Abstract
The evolutionary diversification of animal behavior is often associated with changes in the structure and function of nervous systems. Such evolutionary changes arise either through alterations of individual neural components (“mosaically”) or through scaling of the whole brain (“concertedly”). Here we show that the evolution of a courtship behavior in Malawi cichlid fish is associated with rapid, extensive, and specific diversification of orosensory, gustatory centers in the hindbrain. We find that hindbrain volume varies significantly between species that build pit (depression) compared to castle (mound) type bowers and that this trait is evolving rapidly among castle-building species. Molecular analyses of neural activity via immediate early gene expression indicate a functional role for hindbrain structures during bower building. Finally, comparisons of bower building species in neighboring Lake Tanganyika suggest parallel patterns of neural diversification to those in Lake Malawi. Our results suggest that mosaic brain evolution via alterations to individual brain structures is more extensive and predictable than previously appreciated.
Similar content being viewed by others
Introduction
Animal behaviors vary widely, as do their neural phenotypes1. Evolutionary neuroscience identifies how the brain diversifies over time and space in response to selective pressures2. A key goal of evolutionary neuroscience has been to identify whether brain structures evolve independently (“mosaically”) or in tandem with each other as they reflect key life history traits, especially behavior3,4,5,6. While a number of studies have linked variation in brain structure with other traits across evolutionary time2,7,8,9, it remains unclear whether or not this variation is predictable. Specifically, when similar behavioral traits evolve among two or more species, do their neural bases evolve correspondingly? If parallel brain evolution is predictable then it may be possible to understand general principles of neural organization and function across animals. This would expand our ability to manipulate brain function, but if this is not true, new strategies will be needed to reveal the mechanisms of brain evolution.
Fishes, as both the most speciose (50% of extant vertebrates) and most varied vertebrate radiation10 offer opportunities to answer these questions. Fish species live in diverse ecological, sensory, and social environments and have evolved elaborate variations in neural structure and function from a common basic ground plan11 making rapid and variable diversification of brain structures a broad and general feature of their evolution10.
The cichlid fishes of Lake Malawi, Africa offer a particularly striking model of these patterns of diversification. Although geologically young (less than 5 million years old), Lake Malawi contains at least 850 species of cichlids12 that, based on molecular phylogenetic analyses, can be sorted into six diverse clades13: sand-dwelling shallow benthic species (287 species), deep benthic species (150 species), rock-dwelling ‘mbuna’ (328 species), the deep-water pelagic genus Diplotaxodon (19 species), the pelagic genus Rhamphochromis (14 species), and the sand-breeding pelagic ‘utaka’ species (55 species)14. These clades exhibit substantial variation in habitat use, visual sensitivity, diet, behavior, and coloration as well as craniofacial morphology and tooth shape, presumably arising through repeated divergence in macrohabitat, trophic specializations, diet, and coloration15,16,17.
We studied the behavior, brain structures and bower construction of ~200 sand-dwelling shallow living benthic species with males that build species-specific mating nests, known as bowers, to court females in competition with conspecifics17,18,19. Two basic types of bowers are pits or depressions in the sand, and castles, where sand is heaped into a volcano structure showing species-specific differences in size and shape17,18,19. Pits and castles are constructed via differential scooping and spitting of sand. Pit and castle type bower building is innate and appears to be rapidly evolving and relatively free from phylogenetic constraint; multiple sand-dwelling genera contain both pit and castle-building species17,19. Furthermore, phylogenomic analyses suggest evidence of the repeated evolution of bower types in the Malawi cichlid phylogeny17,19.
How is construction of different bower types reflected in brain anatomy? Do differences in brain structure evolve in tandem with bower building and, if so, how are they organized and functionally related to this behavior? Here we address these questions using neuroanatomical, phylogenetic, functional, and behavioral analyses and compare patterns of diversification in Lake Malawi to other East African cichlid radiations.
