Abstract
This systematic review and meta-analysis aimed to provide a contemporaneous estimate of the global burden of rheumatic heart disease (RHD) from echocardiographic population-based studies. We searched multiple databases between January 01, 1996 and October 17, 2017. Random-effect meta-analysis was used to pool data. We included 82 studies (1,090,792 participant) reporting data on the prevalence of RHD and 9 studies on the evolution of RHD lesions. The pooled prevalence of RHD was 26.1‰ (95%CI 19.2–33.1) and 11.3‰ (95%CI 7.2–16.2) for studies which used the World Heart Federation (WHF) and World Health Organization (WHO) criteria, respectively. The prevalence of RHD varied inversely with the level of a country’s income, was lower with the WHO criteria compared to the WHF criteria, and was lowest in South East Asia. Definite RHD progressed in 7.5% (95% CI 1.5–17.6) of the cases, while 60.7% (95% CI 42.4–77.5) of cases remained stable over the course of follow-up. The proportion of cases borderline RHD who progressed to definite RHD was 11.3% (95% CI 6.9–16.5). The prevalence of RHD across WHO regions remains high. The highest prevalence of RHD was noted among studies which used the WHF diagnostic criteria. Definite RHD tends to progress or remain stable over time.
Similar content being viewed by others
Introduction
Rheumatic heart disease (RHD), an inflammatory heart valve condition, is a chronic sequel of acute rheumatic fever (ARF). ARF is a multisystem disease resulting from an autoimmune reaction to group A streptococcal (GAS) pharyngitis in genetically susceptible individuals1. RHD causes inflammation of the cardiac valves, initially leading to clinically silent valvular disease and ultimately severe permanent damage. Individuals with RHD are at increased risk of complications such as congestive heart failure, arrhythmias including atrial fibrillation, stroke, infective endocarditis, poor maternal and fetal outcomes, and premature death2,3,4. RHD is the most common cause of acquired heart disease in children and young adults globally5.
ARF and RHD are diseases of poverty, driven by poor sanitation, overcrowding, malnutrition and limited access to health care. In 2015, RHD affected 33.4 million people globally, and caused 319,400 deaths, nearly all of which occurred in LMIC5. This heavy burden of RHD contrasts with persistent neglect of the condition on national health agendas in endemic countries6.
To tackle the burden of RHD, the World Heart Federation (WHF) released in 2013 a position statement on the prevention and control of RHD, with the ambitious goal of achieving a 25% reduction in premature deaths from ARF and RHD among individuals aged <25 years by 20257. To achieve this goal, comprehensive and effective national disease programs should be developed and integrated into existing efforts by ministries of health. A major impediment is the lack of “true” burden of disease estimates on local, national, and international levels which can be used for the implementation of existing evidence-based, cost-effective approaches to preventing GAS/ARF and treating RHD7.
Echocardiography is the most cost-effective tool for population screening and estimating the prevalence of RHD8. About five years ago, Rothenbühler et al. published a meta-analysis of the prevalence of RHD among children and adolescents, highlighting the substantial burden of disease in endemic regions9. The major flaw of this meta-analysis was to pool together estimates from studies which used various echocardiographic screening approaches and criteria. Additionally, a large number of echocardiographic screening studies have been published subsequent to the publication of this study. We present herein an updated systematic review and meta-analysis population-based echocardiographic studies, to estimate the prevalence of RHD according to diagnostic protocol and criteria, and to assess the evolution of clinically silent RHD.
Methods
Design and registration
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)10. The protocol was registered in PROSPERO (registration number CRD42017068732).
Search strategy for identifying relevant studies
We performed a comprehensive and exhaustive search of PubMed/MEDLINE, Excerpta Medica Database (EMBASE), African Journals Online (AJOL), the Latin-American and Caribbean System and the Cochrane Database of Systematic Reviews to identify all relevant population-based studies estimating the prevalence of RHD using echocardiography and published between January 01, 1996 and October 17, 2017. We conceived and applied a search strategy based on the combination of terms related to RHD, ARF and echocardiography. The search strategies for all the databases are available in the Appendix (Supplementary Tables 1–5). To supplement these bibliographic database searches, we also scrutinized references of all relevant research articles and reviews to identify additional potential data sources.
Criteria for eligibility
To be included in this systematic review, studies had to be population-based (i.e., school-based or community-based) with a sample size of at least 300 participants, and reporting on the prevalence of RHD detected or confirmed using echocardiography, irrespective of the age of participants. We excluded studies which used only auscultation to screen for RHD with no confirmatory echocardiography, and those reporting primarily on ARF or GAS infections.
Selection of studies for inclusion in the review
Two review authors (JJN and ADK) independently screened the titles and abstracts of articles retrieved from literature search, and the full-texts of articles found potentially eligible were obtained and further assessed for final inclusion. For studies published in more than one report (duplicates), the most comprehensive report of the largest sample size was considered. Disagreements were resolved through discussions between investigators until a consensus was reached.
Appraisal of the methodological quality of included studies
The methodological quality of included studies was assessed using an adapted version of the tool developed by Hoy and colleagues to evaluate the risk of bias in prevalence studies11. Each item was assigned a score of 1 (yes) or 0 (no), and scores were summed across items to generate an overall quality score that ranged from 0 to 9. Studies at low risk of bias had scores of 7 or higher, moderate a score of 4–6, and high a score of 3 or lower. Two review authors (VNA and UFN) independently assessed study quality; disagreements were resolved by consensus.
