase) or DMDS alone (5.87 kg m−2, 93.7% increase), but was significantly higher than MNa alone (4.85 kg m−2, 60.1% increase). At trial two, treated MB plots produced the maximum yield (10.12 kg m−2, 78.8% increase), that was not found significantly different from the DMDS + MNa treatment (10.03 kg m−2, 77.2% increase) or DMDS alone (9.92 kg m−2, 75.3% increase), but significantly higher than MNa alone (8.79 kg m−2, 55.3% increase) (P = 0.05) (Table 4).
Similarly, the gross income of grower through the production of cucumber also varied with chemical treatments (Table 4). Minimum gross income from cucumber cultivation without treatment was obtained (9.67 and 12.50 ¥ m−2, respectively). The plots treated with MB provided the highest gross incomes (22.47 and 26.24 ¥ m−2, with 132.0% and 109.9% increase, respectively, at trials one and two), which was not different significantly from the DMDS + MNa treatment (22.33 and 25.00 ¥ m−2, with 129.9% and 100.0% increase, respectively, at trials one and two) or DMDS alone (19.14 and 23.31 ¥ m−2, with 96.9% and 86.5% increase, respectively, at trials one and two), but significantly higher than MNa alone (16.93 and 20.05 ¥ m−2, with 74.2% and 60.4% increase, respectively, at trials one and two) (P = 0.05) (Table 4).
In addition, the economic benefits (also called net income) of different soil fumigation treatments were assessed. Fumigation cost of different soil treatments were calculated by the sum of fumigants cost and film cost (Table S2). The DMDS + MNa treatment had a relatively low fumigation cost (1.42 ¥ m−2), which was lower than MB treatment (4.07 ¥ m−2) or MNa alone (1.60 ¥ m−2) (Table 4). Net income of growers was finally calculated by the difference of the gross income and fumigation cost (Table 4). In trial one and two, the treated plots with combination DMDS + MNa both had the highest net income (116.2% and 88.6% increase, respectively), followed by MB treated plots (90.3% and 77.4% increase, respectively), DMDS alone treated plots (85.0% and 76.5% increase, respectively), MNa alone treated plots (58.5% and 47.6% increase, respectively) and the untreated plots (P = 0.05) (Table 4).
Discussion
To control root-knot nematode, DMDS is proven equally effective to MB not only in China but elsewhere16,17,29, which was confirmed in our both field trials. Anyhow, the overall formulations of DMDS exhibit poor efficacy against soilborne fungi and oomycetes, and moderate activity against weeds. So, additional herbicide and fungicide would be needed to improve soilborne fungi, oomycetes and weed control. MNa, a MITC producer, can control soilborne fungi, oomycetes and weeds efficiently. In the current study, it was observed that the MNa was comparatively less effective ton soilborne fungi and oomycetes than weeds. Thus, the combined use of DMDS and MNa as a promising soil fumigant is at least possible theoretically. All of the combinations of DMDS and MNa in laboratory studies exhibited positive synergy effects on root-knot nematodes, 2 major weed seeds and key soilborne fungi and oomycetes.
The mechanism of specific synergistic activity of DMDS and MNa was still not clearly understood but the laboratory outcomes presented above, preliminarily indicated the possibility of their synergistic usage and affirmed the synergy activity of DMDS and MNa as previously reported by Gerik and Hanson28. DMDS is found to inhibit complex IV of mitochondrial electron transport chain (ETC), decrease the intracellular ATP concentration subsequently, which thereby activated neuronal KATP channels mediating membrane hyperpolarization and reduction of neuronal activity30. MITC as the active biocidal product of MNa is believed to react with amines and thiols in biological molecules31. It will be very difficult to assess a single mechanism of action that accounts for the synergism observed for the combination of DMDS plus MNa. However, two possible mechanisms may offer insight into the synergistic response of the fumigant combination32. One mechanism suggested is that sub-lethal exposure of soilborne pests to one fumigant may alter cell processes to such an extent that exposure to a second fumigant is more deleterious than would normally be expected33. Another suggested mechanism is that one fumigant causes changes in cell-wall permeability that increases the uptake or decreases the efflux of a second fumigant from the cell34. Further understanding of the activity of DMDS and MNa is necessary before the specific synergism mechanism can be deduced.