Results
Bower type predicts hindbrain volume
To assess patterns of neural evolution among the cichlids of Lake Malawi we compiled measurements of six brain regions (telencephalon, hypothalamus, cerebellum, optic tectum, olfactory bulb, and hindbrain) from a phenotypic data set comprising brain measurements for 189 cichlid species from East Africa and Madagascar20. We identified 37 bower building species within the data set and classified them as either pit or castle building species (Table S1). For each brain region, we calculated a volume estimate using an ellipsoid model21 and controlled for size differences between species by normalizing the results using mean standard length for each species (Supplementary material). Pairwise comparisons of normalized volumetric measures for each region revealed a significant difference in hindbrain volume between pit digging and castle building species (Kruskal-Wallis test; H = 13.87, 1 d.f., p = 0.00019) but not for any other brain structure (Table 1). Plotting structural volume against standard length demonstrated strong evidence for allometric scaling of the pit and castle hindbrain (Fig. 1A). Further, an analysis of variance (ANOVA) comparing the slopes of standard length compared to those of hindbrain volume indicated that pit and castle species significantly differ in this relationship (F = 25.04, 1 d.f., p = 1.82 × 10−05) as also confirmed by post hoc Tukey test (p < 0.05). Analysis of gross brain morphology highlighted the extent to which this hindbrain diversification could occur, as evidenced by the extreme hindbrain differences between by the relatively size matched Tramitichromis brevis (castle) and Copadichromis virginalis (pit) (Fig. 1B; species highlighted in Fig. 1A).
Evolution and diversification of hindbrain volume among Malawi cichlids. (A) Scatterplot comparing standard length and hindbrain volume for castle building species (green) and pit digging species (yellow). Species subsequently presented in greater depth are highlighted by gray circles: Tramitichromis brevis (Displayed in (B) larger hindbrain castle building species, green), Copadichromis virginalis (Displayed in Figs. 1B, 2B; pit digging species, yellow), and Mchenga conophoros (smaller hindbrain castle building species, green; See Fig. 2D). (B) Representative photos of whole brain morphology and hindbrain size (location indicated by arrows) of T. brevis (castle) and C. virginalis (pit). (C) Maximum likelihood phylogeny for 19 Lake Malawi sand and rock species. Hindbrain volumes normalized by standard length are represented by dots (castle = green, pit = yellow, rock = purple; for species labels and node support see Fig. S2). (D–F) Normalized hindbrain volume diversification rates for rock and sand (D), sand alone (E), and rock alone (F). (G) Boxplot comparing per-species normalized hindbrain volume diversification rates between sand and rock clades.
Hindbrain volume varies independent of phylogeny
If hindbrain volume has evolved mosaically among closely related species, as suggested by our allometric analyses, then pairwise tests of phenotypic variation controlling for phylogeny should reveal a lack of similarity among closely related species in the size of this brain region. To test this, we employed a genome-wide maximum likelihood phylogeny composed of 13 bower-building species and 6 non-bower building rock-dwellers19. Phylogenetic ANOVA revealed significant variation between pit and castle species independent of relatedness (F = 13.18, 1 d.f., p < 0.05). Assessing the distribution of volumetric measures along the full tree demonstrates this pattern (Fig. 1C). We next calculated more explicit measures of phylogenetic signal among the 13 bower-building species using Pagel’s λ (Pagel 1999) and Blomberg’s K22. Pagel’s λ ranges from 0 to 1 with values closer to 1 representing stronger phylogenetic signal while values of Blomberg’s K < 1 suggest less phylogenetic signal in the trait. Both measures indicated a lack of phylogenetic signal among the species tested (λ = 0.25; K = 0.29), suggesting that hindbrain volume may be rapidly evolving but not correlated to phylogeny22.
Since recently evolved clades such as Lake Malawi cichlids are prone to processes such as introgression and incomplete lineage sorting and cannot be described by a single species tree12, we re-performed the phylogenetic ANOVAs and tests of phylogenetic signal on non-overlapping genomic windows each containing 10,000 SNPs (1,029 windows; Fig. S1A). We reasoned that if the patterns revealed by the genome-wide tests held in a substantial number of these windows then the observed results should be robust to alternative phylogenetic scenarios among the 13 species sampled. Applying phylogenetic ANOVAs across the windows we found that all tested yielded a p-value less than 0.05 with the median of 0.006 (Fig. S1B). Similarly, median values of phylogenetic signal were less than 1 across all windows (median λ = 0.011; median K = 0.728; Fig. S1C,D). Of note, the distributions of K and λ are multimodal, suggesting that multiple phylogenetic patterns may still exist within the genomes of these species. This is reflective of the genomic complexity found among Malawi cichlids mentioned above and may have been captured by the relatively large window size used for these tests. Nonetheless, taken together these results support a model in which variation in hindbrain volume, at least among the species tested, does not follow phylogenetic expectations.