Data extraction and management
Two review authors (VNA and UFN) independently extracted relevant data from included studies using a preconceived and standardized abstraction form. Disagreements between these authors were reconciled through discussion and consensus. Two review authors (JJN and JJB) cross-checked the database for errors. Data were extracted from each study on: the surname of the first author, year of publication, area (rural vs urban), country of recruitment of participants, study design, sampling method, male proportion, age distribution, setting (school-based vs community-based), diagnostic approach (auscultation only for screening with second-line echocardiographic confirmation [auscultation > echo], first-line echocardiography with or without auscultation with second line echocardiographic confirmation [echo > echo], and first line echocardiography without confirmation [echo > nothing]), diagnostic criteria (World Health Organization, WHF criteria, and others), the number of participants with clinically silent and manifest RHD, and with borderline and definite RHD. Clinically manifest RHD was defined as the presence of a heart murmur on cardiac auscultation with evidence of RHD on echocardiography (pathological mitral regurgitation or stenosis and/or morphological features of RHD), while absence of heart murmur with echocardiographic evidence of RHD were considered as cases of clinically silent RHD7. Where relevant data were not available, we contacted the corresponding author to request the information. Using the country in which the study was conducted in and year of recruitment, we assigned gross domestic product per capita (GDP) in United State dollars12, WHO regions13, United Nations Statistics Division (UNSD) of countries by continent14, situation or not in endemic area15, human development index (HDI)16, the 2016 level of income17, and GINI coefficient18. For multinational studies, data were presented according to the country where the study was conducted in.
Data synthesis and analysis
All analyses were performed using ‘meta’ packages of R (version 3.3.3) (The R foundation for statistical computing, Vienna, Austria). Freeman-Tukey double arcsine transformation was used to pool data by random effect meta-analysis19. Following crude overall prevalence, a sensitivity analysis was conducted considering only studies with a low risk of bias. We assessed inter-rater agreement for inclusion and quality assessment using Cohen’s kappa (κ) coefficient. All prevalence estimates were reported per 1000 people (‰) with their 95% confidence interval (95%CI) and their 95% predictive interval (95%PI).
We appraised heterogeneity between studies using Cochran’s Q statistic, H and the I2 statistics20,21, which estimate the percentage of total variation across studies due to true between-study difference rather than chance, with I2 values of 25%, 50% and 75% representing low, medium and substantial heterogeneity, respectively. We explored sources of heterogeneity through subgroup and meta-regression analyses defined by mean/median age, proportion of males, diagnostic approach, diagnostic criteria, study setting, area, WHO regions, UNSD of countries, clinical significance of RHD, level of income, GINI, HDI, GDP, and situation in endemic area. Comparisons between subgroups were performed using the Q-test based on the Analysis of the Variance (ANOVA). Univariable and multivariable meta-analyses were used. To be included in the multivariable analysis, a p value < 0.20 was required in univariable analysis. For categorical variables, the global p value was considered for inclusion in the multivariable model. We applied a manual backward selection procedure to identify factors independently associated with the variation of overall prevalence of RHD. We successively removed variables from the model if p value > 0.10. In the case of non-linear distribution, we log-transformed the covariate before conducting the meta-regression analyses. Publication bias was evaluated with funnel plots supplemented by formal statistical assessment using Egger’s test22. A p value < 0.10 was considered statistically significant to detect publication bias.
Results
The review process
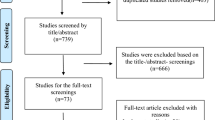
A total of 7969 records were retrieved via databases searches. After removing duplicates, we screened the titles and abstracts of 5592 records, of which 215 were selected for full-text review. Of these, 81 articles were included in the review, 72 providing data on the prevalence of RHD (in a total of 82 individual studies), 6 reporting data on the evolution of RHD lesions, and 3 on both the prevalence and the evolution of RHD (Fig. 1), all published from 1996 to 201723,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103. Inter-rater agreements for inclusion based on titles and abstracts, full texts, and for assessment of the methodological quality of finally included studies between review authors were κ = 0.68, 0.98, and 0.87 respectively.
Characteristics of included studies reporting on the prevalence of RHD
Regarding methodological quality, 51 (62%) had low risk of bias, 30 (37%) had moderate risk of bias, and one (1%) had high risk of bias. The inter-rater agreement for quality assessment was excellent (κ = 0.97). The characteristics of included studies from 35 countries are summarized in the Appendix (Supplementary Tables 6–8). Most of studies were school-based (78%, n = 64), conducted both in urban and rural settings, in endemic areas (84.1%, n = 69) of low-middle income countries (41.5%, n = 34). The most represented WHO regions were Africa (26.8%, n = 22), South-East Asia (26.8%, n = 22) and Western Pacific (24.4%, n = 20). On the other hand, the most represented UNSD continent were Asia and Africa. The majority of studies used the WHF (39%, n = 32) and WHO criteria (36.6%, n = 30), and the echo > echo procedure (51.1%, n = 46).
Participants were recruited from 1991 to 2015. The mean or median age of participants varied from 8 to 48 years (48 studies); range from 3 to 74 years (74 studies). The proportion of male participants varied from 30% to 65% (58 studies). The sample size in included studies varied from 337 to 229,829 participants. The median GDP, HDI and GINI coefficient were 1346 USD (range: 140–40911), 0.624 (range: 0.418–0.939; 79 studies) and 0.36 (range: 0.31–0.63; 76 studies), respectively.