Our field trials showed that the combined use of DMDS and MNa at 30 + 21 g a.i. m−2 rate successfully inhibited Meloidogyne spp. root galling, decreased the colony-forming units (cfu) of Fusarium spp. sharply and Phytophthora spp. on media, sustained maximum production of cucumbers at the same time, and was not different significantly from 40 g m−2 MB dose or DMDS alone at 60 g m−2 dose, but improved than MNa alone at 42 g m−2 dose (P = 0.05). The results of field trials above confirmed that the combinations of DMDS and MNa are good choices than alone MB application. Furthermore, we compared the combination of DMDS plus MNa with the used soil fumigant combinations, such as 1,3-D/DZ, 1,3-D/Pic, DMDS/DZ, 1,3-D/DMDS and 1,3-D/MNa (Table S3). Indeed, the combination of DMDS plus MNa is not the best alternative of MB, if only considering the control efficacy. However, considering the application methods and forbidden use issue at the same time, the combination of DMDS plus MNa is more promising than other combinations to replace MB.
Commercial preparations of MNa typically consist of 42% MNa in an aqueous solution at a self-buffered pH > 7. True MNa and halogenated chemicals mixtures (e.g. 1,3-D and Pic) are not available currently due to chemical incompatibility arising when combining them35,36. However, the different combinations of DMDS and MNa did not show any degradation according to the synergy effect in our laboratory in the current tests, which may be attributed to the relatively high rates of application4.
MNa requires relatively rigorous prerequisites just like DZ for the effective application. The aqueous solution of MNa is diluted by water and applied to the moist soil, where the compound rapidly decomposes to produce MITC. In the vapor and liquid phase of the soil, MITC is toxic to various soilborne pests. However, the physical and chemical properties of MITC (low water solubility, low vapor pressure, and low Henry’s constant) limit its movement and distribution in the soil following MNa application37,38. DMDS is highly volatile and a small molecule, so it diffuses and moves in the soil more quickly than MITC30. In this point, DMDS could make up the disadvantage of MITC and improve the control efficacy of the combination. Anyhow, uniform distribution in soil is needed by effectual mechanical injection or chemigation system. For better distribution of fumigant in soil, MNa is suitable to be applied by broadcast application or shank injection rather than chisel injection. If there was a good chemigation system available in the field, applying the fumigants by chemigation couldn’t be better.
In concluding remarks, the combination of DMDS and MNa fumigant applied by chemigation was almost equally to MB in terms of controlling knot-nematodes along with soilborne fungus while sustaining high cucumber yields and income for growers. However, much of the detailed work on its application type including its formulations and suitable composites with biological agents (eg. Trichoderma asperellum, Bacillus subtilis or others) are desirable before the DMDS and MNa combination can be suggested as an efficient substitute to MB for the yield of cucumber in China. The side effects of the combinations on soil beneficial organisms (eg. earthworms, actinomycetes, etc.) should also be considered in further studies.
Materials and Methods
Laboratory studies
The combined and single efficacy of DMDS and MNa, was assessed under laboratory study. The collection of soil samples was taken from the 20 cm top in a cucumber field at Shunyi, Beijing, where nematodes and soilborne pests’ presence was abundant. The composition of soil sample was 20.34% sand, 76.25% silt, and 3.41% clay, with pH 6.40 and organic matter content 35.64 g kg−1 soil. A soil sieve of 2-mm mesh was used and then mixing was done thoroughly. The moisture of soil was recorded 23.80% (w/w). Analysis for particle size were estimated through pipette method39. pH was determined in a 1:2.5 soil to water extract. Organic carbon contents were determined by wet oxidation followed the method of Walkley and Black40. Soil moisture contents were performed by remaining the soil at 105 ± 5 °C for 6 hours until constancy of mass was reached41.