Castle building is associated with increased rates of hindbrain diversification
We analyzed rates of trait diversification between rock and sand Malawi cichlid species with a whole-genome phylogeny using Bayesian Analysis of Macroevolutionary Mixtures (BAMM) v.223, showing that hindbrain volume has increasingly diversified since the split between rock and sand lineages around 800,000 years ago (Fig. 1D). Separating the overall phylogenetic tree into rock and sand clades revealed extremely different rates of diversification between these two groups with the sand group (including bower builders) displaying a 7.4-fold faster rate of phenotypic diversification (Fig. 1E,F).
Given this rapid evolution, we hypothesized that hindbrain diversification rates would be greatest in more recently diverging clades. Consistent with this hypothesis, the model with the best rate shift configuration in BAMM included two significant shifts in younger bower-building subclades within the sand-dwelling lineage (Posterior probability = 31.58; Fig. S2). We further found that three of the four best shifts accounting for the majority of posterior probabilities sampled within the 95% credible shift set significant shifts on at least one of these branches (Fig. S2). These subclades were enriched for castle building species, including one that was entirely composed of castle-builders, suggesting that the evolution of castle building is associated with increased diversification rates and trait values for hindbrain size. The phylogenetic analyses conducted here, and previously17, suggest that the construction of pit bowers may be ancestral from which castle building may have arisen multiple times. We then hypothesized that hindbrain volumes among pit digging species should be more similar to those of the rock lineage while castle building species should differ significantly. Indeed, hindbrain volumes of pit digging and rock dwelling species are statistically indistinguishable (Kruskal-Wallis test; H = 0.66, 1 d.f., p = 0.42) while volumes of castle building and rock dwelling species differ substantially (Kruskal-Wallis test; H = 16.74, 1 d.f., p = 4.28 × 10−5). Taken together these results demonstrate that hindbrain volume is evolutionary labile and appears to have rapidly diversified in concert with bower type, suggesting extensive mosaic evolution of this structure within the sand-dwelling lineage.
Pits and castles are associated with differences in gustatory structures and hindbrain architecture but not connectivity
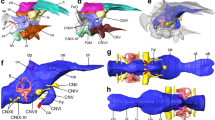
Are the observed evolutionary differences in hindbrain size associated with accompanying regional differences in architecture or connectivity? To address this we compared the neuroanatomy of two closely-related species - Copadichromis virginalis (CV; pit-digging) and Mchenga conophoros (MC; castle building) - that build divergent bower types but possess similar body sizes. For these analyses we focused on the vagal lobe of the dorsal medulla, a key center for taste and oropharyngeal sensation and processing that has been shown to be associated with hindbrain diversification in other fish species and receives projections from sensory structures involved in gustation24.
nerve complex and associated vagal sensory structures of the brainstem. Notably, CV was similar to that of most teleost genera examined, with a smooth epithelium in the posterior oropharynx and small masticatory pads situated in the posterior-most portion of the oropharynx (Fig. 2A). In contrast, the oral apparatus and vagal complex of MC was more highly developed. A distinct palatal organ (PO) was associated with the gill arches located most caudally and the oral palatal surface morphology was more convoluted (Fig. 2B). The vagus nerve of CV was correspondingly small, with a single main root, whereas in MC, the vagal nerve was larger and had multiple roots at the point of entrance to the brainstem. Similarly, brainstem vagal complex of CV was smaller than in MC (Fig. 2C,D).
Oral cavity and brain morphology of M. conophoros and C. virginalis. (A) The dorsal oral cavity of the pit-digger C. virginalis is comparatively simple and lacks a distinct palatal organ. (B) The dorsal oral cavity of the castle-builder M. conophoros displays a more convoluted structure and caudal palatal organ labeled ‘PO’. (C) Photograph of in situ caudal brain structures of C. virginalis. The approximate location of the vagal lobe is outlined and labeled ‘VL’ and the cerebellum is labeled ‘Cblm’. (D) Photograph of in situ caudal brain structures of M. conophoros. As in (C) the approximate location of the vagal lobe is outlined and labeled ‘VL’ and the cerebellum is labeled ‘Cblm’.