Overall prevalence of rheumatic heart disease
Table 1 shows the pooled prevalence of RHD in the included studies. In a total sample of 1,090,792 participants from 82 studies, the overall RHD prevalence estimates were 26.1‰ (95%CI 19.2–33.1), 11.3‰ (9%CI 7.2–16.2), and 5.2‰ (95%CI 3.0–8.0) with WHF, WHO and other criteria respectively (p < 0.0001) (Fig. 2). The overall RHD prevalence estimates were 6.4‰ (95%CI 4.0–9.2) and 21.2‰ (95%CI 15.3–28.1) with auscultation > echo and echo > echo procedures, respectively (p < 0.0001) (Fig. 3). Prevalence estimates of sensitivity analysis including only studies with low risk bias were in the range of the crude analysis. There was a wide variation of the range of the 95% PI.
Definite RHD prevalence varied from 3.7‰ to 11.4‰ depending on the diagnostic criteria or procedure used with significant difference between diagnostic criteria and procedure. Borderline or probable RHD prevalence varied from 5.6‰ to 15.2‰ with difference between diagnostic criteria and without difference between diagnostic procedures. Clinically manifest RHD prevalence varied from 1.7‰ to 2.6‰ without difference between diagnostic criteria and procedure. Clinically silent RHD prevalence varied from 2.6‰ to 15.5‰ with difference between diagnostic procedures but not between diagnostic criteria (Table 1).
Subgroup analyses
When considering WHF and WHO criteria, the prevalence differed between levels of income but this difference was not found when considering diagnostic procedure. Regardless of diagnostic criteria or procedure, there was no difference between endemic and non-endemic areas, between rural and urban areas, and between community-based and school-based studies. Regardless of diagnostic criteria or procedure, there was difference between UNSD regions and WHO regions (Appendix, Supplementary Table 9).
Factors associated with the prevalence of rheumatic heart disease
In the univariable meta-regression analysis, the RHD prevalence was associated with the year of publication, GDP, GINI coefficient, proportion of males, diagnostic procedure, diagnostic criteria, WHO regions, UNSD of countries, and level of income. In the final multivariable model, the prevalence of RHD was lowest in South-East Asia compared to WHO regions. The RHD significantly decreased with rising level of income; low income countries had higher RHD prevalence. Compared with the WHF diagnostic criteria, the prevalence of RHD estimated with WHO criteria was significantly lower. UNSD of countries by continent was not associated with RHD prevalence. Variables included in the final model explained 57.3% of the 98.4% residual heterogeneity of the RHD prevalence (Table 2).
For each meta-analysis, we found some substantial heterogeneity across the included studies overall and within subgroups (Table 1 and Supplementary Table 9 in the Appendix,). There was some evidence of publication bias across the contributing studies (Appendix, Supplementary Table 9). For overall RHD prevalence analyses, the publication bias was found for all analyses except for WHF criteria and echo > nothing procedure (Table 1, Supplementary Figs. 1–6 in the Appendix).
Evolution of clinically silent rheumatic heart disease
Supplementary Fig. 8 in the Appendix summarizes studies reporting on the evolution of definite RHD and borderline RHD. The majority of studies were from the Western Pacific and Africa, and published from 2011 to 2017. The ages of a total of 824 participants ranged from ranged from 5–18 years. The proportion of participants on penicillin prophylaxis ranged from 18.8–100% with only three studies reporting on the adherence rate to secondary prophylaxis. The median duration of follow-up ranged from 3.7–90 months.
Table 3 depicts the evolution of definite and borderline RHD, and nonspecific valvular abnormalities. Definite RHD progressed in 7.5% (95% CI 1.5–17.6) of the cases, while 60.7% (95% CI 42.4–77.5) of cases remained stable over the course of follow-up. On the other hand, the progression rate for borderline RHD was 11.3% (95% CI 6.9–16.5). Moderate and substantial heterogeneity was noted across included studies overall, with no evidence of publication bias. Stable or progressed lesions were mostly determined by increasing age, presence of a functional aortic valve abnormality, higher durations from diagnosis, receipt of secondary prophylaxis, and presence of a pathological mitral regurgitation murmur (Fig. 3).
Discussion
This systematic review and meta-analysis provides a critical summary of the global prevalence of RHD based on data pooled from 82 observational community- and school-based studies involving 1,090,792 individuals. There were several key findings: (1) we found a high overall prevalence of RHD of varying from 5.2‰ to 26.1‰ depending on diagnostic criteria and procedure used. This prevalence was highest in studies which employed WHF criteria followed by those which used the WHO criteria (21.6‰ versus 11.3‰) and was also higher with echo > echo (21.2‰) procedure compared with auscultation > echo (15.6‰) procedure. (2) The prevalence RHD varied significantly with the level of income at country level, diagnostic criteria used and by region (i.e., higher in Africa than South East Asia); and (3) The lesions of over three-quarters of persons diagnosed with definite RHD either remained stable or progressed, while 11% of those diagnosed with borderline RHD progressed to definite RHD over 3.7–90 months of follow-up.