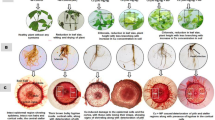
Soil samples with six hundred grams weight were placed into each of 27 (2.5 L) desiccators. In each desiccator, 10 Abutilon theophrasti seeds and fifteen Digitaria sanguinalis seeds were buried at 2 cm depth, respectively. The following fumigant treatments were performed with three replicates: DMDS alone (40, 80 mg a.i. kg−1 soil), MNa alone (20, 40 mg a.i. kg−1 soil), DMDS + MNa (40 + 20, 40 + 40, 80 + 20, 80 + 40 mg a.i. kg−1 soil) and untreated group of control. DMDS or MNa was (Eppendorf, Germany) injected into the soil by pipette, and then the desiccator was sealed immediately. The laboratory study photo (see Fig. 1a.) was taken by the authors. The desiccators were placed at 28°C for five days, and then covers were remained open for one day to release the fumigant residues. The height of weed was then determined. Fusarium and Phytophthora spp. were both separated from the fumigated soil quantitatively based on the methods of Komada42 and Masago et al.43, respectively. Meloidogyne spp. were isolated from one hundred subsample based on Liu methods44.
Field trials
In years 2012 and 2013, two trials demonstration were performed in cucumber growing field at Tongzhou, Beijing and Shunyi, Beijing, respectively, where were both important production areas for cucumber. The farms, which have grown this vegetable for more than five years, are facing serious problems of soilborne pests including soilborne fungi, oomycetes, root-knot nematode, and other pests. Necessary details are given in Tables S4.
99 DMDS TC (Hohhot Guangxin Chemical Trading Co., Ltd., China, containing DMDS of 99%), 42% MNa AS (Shenyang Harvest Agrochemical Co., Ltd., China, a commercial product of 42% MNa) and 98 MB TC (Changyi Chemical Plant, China, containing 98% MB) were selected in the current study. For soil mulch polyethylene film (PE) (0.04 mm thick, from Shouguang Longxing Plastic Co., Ltd., Shandong Province, China) was used.
Randomized blocks design of chemical treatments was performed with three replicates (Table 5). In field trial one, the size of each plot was 17.92 m2 (3.2 m wide by 5.6 m long). A treatment as a reference of MB, DMDS alone, MNa alone, DMDS combination with MNa and untreated group of control were established. The application of DMDS was done through chemigation at 60 g a.i. m−2 rate; in order to increase the solubility of DMDS in the water, approximate 4% addition of Tween 80 is necessary13. MNa was practiced by chemigation at 42 g a.i. m−2 rate. Combined treatment of DMDS and MNa was practiced as follows: apply MNa by chemigation at 21 g a.i. m−2 rate, and then apply DMDS by chemigation 30 g a.i. m−2 rate. To cover the above treatments, PE film was placed. Application of MB between PE sheet and soil was through the hot gas method at 40 g m−2 rate. The fumigants were applied on 27 July 2012, and the tarps were removed on 17 August 2012. The cucumbers (cultivar “No.16, Zhongnong”) were transplanted on 28 August 2012, and the final root disease investigation was conducted on 3 December 2012. In field trial two, the size of each plot was 20.16 m2 (3.6 m wide by 5.6 m long). All of the treatments in field trial two were the same as those in field trial one (Table 5). The fumigation period was from 10 September 2013 to 26 September 2013. The field trial photo (see Fig. 1b.) was also taken by the authors. The cucumbers (cultivar “No.12, Jinyou”) were transplanted on 5 November 2013, and the final root disease evaluation was conducted on 28 May 2014.
Populations of soil fungi [colony-forming units (cfu) g−1 soil] were evaluated after fumigation treatment from soil samples at 0–20 cm depth from the surface of soil. Soil samples were collected from 3 spots in each plot along the diagonal lines. Root-knot nematode densities in the soil were estimated after soil fumigation from 0–20 cm depth. Soil samples from every plot was collected from 3 spots as above. Populations of root-knot nematode and soil fungi were respectively estimated by the same methods as in the laboratory studies.