The cellular organization of the vagal lobe complex of CV and MC showed differences corresponding to the differences in oral anatomy (Fig. 3, S3). In CV, the vagal lobe although clearly laminated exhibited relatively poorly defined layers of cells lying between the superficial and periventricular layers. The vagal lobe of MC was larger and although more highly structured than that of CV, the essential laminated organization was similar.
Vagus nerve termination. Composite micrographs comparing the laminar pattern of termination of vagus nerves in the vagal lobes of C. virginalis and M. conophoros. The sensory roots of the vagus nerve, labeled by DiI and rendered in magenta, terminate similarly in the two species: in superficial and deep layers leaving the central region void of terminals but crossed by fascicles running from the superficial root into the deeper layers. Green = Nissl staining inverted and rendered in green as if a fluorescent Nissl stain; magenta = diI label; image superimposed on the image of Nissl staining from another specimen.
Tracing the vagus nerve input with DiI showed the pattern of termination was similar in both species. The sensory root entered the lobe ventrolaterally and divided into superficial and deep roots terminating respectively in the superficial half and in the deeper one-third of the lobe with an intervening zone relatively free of vagal nerve terminals (Fig. 3) but penetrated by fascicles running between the superficial and deeper terminal layers. In both species, the motorneurons of the vagus nerve lay in a periventricular position ventral to the vagal lobe proper, as in most other species of fish (e.g. zebrafish)25.
These lines of evidence suggest that differences in hindbrain size among bower building species are accompanied by variation in gustatory and orosensory capabilities – notably the presence or absence of a palatal organ – and the elaboration of associated nerve complexes while patterns of hindbrain connectivity appear conserved but scale with the overall size of the structure.
The vagal lobe is functionally involved in bower building and feeding
We used cFos mRNA expression as a proxy for neural activity to assess which vagal lobe neurons are activated during bower building26. Comparing the castle-building species M. conophoros (MC) and the pit-digging species C. virginalis (CV), we measured cFos expression in the vagal lobes of males after bower construction and in control individuals who were kept in tanks without sand or female conspecifics (Fig. S4A–F). Cells in the vagal lobe of bower building MC males showed greater cFos expression than control (2.601-fold difference, p = 0.019, bootstrap 1-way ANOVA, n/group >3) (Fig. 4A). The level of cFos expression in the vagal lobe of CV also increased significantly (2.517-fold difference, p = 0.034). Likewise, cFos expression in the vagal lobe increased during feeding in both MC (2.254-fold difference, p = 0.046) and CV (2.438-fold difference, p = 0.033) (Fig. 4B).
Molecular correlates of vagal lobe activity associated with bower building. (A) Barplot comparing cFos mRNA staining in MC across control, feeding, and building conditions across the VL. *P < 0.05. (B) Barplot comparing cFos mRNA staining in CV across control, feeding, and building conditions across the VL. *P < 0.05. (C) Barplot comparing pS6 positive cell abundance during bower building in C. virginalis and M. conophoros. *P < 0.05.
We measured vagal lobe activation during bower building using in situ hybridization of cFos and immunohistochemical labeling of phosphorylated ribosomal protein S6 (pS6). Labeling of pS6 indicates recent neural activity and displays punctate staining, allowing for granular quantification in individual neurons27. Staining of pS6 revealed that the number of VL neurons activated during bower building differed significantly between CV and MC. As with cFos, pS6 cell labeling was robust throughout the vagal lobe of both CV and MC after bower building (Fig. S5A–H). Quantification of pS6 labeling in the vagal lobe showed significant differences during bower building in both species (CV: 4.12 fold difference, p = 0.015; MC: 13.84 fold difference, p = 0.022). Moreover, we found that MC bower building males displayed an almost four-fold greater abundance of pS6 positive neurons compared to CV (3.68 fold increase, p = 0.04) (Fig. 4C).