Globally, the prevalence of RHD was about three times greater for studies which used the echo > echo procedure compared with those which used the auscultation > echo diagnostic procedure. Echocardiography has been reported to be far more sensitive than cardiac auscultation in screening for RHD43,50,56. Marijon reported a failure of auscultation to detect more than 90% of RHD cases detected with echocardiography38. Auscultation is therefore an ineffective screening method for RHD, especially in endemic regions. This finding in accordance with endorsement of echocardiography as a screening tool for RHD in endemic areas by the WHO104. In this light, a number of studies have evaluated the sensitivity of a more conducive echocardiographic screening tool, the handheld echocardiography (HAND), which has demonstrated its superiority over auscultation42,76. HAND is particular useful as it is associated with an acceptable sensitive and specificity for both borderline and definite RHD, and a sensitivity of greater than 90% of definite RHD when used by nurses77, and an excellent sensitivity and specificity when used by experienced physicians81,98. In addition, it is less costly, and portable when compared with the standard echocardiography. This is critical in overcoming the limitation of large-scale echocardiographic screening in resource-limited settings.
The prevalence of RHD was over two times greater for studies employing the WHF diagnostic criteria than those using the WHO criteria. This discrepancy could be explained by: a difference in definition for case detection and diagnostic criteria for RHD99,104; the difficulties associated with large-scale screening; method of screening; and the period during which data was collected for the included studies. These findings could signify that the true prevalence of RHD is underrated or overrated by studies employing the WHO or WHF criteria, respectively. Indeed, the prevalence of definite RD was about twice greater for WHF studies than WHO studies. Furthermore, the prevalence of borderline RHD was over three times greater than that of probable RHD. The major reasons for moderate to high risk of bias in the prevalence of RHD from studies included in this systematic review and meta-analysis were: the use of auscultation in screening for RHD, non-randomized sampling, and failure to report on the study setting, appropriate numerator (number of cases of RHD) to compute the prevalence of RHD; the use of an acceptable case definition for RHD or study setting.
The relatively high prevalence of borderline RHD could be due to a high false-positive rate associated with the WHF criteria76. It is noteworthy that the clinical significance of borderline RHD in individuals with no prior history of ARF remains obscure. In fact, the WHF affirms that the recent criteria was “established to improve sensitivity at the expense of specificity,” and does not necessarily represent a diseased state7. Roberts et al.76 in 2014 after screening a group of 3946 and 1053 children considered to be at high- and low-risk of RHD respectively, demonstrated that borderline RHD could be found in about 0.5% of the low-risk population. High positive rates are crucial because it may lead to unnecessary health expenditures on the part of individual and his/her family as they might be dealing with the wrong diagnosis of a chronic disease. Also, this further weighs on the healthcare system as there is the need to allocate adequate resources required for further evaluation of such potential cases, which is usually difficult in resource-poor settings. The high false-positive rate of the WHF diagnostic criteria cannot be completely disregarded due to its stronger association with populations at high-risk, compared with those at low-risk of RHD76.
There was no significant difference in the prevalence of RHD among school-based compared with community-based studies, and did not influence the variability of the prevalence of RHD globally. This is contrary to the hypothesis that school-based studies are likely to underrate the actual burden of RHD due to the association between school attendance and socioeconomic status; which in turn, is a principal risk factor for RHD100. Our finding ties with that of Rothenbühler et al. where no significant difference in the prevalence of RHD was noted among children sampled in schools and the community9. However, with the inclusion of an unbalanced proportion of studies which used community- (64/82, 78%) compared with school-based interventions, this claim warrants further investigation. Engel et al.30, 2015 and Gemechu et al.31, 2017 estimated the prevalence of RHD in Ethiopian scholars and at population level respectively, two years apart. They noted a higher prevalence of RHD at population level (56.7 cases per 1000) than schools (31 cases per 1000).
The prevalence of clinically silent RHD was over twice greater than that of clinically manifest RHD. A higher ratio was noted by Rothenbühler et al.9. Asymptomatic persons with no history of acute rheumatic fever but presenting with echocardiographic signs of RHD are said to have a clinically silent RHD, which is a latent stage of RHD101. The sensitivity of cardiac auscultation in detecting cases of latent RHD is very low, with a greater majority of the cases detected by active surveillance using echocardiography42,43,50,102. The natural progression of latent RHD, which is still obscure, is associated with clinical and economic implications as to whether the affected individual should be placed on penicillin prophylaxis or not105. Although, it has been suggested that the progression of latent RHD can be halted by early institution of penicillin prophylaxis, this claim is yet to be confirmed by appropriate prospective studies106,107. The natural progression of latent RHD has been evaluated by some prospective studies40,102,105,108. However, appropriate comparison across cohorts is limited by small sample sizes, the use of non-standardized criteria for diagnosis of RHD, short duration of follow up, varying proportion of participants on penicillin prophylaxis and rates of adherence to penicillin prophylaxis. We noted that about 70% of children diagnosed with definite RHD either progressed or remained stable over time. It is crucial to report disease progression according to disease severity (mild, moderate and severe). Indeed, participants with moderate and/or severe definite RHD have been reported to have a greater progression rate and poorer outcome than those with mild definite RHD109,110. Secondary prophylaxis coupled with adherence enhancement as proposed by the WHF in 2012 is advised by some experts that individuals with moderate to severe forms of definite RHD5. Whether penicillin prophylaxis is able halt the progression of RHD still remains obscured and warrants further investigation111. In addition, about 11% of participants initially diagnosed with borderline RHD progressed to definite RHD. This finding suggests, to a minimum, that individuals with borderline disease need surveillance for disease progression as the process persists with time112,113.