Height of cucumber plant was determined at 4 weeks after transplant (WAT) (per plot 20 plants). Calculation of root galls and the severity of cucumber root disease were done at the end of the trials (per plot 20 plants). Plants were rated on a scale of 0–4: 0 = 0%, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100% roots galled4. Cucumber root disease severity was also rated on a scale of 0−4, 0 = healthy plant and root, without disease; 1 = black brown rotten roots comprise < 25% of the entire root system; 2 = 26−50%; 3 = 51−75%; and 4 = 76−100% black brown rotten roots45. Cucumber fruit yields and income of growers were collected after every harvest and summed together at the end of the trials.
Statistical analyses
Laboratory studies
Mortality of nematode was calculated by the equation following.
where X is % nematode mortality, N1 is the number of dead nematodes, and N2 is the number of the live nematodes.
Corrected mortality of nematode was considered by the equation following.
where Y is the % corrected nematode mortality, while X1 is the % nematode mortality of fumigated treatments, and X2 is the % nematode mortality of untreated control.
Controlling fungi or weed seeds efficacy was measured according to the equation following.
where Y is the fungi or weed seeds control efficacy, X1 is the fungal populations or weed height in untreated control group, X2 is the fungal populations or weed height in treated fumigated plots.
The DMDS plus MNa control efficacy was calculated by the equation following, using methods described by Limpel et al.46.
where E0 is the expected control efficacy of DMDS plus MNa, X1 is the actual measured control efficacy of DMDS, X2 is the actual measured control efficacy of MNa. E is the actual measured control efficacy of DMDS plus MNa. If E > E0, the combination of DMDS plus MNa was synergistic; if E < E0, the combination of DMDS plus MNa was antagonistic.
Field trials
The effectiveness of controlling root-knot nematode or soilborne fungi or oomycetes was measured according to the Eq. (3).
Root galls scores or disease scores recorded for each plot were respectively converted into root galling index (% RGI) or root disease indices (% RDI) using the formula described by McKinney45:
where ƒ = number of plants in each class, v = class value, N = number of observed plants, and X = highest value of the evaluation scale.
Statistical software SAS (SAS, version 8.0 for Windows) was run to conduct analysis of variance (ANOVA) for data analysis. Data for populations of soilborne fungus and knot-nematode were transformed as necessary (log10 for large numbers [>100] and square root transformations for small numbers [<100] for statistical analyses), however, all of the data reported here as non-transformed values. Fisher’s LSD test at P = 0.05 was used for significant differences determination among means.
References
Giannakou, I. & Anastasiadis, I. Evaluation of chemical strategies as alternatives to methyl bromide for the control of root-knot nematodes in greenhouse cultivated crops. Crop protection 24, 499–506 (2005).
Cao, A. et al. Evaluation of sulfuryl fluoride as a soil fumigant in China. Pest management science 70, 219–227 (2014).
Mao, L., Jiang, H., Wang, Q., Yan, D. & Cao, A. Efficacy of soil fumigation with dazomet for controlling ginger bacterial wilt (Ralstonia solanacearum) in China. Crop protection 100, 111–116 (2017).
Desaeger, J. A., Seebold, K. W. & Csinos, A. S. Effect of application timing and method on efficacy and phytotoxicity of 1,3-D, chloropicrin and metam-sodium combinations in squash plasticulture. Pest management science 64, 230–238 (2008).
Gilreatha, J. P., Nolingb, J. W. & Santosa, B. M. Methyl bromide alternatives for bell pepper (Capsicum annuum) and cucumber (Cucumis sativus) rotations. Crop protection 23, 347–35 (2004).
Gilardi, G., Gullino, M. L. & Garibaldi, A. Soil disinfestation with dimethyl disulfide for management of Fusarium wilt on lettuce in Italy. Journal of plant diseases and protection 124, 361–370 (2017).
Mao, L. et al. Evaluation of the combination of 1,3-dichloropropene and dazomet as an efficient alternative to methyl bromide for cucumber production in China. Pest management science 68, 602–609 (2012).
Qiao, K., Shi, X., Wang, H., Ji, X. & Wang, K. Managing root-knot nematodes and weeds with 1,3-dichloropropene as an alternative to methyl bromide in cucumber crops in china. Journal of agricultural and food chemistry 59, 2362–2367 (2011).
Qiao, K. et al. Effectiveness of 1,3-dichloropropene as an alternative to methyl bromide in rotations of tomato (Solanum lycopersicum) and cucumber (Cucumis sativus) in China. Crop protection 38, 30–34 (2012).