Hindbrain volume varies with bower building among Lake Tanganyika cichlids
To ask whether parallel diversification of hindbrain volumes may have occurred in non-Malawi cichlids, we measured variation in hindbrain volumes in the cichlid radiations of Lakes Victoria and Tanganyika. We found that the Lake Victoria species sampled had hindbrain volumes similar to those of the rock-dwelling cichlids of Lake Malawi (Fig. 5A) whereas normalized hindbrain volumes of Lake Tanganyika species were distributed similar to those of Malawi species (Kruskal-Wallis test, H = 0.043, 1 d.f., p = 0.84). This observation was notable given that Lake Tanganyika has a number of reported bower building species, in contrast with Lake Victoria where few, if any, are known. This suggests that hindbrain diversification has occurred where cichlids have evolved this specific behavior (Malawi and Tanganyika), but not in lakes where bower-building does not occur (Lake Victoria). Accordingly, differences in the hindbrain volumes of Tanganyikan bower building species compared to non-bower builders were comparable to those found between the Malawi rock and sand lineages (Fig. 5A).
Evolution and diversification of hindbrain volume across East African Cichlids. (A) Violin plots of normalized hindbrain volumes for Malawi rock-dwelling species (n = 47), Lake Tanganyika non-bower building species (n = 14), Lake Victoria species (n = 55), Malawi sand-dwelling species (n = 37), and Tanganyika bower building species (n = 12). Lakes are represented by cartoon outlines accompanying their respective violin plots. (B) Maximum likelihood phylogeny of Lake Tanganyika species sampled with accompanying normalized hindbrain volumes. Red represents the Ectodini tribe, yellow represents non-Ectodini, stars indicate known bower building species (for species and node labels see Fig. S10). (C–E) Normalized hindbrain volume diversification rates for Ectodini and non-Ectodini (C), Ectodini (D), and non-Ectodini (E). (F) Boxplot comparing per-species normalized hindbrain volume diversification rates between Ectodini and non-Ectodini tribes.
Given these observations, we next queried which phylogenetic patterns might be associated with this trait among Lake Tanganyika cichlids. Analyzing hindbrain volume on the Tanganyika phylogeny revealed that diversification was largely limited to the Ectodini tribe, a clade possessing a number of bower building species (Fig. 5B). Unlike among bower-building clades in lake Malawi, a phylogenetic ANOVA revealed strong phylogenetic signal associated with hindbrain volume among these Tanganyika bower-species (ANOVA (F = 9.79, 1 d.f., p = 0.12). Similarly, Blomberg’s K and Pagel’s λ confirmed significant amounts of phylogenetic signal in this trait (K = 0.53, p = 0.035; λ = 0.65, p = 0.048). Analyses of diversification rates using BAMM showed hindbrain diversification has increased within the Lake Tanganyika Ectodini tribe as compared with non-Ectodini species (Fig. 5C–E). These observations suggest a relationship between bower-building and hindbrain volume among Lake Tanganyika cichlids but without the patterns of rapid and recurrent diversification apparent among Malawi cichlids, possibly arising from Tanganyika’s greater age and accordingly greater divergence times between species and clades.
Discussion
We show here that hindbrain volume increases in tandem with the behavioral evolution of castle-type bower building, both within the dozens of species sampled from Lake Malawi here and also as compared to species in Lake Tanganyika but not Victoria, which seems to contain no known builders. Despite previous results identifying mosaic evolution of the brain, whether this may occur repeatedly given common ecological, evolutionary, or phylogenetic pressures is unclear. Our findings suggest that in certain scenarios brain evolution may proceed in a predictable manner, as previously proposed for systems such as bony fish10 and vocal learning birds and mammals8, but has rarely has this been tested explicitly in a controlled phylogenetic context. However, resolving phylogenetic relationships among Malawi cichlid species is difficult given their close genetic relationships and complex histories of gene flow and incomplete lineage sorting14. Therefore, future work attempting to disentangle further the differential roles of ecology, behavior, and evolution on brain morphology in Lake Malawi should include wider, more targeted sampling from the phylogeny in addition to comprehensive collection of demographic traits for each species. Sequencing more Malawi species would allow useful phylogenomic analyses of genetic association and identification of the roles of gene flow and/or incomplete lineage sorting in the context of brain evolution.