Despite the drastic drop in the prevalence of RHD in most high-income and some low-income countries such as Cuba, it still remains high in many developing countries5,114. With the absence of an effective vaccine to prevent RHD, WHO experts endorsed a multilevel approach for the control and/or eradication of RHD which consists of: the improving the social economic and environmental conditions of at-risk populations, referred to as ‘primordial prevention’; treating all patients with strep throat using penicillin – ‘primary prevention’; using antibiotic prophylaxis in persons with history of rheumatic fever or RHD prevent recurrence of an hence reduce progression of already established cardiac lesions, referred to as ‘secondary prevention’; and treatment medically and/or surgically the complications of RHD, known as ‘tertiary prevention’7,104. However, the implementation of these recommendations faces several challenges7.
Though the meta-analytic techniques used in this study were robust, the findings herein should be interpreted with care taking into account the study limitations. Firstly, we noted substantial heterogeneity in the prevalence of RHD across countries and regions, however more than half of the heterogeneity was explained by the final multivariable model. Indeed, studies have shown significant differences in the prevalence of RHD, when the WHO diagnostic criteria of 2006 is compared with the WHF criteria of 201276. Also, the WHF diagnostic criteria of 2012 have not been universally accepted52,53,59. Consequently, alternative guidelines have been adopted in many countries115. This might explain the heterogeneity of RHD observed across studies. Secondly, despite the superiority of echocardiography over auscultation in diagnosing RHD, there is still no gold standard for diagnosing RHD as echocardiography relies on criteria-specific sensitivity and specificity which needs to be improved. In fact, there are concerns that available echocardiographic criteria for diagnosing RHD might overestimate the actual burden of RHD76. Though, the age of participants included in this study spanned from 3 to 74 years, specific criteria for echo-based diagnosis of RHD and studies on the prevalence of RHD in adults, in whom we expect to be the vast majority in developing countries, are sparse. The current criteria for the diagnosis of subclinical RHD might not be appropriate in adults, as such criteria were developed and validated in children in which early diagnosis and intervention can positively impact the natural history of RHD. Thirdly, all WHO regions were not uniformly represented partly due to difficulties in retrieving the full-text of articles published in local journals. For example, there are regions represented by just a single study or two. The authors will advise the prevalence rates obtained for such regions not be taken into consideration when comparing the global prevalence of RHD according to the different WHO regions. More studies are needed in these regions reliable statistics concerning the prevalence of RHD in these regions. Finally, evidence from studies reporting on the evolution of clinically silent RHD are limited by small sample sizes, short duration of follow-up and non-standardized criteria for diagnosis and classification of RHD.
This study reveals a high prevalence of RHD among WHO regions, with the highest rates recorded in Africa. The prevalence of RHD is highest with studies employing the WHF diagnostic criteria and those which use echocardiography for both screening and confirmation of RHD. Well-designed prospective studies with longer periods of follow-up, larger sample sizes to provide adequate data on the evolution of RHD and standardization of criteria to diagnose and classify RHD, preferably in the context of randomized trials of the effectiveness of antibiotic prophylaxis, are warranted to inform the management of asymptomatic RHD detected on screening echocardiography.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Engel, M. E., Stander, R., Vogel, J., Adeyemo, A. A. & Mayosi, B. M. Genetic susceptibility to acute rheumatic fever: a systematic review and meta-analysis of twin studies. PLoS One 6, e25326, https://doi.org/10.1371/journal.pone.0025326 (2011).
Zuhlke, L. et al. Clinical Outcomes in 3343 Children and Adults With Rheumatic Heart Disease From 14 Low- and Middle-Income Countries: Two-Year Follow-Up of the Global Rheumatic Heart Disease Registry (the REMEDY Study). Circulation 134, 1456–1466, https://doi.org/10.1161/circulationaha.116.024769 (2016).
Agbor, V. N. et al. Heart failure in sub-Saharan Africa: A contemporaneous systematic review and meta-analysis. International journal of cardiology 257, 207–215, https://doi.org/10.1016/j.ijcard.2017.12.048 (2018).
Nyaga, U. F. et al. Data on the epidemiology of heart failure in Sub-Saharan Africa. Data in brief 17, 1218–1239, https://doi.org/10.1016/j.dib.2018.01.100 (2018).
GBD. 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544, https://doi.org/10.1016/s0140-6736(16)31012-1 (2016).
Watkins, D. A., Zuhlke, L. J., Engel, M. E. & Mayosi, B. M. Rheumatic fever: neglected again. Science (New York, N.Y.) 324, 37, https://doi.org/10.1126/science.324.5923.37b (2009).
Remenyi, B., Carapetis, J., Wyber, R., Taubert, K. & Mayosi, B. M. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nature reviews. Cardiology 10, 284–292, https://doi.org/10.1038/nrcardio.2013.34 (2013).
Zuhlke, L. J. & Steer, A. C. Estimates of the global burden of rheumatic heart disease. Global heart 8, 189–195, https://doi.org/10.1016/j.gheart.2013.08.008 (2013).
Rothenbuhler, M. et al. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta-analysis of prevalence among children and adolescents. The Lancet. Global health 2, e717–726, https://doi.org/10.1016/s2214-109x(14)70310-9 (2014).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62, 1006–1012, https://doi.org/10.1016/j.jclinepi.2009.06.005 (2009).
Hoy, D. et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 65, 934–939, https://doi.org/10.1016/j.jclinepi.2011.11.014 (2012).