Yates, S. R., Gan, J., Papiernik, S. K., Dungan, R. & Wang, D. Reducing fumigant emissions after soil application. Phytopathology 92, 1344–1348 (2002).
Yan, D. D. et al. Gelatin encapsulation of chloropicrin and 1,3-dichloropropene as fumigants for soilborne diseases in the greenhouse cultivation of cucumber and tomato. Journal of integrative agriculture 16, 1758–1766 (2017).
Mao, L. et al. Evaluation of the combination of dimethyl disulfide and dazomet as an efficient methyl bromide alternative for cucumber production in China. Journal of agricultural and food chemistry 62, 4864–4869 (2014).
Mao, L. G. et al. Application of the combination of 1, 3-dichloropropene and dimethyl disulfide by soil injection or chemigation: effects against soilborne pests in cucumber in China. Journal of integrative agriculture 15, 145–152 (2016).
Mao, L. et al. Replacing methyl bromide with a combination of 1, 3-dichloropropene and metam sodium for cucumber production in China. PloS one 12, e0188137 (2017).
Wang, Q. X. et al. Efficacy of 1,3-dichloropropene plus chloropicrin gelatin capsule formulation for the control of soilborne pests. Crop protection 48, 24–28 (2013).
Santos, B. M. et al. Methyl bromide alternatives for high tunnel strawberry production in southern Spain. HortTechnology 19, 187–192 (2009).
De Cal, A., Martinez-Treceño, A., Lopez-Aranda, J. M. & Melgarejo, P. Chemical alternatives to methyl bromide in Spanish strawberry nurseries. Plant disease 88, 210–214 (2004).
Gilreath, J., Santos, B., Busacca, J., Egerjr, J. & Gilreath, P. Validating broadcast application of Telone C-35 complemented with chloropicrin and herbicides in commercial tomato farms. Crop protection 25, 79–82 (2006).
Minuto, A. et al. Application of an emulsifiable mixture of 1,3-dichloropropene and chloropicrin against root knot nematodes and soilborne fungi for greenhouse tomatoes in Italy. Crop protection 25, 1244–1252 (2006).
UNEP, Report of the methyl bromide technical options committee, in 2010 assessment, United Nations Environment Programme, Nairobi, Kenya, pp 101–158 (2010).
Santos, B. M. et al. Comparing methyl bromide alternatives for soilborne disease, nematode and weed management in fresh market tomato. Crop protection 25, 690–695 (2006).
Van Wambeke, E. Combinations of reduced rates of 1,3-dichloropropene and dazomet as a broad spectrum soil fumigation strategy in view of methyl bromide replacement. Communications in agricultural and applied biological sciences 72, 61–70 (2007).
Thomson W. T., Agricultural chemicals. Thomson Publications, Fresno, Ca., U.S.A., p 206 (1992).
Fennimore, S. et al. Methyl bromide alternatives evaluated for California strawberry nurseries. California agriculture 62, 62–67 (2008).
Li, Y. et al. Control of soilborne pathogens of Zingiber officinale by methyl iodide and chloropicrin in China. Plant disease 98, 384–388 (2014).
Van Wambeke, E., Ceustermans, A., De Landtsheer, A. & Coosemans, J. Combinations of soil fumigants for methyl-bromide replacement. Communications in agricultural and applied biological sciences 74, 75–84 (2009).
Stevens, M. & Freeman, J. Efficacy of dimethyl disulfide and metam sodium combinations for the control of nutsedge species. Crop protection 110, 131–134 (2018).
Gerik, J. S. & Hanson, B. D. Drip application of methyl bromide alternative chemicals for control of soilborne pathogens and weeds. Pest management science 67, 1129–1133 (2011).
Gómez-Tenorio, M. A., Tello, J. C., Zanón, M. J. & de Cara, M. Soil disinfestation with dimethyl disulfide (DMDS) to control Meloidogyne and Fusarium oxysporum f. sp. radicis-lycopersici in a tomato greenhouse. Crop protection 112, 133–140 (2018).