Our results also indicate that variation in hindbrain size is associated specifically with diversification of the vagal lobe, a key gustatory region of the fish hindbrain. The vagal lobe differs significantly between pit and castle species in size and structure, but not connectivity. This contrasts starkly with observations in cyprinid fish, another teleost clade with extensive vagal lobe diversification24,25,28. Among typical food-sorting cyprinids that have a palatal organ, such as goldfish and carp, the vagal lobe is highly laminated with clear motor and sensory zones that extend dorsally in parallel along the deep and superficial layers of the lobe24,25,28. Similarly, the vagal lobe of Heterotis niloticus, an unrelated fish of the order Osteoglossiformes, has a laminated vagal lobe with sensory layers overlying motor layers29. In fish species that lack an elaborate palatal organ, such as catfish and zebrafish, the vagal lobe is cytologically simpler with less obvious lamination and a motor nucleus which is restricted to a sub-ventricular area with little penetration into the lobe itself 30. Malawi cichlid hindbrain diversification is an intermediate between the vagal lobes of food sorting and non-food sorting cyprinids. While the vagal motor nuclei of pit and castle species are similar in location to that of catfish, histology and DiI labeling indicate that castle building species tend to show increases in vagal lobe size, lamination, and manner of termination of primary vagal sensory fibers. These patterns of vagal lobe elaboration are supported by the presence of palatal organs in the castle building species sampled. Furthermore, our studies of immediate early gene expression and ps6 abundance during behavior reveal not only that the vagal lobe is involved in both bower building and feeding, but that there are detectable species differences in neuronal activity during bower building. Such differences may merely reflect differences in the degree to which oral manipulation of substrate is necessary for the different types of bower: pit versus castle.
Evidently, the largest differences in hindbrain structure and function among bower builders are determined mostly by activity (via behavioral state) and size, but only moderately by changes in connectivity and histological structure. Any functional differences in the vagal lobe associated with pit and castle bower types, then, likely arise through both variations in developmental patterning and, in adulthood, modulation of vagal lobe function. It does not appear that bower building is associated with the evolution of novel, behavior-specific hindbrain circuits. Support for this comes from previous work showing that ecologically-relevant differences in forebrain size among rock- and sand-dwelling Malawi cichlids arise from a common blueprint that is differentially modified by patterning genes31. Similarly, the hindbrains of the sand-dwelling cichlids analyzed here appear to have a relatively conserved brain bauplan at base, but with substantial elaboration in size and modulation among species. The high degree of relatedness and recent evolution of Malawi cichlid species likely constrains the phenotypic possibilities available for the evolution of brain and behavior. The significant increase in diversification rate of the hindbrain among castle-builder clades in both Malawi and Tanganyika suggests that behavioral evolution in these lakes is likely supported by neural variation producing bigger brain structures that generate more activity.
The degree of phenotypic predictability displayed by the hindbrain in Malawi cichlids suggests that, given a common phylogenomic context, diversifying species may present some degree of commonality in the evolution of neural and behavioral traits in response to similar pressures. Of particular interest will be the study of how evolution acts on conserved and novel genes and genetic networks to regulate the brain and behavior across evolutionary distances, potentially revealing common principles of the evolution of behavior.
Methods
Fish were bred, housed, and maintained at Stanford University following established Stanford University IACUC protocols.
Volumetric analyses were performed using measurements from a phenotypic data set comprising brain measurements for 189 cichlid species from East Africa and Madagascar19. Phylogenetic comparisons were conducted in R. The packages SNPhylo32 and ape33 were used to construct ultrametric phylogenies for the Lake Malawi species from whole genome SNP data21. A previously published phylogeny was used to infer phylogenetic relationships of the Lake Victoria and Tanganyika species34. Phylogenetic ANOVAs were performed with the package geiger35 while phylogenetic signal and trait diversification rates were inferred from the packages phyloSignal36 and BAMMtools23, respectively.
Oropharyngeal anatomy was assayed by scanning electron microscopy of the oral cavities of C. virginalis (CV), M. conophoros (MC), and T. brevis (TB) using a Hitachi S-3400N VP scanning electron microscope. Taste buds and innervation patterns were identified by immunocytochemical labeling using antisera against human calretinin (CR 7697; AB_2619710) and acetylated tubulin (Sigma T7451; AB_609894).