The World Bank. GDP per capita (current US$), https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (2017).
WHO. Global Burden of Disease Regions used for WHO-CHOICE Analyses, http://www.who.int/choice/demography/regions/en/ (2018).
United Nations Statistical Division. Methodology, https://unstats.un.org/unsd/methodology/m49/ (2018).
Watkins, D. A. et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990-2015. The New England journal of medicine 377, 713–722, https://doi.org/10.1056/NEJMoa1603693 (2017).
United Nations Development Program. Human Development Reports, http://hdr.undp.org/en/countries (2019).
The World Bank. World Bank Country and Lending Groups, https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (2018).
The World Bank. GINI index (World Bank estimate), https://data.worldbank.org/indicator/SI.POV.GINI (2018).
Barendregt, J. J., Doi, S. A., Lee, Y. Y., Norman, R. E. & Vos, T. Meta-analysis of prevalence. Journal of epidemiology and community health 67, 974–978, https://doi.org/10.1136/jech-2013-203104 (2013).
Cochran, W. G. The Combination of Estimates from Different Experiments. Biometrics 10, 101–129, https://doi.org/10.2307/3001666 (1954).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, https://doi.org/10.1002/sim.1186 (2002).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–634, https://doi.org/10.1136/bmj.315.7109.629 (1997).
Engelman, D. et al. Screening for rheumatic heart disease: quality and agreement of focused cardiac ultrasound by briefly trained health workers. BMC cardiovascular disorders 16, 30, https://doi.org/10.1186/s12872-016-0205-7 (2016).
Kwok, M. et al. Prevalence of rheumatic heart disease in urban and rural angola by echocardiography. Journal of the American Society of Echocardiography 27, B17 (2014).
Ledos, P. H., Kamblock, J., Bourgoin, P., Eono, P. & Carapetis, J. R. Prevalence of rheumatic heart disease in young adults from New Caledonia. Archives of cardiovascular diseases 108, 16–22, https://doi.org/10.1016/j.acvd.2014.07.053 (2015).
Mirabel, M. et al. Echocardiography screening to detect rheumatic heart disease: A cohort study of schoolchildren in French Pacific Islands. International journal of cardiology 188, 89–95, https://doi.org/10.1016/j.ijcard.2015.04.007 (2015).
Mucumbitsi, J. et al. Prevalence of rheumatic valvular heart disease in Rwandan school children: echocardiographic evaluation using the World Heart Federation criteria. Cardiovascular journal of Africa 28, 1–8, https://doi.org/10.5830/cvja-2017-007 (2017).
Nascimento, B. R. et al. Echocardiographic prevalence of rheumatic heart disease in Brazilian schoolchildren: Data from the PROVAR study. International journal of cardiology 219, 439–445, https://doi.org/10.1016/j.ijcard.2016.06.088 (2016).
Ngaide, A. A. et al. Prevalence of rheumatic heart disease in Senegalese school children: a clinical and echocardiographic screening. Heart Asia 7, 40–45, https://doi.org/10.1136/heartasia-2015-010664 (2015).
Engel, M. E. et al. Prevalence of rheumatic heart disease in 4720 asymptomatic scholars from South Africa and Ethiopia. Heart (British Cardiac Society) 101, 1389–1394, https://doi.org/10.1136/heartjnl-2015-307444 (2015).
Gemechu, T., Mahmoud, H., Parry, E. H., Phillips, D. I. & Yacoub, M. H. Community-based prevalence study of rheumatic heart disease in rural Ethiopia. European journal of preventive cardiology 24, 717–723, https://doi.org/10.1177/2047487316687104 (2017).
Yadeta, D. et al. Prevalence of rheumatic heart disease among school children in Ethiopia: A multisite echocardiography-based screening. International journal of cardiology 221, 260–263, https://doi.org/10.1016/j.ijcard.2016.06.232 (2016).
Gleason, B. et al. Prevalence of Latent Rheumatic Heart Disease Among HIV-Infected Children in Kampala, Uganda. Jaids-Journal of Acquired Immune Deficiency Syndromes 71, 196–199, https://doi.org/10.1097/qai.0000000000000827 (2016).
Kennedy, L. et al. RHD prevention and control programme in Tuvalu. Global heart 9, e119, https://doi.org/10.1016/j.gheart.2014.03.1646 (2014).
Kumari, N. R. et al. Prevalence of rheumatic and congenital heart disease in school children of Andhra Pradesh, South India. Journal of cardiovascular disease research 4, 11–14, https://doi.org/10.1016/j.jcdr.2012.11.003 (2013).
Longo-Mbenza, B. et al. Survey of rheumatic heart disease in school children of Kinshasa town. International journal of cardiology 63, 287–294 (1998).
Mulatu, H. A., Woldemichael, M. & Aberra, M. Prevalence of Rheumatic Heart Disease among Primary School Students in Mid-Eastern Ethiopia. Biol syst Open Access 5, 1000149, https://doi.org/10.4172/2329-6577.1000149 (2016).
Marijon, E. et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. The New England journal of medicine 357, 470–476, https://doi.org/10.1056/NEJMoa065085 (2007).
Negi, P. C. et al. Epidemiological trends of RF/RHD in school children of Shimla in north India. The Indian journal of medical research 137, 1121–1127 (2013).
Paar, J. A. et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. The American journal of cardiology 105, 1809–1814, https://doi.org/10.1016/j.amjcard.2010.01.364 (2010).