Dugravot, S. et al. Dimethyl disulfide exerts insecticidal neurotoxicity through mitochondrial dysfunction and activation of insect KATP channels. Journal of neurophysiology 90, 259–270 (2003).
Pruett, S. B., Myers, L. P. & Keil, D. E. Toxicology of metam sodium. Journal of toxicology and environmental health part B: critical reviews 4, 207–222 (2001).
Hutchinson, C. M., McGiffen, M. E. Jr, Ohr, H. D., Sims, J. J. & Becker, J. O. Efficacy of methyl iodide and synergy with chloropicrin for control of fungi. Pest Management Science: formerly Pesticide Science 56, 413–418 (2000).
Samoucha, Y. & Gisi, U. Possible explanations of synergism in fungicide mixtures against Phytophthora infestans. Annals of applied biology 110, 303–311 (1987).
Cohen, Y. & Levy, Y. Joint action of fungicides in mixtures: theory and practice. Phytoparasitica 18, 159–169 (1990).
Guo, M., Yates, S. R., Papiernik, S. K. & Zheng, W. Incompatibility of metam sodium with halogenated fumigants. Pest management science 61, 467–476 (2005).
Zheng, W., Yates, S. R., Guo, M., Papiernik, S. K. & Kim, J. H. Transformation of chloropicrin and 1,3-dichloropropene by metam sodium in a combined application of fumigants. Journal of agricultural and food chemistry 52, 3002–3009 (2004).
Ajwa, H. A. et al. Application of alternative fumigants through drip irrigation systems. Phytopathology 92, 1349–1355 (2002).
Triky-Dotan, S. et al. Generation and dissipation of methyl isothiocyanate in soils following metam sodium fumigation: impact on Verticillium control and potato yield. Plant disease 91, 497–503 (2007).
Schinner, F., Öhlinger, R., Kandeler, E. & Margesin, R. Methods in soil biology. Springer: Verlag Berlin Heidelberg, pp 386-389 (1995).
Nelson, D. W. & Sommers, L. E. Total carbon, organic carbon and organic matter, in Methods of soil analysis; Page. A. L., Miller, R. H. & Keency, O. R. Eds. American Society of Agronomy Publishers: Madison, Wisconsin, pp 539–576 (1985).
Margesin, R. & Schinner, F. Manual for soil analysis - Monitoring and assessing soil bioremediation. Springer: Verlag Berlin Heidelberg, pp 47–49 (2005).
Komada, H. Development of a selective medium for quantitative isolation of Fusarium oxvsporum from natural soil. Review of plant protection research 8, 114–125 (1975).
Masago, H., Yoshikawa, M., Fukada, M. & Nakanishi, N. Selective inhibition of Pythium spp. on a medium for direct isolation of Phytophthora spp. from soils and plants. Phytopathology 67, 425–428 (1977).
Liu, W. Plant pathogenic nematodes. China Agriculture Press: Beijing, China, p 373 (2000).
McKinney, H. H. Influence of soil, temperature and moisture on infection of wheat seedling by Helminthosporium sativum. Journal of agricultural research 26, 195–217 (1923).
Limpel, L. E., Schuldt, P. H. & Lamont, D. Weed control by dimethyl tetrachloroterephthalate alone and in certain combinations. Proceedings of the northeast weed control conference 16, 48–53 (1962).
Acknowledgements
This research was supported by National Key R&D Program of China (2016YFD0200500) and the National Natural Science Foundation of China (31801769).
Author information
Authors and Affiliations
Contributions
L.M., H.J. and A.C. designed the study. L.M., L.Z. and H.Y. performed the experiments. L.M., L.Z. and M.U.S. analyzed data and wrote the manuscript. Y.Z. provided experimental materials. All authors have read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mao, L., Jiang, H., Zhang, L. et al. Assessment of the potential of a reduced dose of dimethyl disulfide plus metham sodium on soilborne pests and cucumber growth. Sci Rep 9, 19806 (2019). https://doi.org/10.1038/s41598-019-56450-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56450-7
This article is cited by
-
Potato Nitrogen Response and Soil Microbial Activity as Affected by Fumigation
American Journal of Potato Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.