DiI tracing was performed on CV, MC, and TB by placing DiI crystals on either the vagus nerve root or the surface of the vagal lobe. After a diffusion period of 1–6 months the brains were sectioned at 75–100 um on a vibratome and then imaged.
Behaviorally-induced neural activity was assessed by running CV and MC males through one of three behavioral paradigms: bower building, feeding, and control. For ISH we used RT-PCR to amplify a portion of coding sequence from cFos (NM_001286320), and subcloned products into pCR-TOPO4 (Life Technologies). cFos forward, 5′-AAT TGG ATC CAA GCC CAG ATC TTC AGT GG-3′; cFos reverse, 5′-AAT TGA ATT CAT AGC CCT GTG ATC GGC AC-3′. Abundance of pS6 was inferred by immunohistochemical labelling with with rabbit anti Phospho-S6 Ser244 Ser247 (ThermoFIsher Scientific 44-923G, RRID AB_2533798). ISH and immunohistochemical labeling were imaged in FIJI and quantified with custom scripts in R.
Additional information can be found in the supplemental experimental methods.
Vertebrate animal use
All animal experiments were approved by the Stanford University Animal Care and Use Committee (protocol number APLAC-28757) and performed in accordance with the guidelines and regulations of this institution.
Data availability
All sequencing data in support of the findings of this study have been deposited in the Short Read Archive under accessions SRR6314224, SRR6314225, SRR6314226, SRR6314228, SRR6314230, SRR6322515. All morphometric, phylogenetic, and histological data are available as electronic supplements to this manuscript.
References
Zupanc, Gn. K. H. Behavioral neurobiology: an integrative approach. (Oxford University Press, Oxford; New York, 2004).
Striedter, G. F. Principles of brain evolution. (Sinauer Associates, Sunderland, Mass., 2005).
Finlay, B. L. & Darlington, R. B. Linked regularities in the development and evolution of mammalian brains. Science. 268, 1578–1584 (1995).
Barton, R. A. & Harvey, P. H. Mosaic evolution of brain structure in mammals. Nature. 405, 1055–1058 (2000).
Yopak, K. E. et al. A conserved pattern of brain scaling from sharks to primates. Proc. Natl. Acad. Sci. USA 107, 12946–12951 (2010).
Hager, R., Lu, L., Rosen, G. D. & Williams, R. W. Genetic architecture supports mosaic brain evolution and independent brain-body size regulation. Nat. Commun. 3, 1079 (2012).
Krebs, J. R., Sherry, D. F., Healy, S. D., Perry, V. H. & Vaccarino, A. L. Hippocampal specialization of food-storing birds. Proc. Natl. Acad. Sci. USA 86, 1388–1392 (1989).
Lefebvre, L., Reader, S. M. & Sol, D. Brains, innovations and evolution in birds and primates. Brain. Behav. Evol. 63, 233–246 (2004).
Dunbar, R. I. & Shultz, S. Understanding primate brain evolution. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 362, 649–658 (2007).
Kotrschal, K., Staaden, M. J. V. & Huber, R. Fish brains: evolution and environmental relationships. Rev. Fish. Biol. Fisher. 8, 373–408 (1998).
Nieuwenhuys, R., Donkelaar, H. Jt. & Nicholson, C. The central nervous system of vertebrates. (Springer, Berlin; New York, 1998).
Brawand, D. et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 513, 375–381 (2014).
Moran, P., Kornfield, I. & Reinthal. P. N. Molecular Systematics and Radiation of the Haplochromine Cichlids (Teleostei, Perciformes) of Lake Malawi. Copeia. 274–288 (1994).
Malinsky, M. et al. Whole Genome Sequences Of Malawi Cichlids Reveal Multiple Radiations Interconnected By Gene Flow. Nat. Eco. Evo. 2, 1940–1955 (2018).
Kocher, T. D. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 5, 288–298 (2004).
Streelman, J. T. & Danley, P. D. The stages of vertebrate evolutionary radiation. Trends in Ecology &. Evolution. 18, 126–131 (2003).
York, R. A. et al. Evolution of bower building in Lake Malawi cichlid fish: phylogeny, morphology, and behavior. Frontiers in Ecology and Evolution. https://doi.org/10.3389/fevo.2015.00018 (2015).