Ba-Saddik, I. A. et al. Prevalence of rheumatic heart disease among school-children in Aden, Yemen. Annals of tropical paediatrics 31, 37–46, https://doi.org/10.1179/1465328110y.0000000007 (2011).
Beaton, A. et al. Echocardiography screening for rheumatic heart disease in Ugandan schoolchildren. Circulation 125, 3127–3132, https://doi.org/10.1161/circulationaha.112.092312 (2012).
Bhaya, M., Panwar, S., Beniwal, R. & Panwar, R. B. High prevalence of rheumatic heart disease detected by echocardiography in school children. Echocardiography (Mount Kisco, N.Y.) 27, 448–453, https://doi.org/10.1111/j.1540-8175.2009.01055.x (2010).
Colquhoun, S. M. et al. Echocardiographic screening in a resource poor setting: borderline rheumatic heart disease could be a normal variant. International journal of cardiology 173, 284–289, https://doi.org/10.1016/j.ijcard.2014.03.004 (2014).
Thakur, J. S., Negi, P. C., Ahluwalia, S. K. & Vaidya, N. K. Epidemiological survey of rheumatic heart disease among school children in the Shimla Hills of northern India: prevalence and risk factors. Journal of epidemiology and community health 50, 62–67 (1996).
Bodian, M. et al. Prévalence des cardiopathies congénitales en milieu scolaire coranique (daara) à Dakar: étude transversale basée sur le dépistage clinique et échographique de 2019 élèves. Bulletin de la Société de pathologie exotique 108, 32–35, https://doi.org/10.1007/s13149-014-0410-5 (2015).
Cramp, G. et al. Undetected rheumatic heart disease revealed using portable echocardiography in a population of school students in Tairawhiti, New Zealand. The New Zealand medical journal 125, 53–64 (2012).
Chaikitpinyo, A. et al. Rheumatic and congenital heart diseases among school children of Khon Kaen, Thailand: Declining prevalence of rheumatic heart disease. Asian Biomedicine 8, 645–650, https://doi.org/10.5372/1905-7415.0805.339 (2014).
Jose, V. J. & Gomathi, M. Declining prevalence of rheumatic heart disease in rural schoolchildren in India: 2001-2002. Indian heart journal 55, 158–160 (2003).
Kane, A. et al. Echocardiographic screening for rheumatic heart disease: Age matters. International journal of cardiology 168, 888–891, https://doi.org/10.1016/j.ijcard.2012.10.090 (2013).
Periwal, K. L. et al. Prevalence of rheumatic heart disease in school children in Bikaner: an echocardiographic study. The Journal of the Association of Physicians of India 54, 279–282 (2006).
Poyyamozhi, J. S., Rushender, C. R. & Dinesh Kumar, G. Prevalence Of Rheumatic Heart Disease In Urban School Going Children In South India, A Community Based Cross Sectional Study. National. Journal of Research in Community Medicine 5, 280–287 (2016).
Sriharibabu, M., Himabindu, Y. & Kabir, Z. Rheumatic heart disease in rural south India: A clinico-observational study. Journal of cardiovascular disease research 4, 25–29, https://doi.org/10.1016/j.jcdr.2013.02.011 (2013).
Ahmed, J., Mostafa Zaman, M. & Monzur Hassan, M. M. Prevalence of rheumatic fever and rheumatic heart disease in rural Bangladesh. Tropical doctor 35, 160–161, https://doi.org/10.1258/0049475054620879 (2005).
Al-Munibari, A. N., Nasher, T. M., Ismail, S. A. & Mukhtar, E. D. A. Prevalence of rheumatic fever and rheumatic heart disease in Yemen. Asian Cardiovascular and Thoracic Annals 9, 41–44 (2001).
Baroux, N. et al. High prevalence of rheumatic heart disease in schoolchildren detected by echocardiography screening in New Caledonia. Journal of paediatrics and child health 49, 109–114, https://doi.org/10.1111/jpc.12087 (2013).
Bhardwaj, R. et al. Prevalence of rheumatic fever and rheumatic heart disease in rural population of Himachal–a population based study. The Journal of the Association of Physicians of India 60, 13–14 (2012).
Grover, A. et al. Epidemiology of rheumatic fever and rheumatic heart disease in a rural community in northern India. Bulletin of the World Health Organization 71, 59–66 (1993).
Zaman, M. M., Choudhury, S. R., Rahman, S. & Ahmed, J. Prevalence of rheumatic fever and rheumatic heart disease in Bangladeshi children. Indian heart journal 67, 45–49, https://doi.org/10.1016/j.ihj.2015.02.009 (2015).
Zhimin, W. et al. Prevalence of chronic rheumatic heart disease in Chinese adults. International journal of cardiology 107, 356–359, https://doi.org/10.1016/j.ijcard.2005.03.048 (2006).
Rizvi, S. F. et al. Status of rheumatic heart disease in rural Pakistan. Heart (British Cardiac Society) 90, 394–399 (2004).
Carapetis, J. R. et al. Evaluation of a screening protocol using auscultation and portable echocardiography to detect asymptomatic rheumatic heart disease in Tongan schoolchildren. Nature clinical practice. Cardiovascular medicine 5, 411–417, https://doi.org/10.1038/ncpcardio1185 (2008).