Keenleyside, M., ed. Cichlid Fishes: Behavior, Ecology, Evolution. (Chapman and Hall, London 1991).
York, R. A. et al. Behavior-dependent cis-regulation reveals genes and pathways associated with bower building in cichlid fishes. Proc. Natl. Acad. Sci. USA 47, E11081–E11090 (2018).
van Staaden, M. J., Huber, R., Kaufman, L. S. & Liem, K. F. Brain evolution in cichlids of African Great Lakes: brain and body size, general patterns, and evolutionary trends. ZACS. 98, 165–178 (1995).
Huber, R., van Staaden, M. J., Kaufman, L. S. & Liem, K. F. Microhabitat use, trophic patterns, and the evolution of brain structure in African cichlids. Brain. Behav. Evol. 50, 167–182 (1997).
Blomberg, S. P., Garland, T. Jr. & Ives, A. R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 57, 717–745 (2003).
Rabosky, D. L. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One. 9, e89543 (2014).
Finger, T. E. Feeding patterns and brain evolution in ostariophysean fishes. Acta. Physiol. Scand. Suppl. 638, 59–66 (1997).
Rupp, B., Wullimann, M. F. & Reichert, H. The zebrafish brain: a neuroanatomical comparison with the goldfish. Anat. Embryol. (Berl). 194, 187–203 (1996).
Maruska, K. P., Zhang, A., Neboori, A. & Fernald, R. D. Social opportunity causes rapid transcriptional changes in the social behavior network of the brain in an African cichlid. J. Neuroendocrinol. 25, 145–157 (2013).
Knight, Z. A. et al. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 151, 1126–1137 (2012).
Herrick, C. J. The central gustatory paths in the brains of bony fishes. J. Comp. Neurol. Psycho. 15, 375–456 (1905).
Braford, M. R. De gustibus non est disputandem: a spiral center for taste in the brain of the teleost fish, Heterotis niloticus. Science. 232, 489–491 (1986).
Finger, T. E. Sorting food from stones: the vagal taste system in Goldfish, Carassius auratus. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 194, 135–143 (2008).
Sylvester, J. B. et al. Competing signals drive telencephalon diversity. Nat. Commun. 4, 1745 (2013).
Lee, T. H., Guo, H., Wang, X., Kim, C. & Paterson, A. H. SNPhylo: a pipeline to construct a phylogenetic tree from huge SNP data. BMC Genomics. 15, 162 (2014).
Paradis, E., Claude, J. & Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 20, 289–290 (2004).
Wagner, C. E., Harmon, L. J. & Seehausen, O. Ecological opportunity and sexual selection together predict adaptive radiation. Nature. 487, 366–369 (2012).
Harmon, L. J., Weir, J. T., Brock, C. D., Glor, R. E. & Challenger, W. GEIGER: investigating evolutionary radiations. Bioinformatics. 24, 129–131 (2008).
Keck, F., Rimet, F., Bouchez, A. & Franc, A. phylosignal: an R package to measure, test, and explore the phylogenetic signal. Ecology and Evolution. 6, 2774–2780 (2016).
Author information
Authors and Affiliations
Contributions
Ryan York conceived of and designed the study, carried out behavioral and molecular experiments, performed phylogenetical and statistical analyses, and wrote the manuscript. Allie Byrne performed behavioral assays and in situ hybridization and immunohistochemical staining protocols. Kawther Abdilleh, Chinar Patil, and Todd Streelman collected and processed tissue samples for next generation sequencing and identified genetic variants for phylogeny construction. Thomas Finger performed neuroanatomical comparisons of Malawi cichlid hindbrain structure and helped write the manuscript. Russell Fernald provide funding, aided in design and coordination of the study and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
York, R.A., Byrne, A., Abdilleh, K. et al. Behavioral evolution contributes to hindbrain diversification among Lake Malawi cichlid fish. Sci Rep 9, 19994 (2019). https://doi.org/10.1038/s41598-019-55894-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55894-1
This article is cited by
-
Brain areas activated during visual learning in the cichlid fish Pseudotropheus zebra
Brain Structure and Function (2023)
-
Evolution of regulatory networks associated with traits under selection in cichlids
Genome Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.