Steer, A. C. et al. High prevalence of rheumatic heart disease by clinical and echocardiographic screening among children in Fiji. The Journal of heart valve disease 18, 327-335; discussion 336 (2009).
Nair, B. et al. Rheumatic Heart Disease in Kerala: A Vanishing Entity? An Echo Doppler Study in 5-15-Years-Old School Children. International journal of rheumatology 2015, 930790, https://doi.org/10.1155/2015/930790 (2015).
El-Aroussy, W. et al. Prevalence of Rheumatic Valvular Heart Disease Among Egyptian School Children: An Echocardiographic Screening. The Medical Journal of Cairo University 81, 139–144 (2013).
Gul, A., Hassan, M. U. & Hafizullah, M. Rheumatic Heart Disease in urban school children of Peshawar. JPMI - Journal of Postgraduate Medical Institute 23, 337–340 (2009).
Kennedy, L. et al. Burden of rheumatic heart disease in the Solomon Islands. Global heart 9, e260, https://doi.org/10.1016/j.gheart.2014.03.2151 (2014).
Allen, M. et al. A rapid echocardiographic screening protocol for rheumatic heart disease in Samoa: a high prevalence of advanced disease. Cardiology in the young 27, 1599–1605, https://doi.org/10.1017/s1047951117000907 (2017).
Viali, S., Futi, V. & Lafaele, T. Prevalence of rheumatic heart disease among secondary school children in Samoa detected by echocardiography screening. Global heart 11, e120, https://doi.org/10.1016/j.gheart.2016.03.416 (2016).
Spitzer, E. et al. Screening for Rheumatic Heart Disease among Peruvian Children: A Two-Stage Sampling Observational Study. PloS one 10, https://doi.org/10.1371/journal.pone.0133004 (2015).
Singh, T. R. et al. Prevalence of subclinical RHD detected by echocardiography - Doppler study using WHF 2012 criteria in school going children aged 5-15 years of Manipur, a North-Eastern Indian state. Annals of pediatric cardiology 7, S35–S36 (2014).
Shrestha, N. R. et al. Prevalence of Subclinical Rheumatic Heart Disease in Eastern Nepal: A School-Based Cross-sectional Study. JAMA cardiology 1, 89–96, https://doi.org/10.1001/jamacardio.2015.0292 (2016).
Saxena, A. et al. Echocardiographic prevalence of rheumatic heart disease in Indian school children using World Heart Federation criteria - A multi site extension of RHEUMATIC study (the e-RHEUMATIC study). International journal of cardiology, https://doi.org/10.1016/j.ijcard.2017.09.184 (2017).
Sanyahumbi, A. S. et al. School and Community Screening Shows Malawi, Africa, to Have a High Prevalence of Latent Rheumatic Heart Disease. Congenital heart disease 11, 615–621 (2016).
Rossi, E., Felici, A. R. & Banteyrga, L. Subclinical rheumatic heart disease in an Eritrean high-school population, detected by echocardiography. The Journal of heart valve disease 23, 235–239 (2014).
Roberts, K. et al. Echocardiographic Screening for Rheumatic Heart Disease in High and Low Risk Australian Children. Circulation 129, 1953–1961, https://doi.org/10.1161/circulationaha.113.003495 (2014).
Ploutz, M. et al. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart (British Cardiac Society) 102, 35–39, https://doi.org/10.1136/heartjnl-2015-308236 (2016).
Huang, J. H. et al. Echocardiographic Screening of Rheumatic Heart Disease in American Samoa. Pediatric cardiology, https://doi.org/10.1007/s00246-017-1724-4 (2017).
Fakakovikaetau, T. et al. Echocardiography screening for RHD of 5-15 years old children saves lives. Global heart 11, e71, https://doi.org/10.1016/j.gheart.2016.03.251 (2016).
Campanale, C. M. et al. Prevalence of rheumatic heart disease in North Madagascar: An echocardiographic screening in young and adult populations. Australasian Medical Journal 10, 620–627, https://doi.org/10.21767/AMJ.2017.3047 (2017).
Beaton, A. et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: a field study. European heart journal cardiovascular Imaging 16, 475–482, https://doi.org/10.1093/ehjci/jeu296 (2015).
Yildiz, A. et al. Echocardiography screening for rheumatic heart disease among schoolchildren in southeastern anatolia region of Turkey. European heart journal 37, 461, https://doi.org/10.1093/eurheartj/ehw432 (2016).
Yadav, P., Joshi, P., Gupta, J., Joseph, D. & Sakhi, P. Prevalence of rheumatic fever and rheumatic heart disease in school children in Malwa region of MP. National. Journal of Community Medicine 1, 156–158 (2010).
Webb, R. H. et al. Optimising echocardiographic screening for rheumatic heart disease in New Zealand: not all valve disease is rheumatic. Cardiology in the young 21, 436–443, https://doi.org/10.1017/s1047951111000266 (2011).
Sadoh, W. E., Omuemu, V. O. & Israel-aina, Y. T. Prevalence of rheumatic heart disease among primary school pupils in Mid-Western Nigeria. East African medical journal 90, 28–32 (2013).
Regmi, P. R. & Pandey, M. R. Prevalence of rheumatic fever and rheumatic heart disease in school children of Kathmandu city. Indian heart journal 49, 518–520 (1997).
Mahmoudi, M. J., Rezaei, N. & Mahmoudi, M. The prevalence of rheumatic heart disease among school children in urban areas of Hamadan province. Annals of Military and Health Sciences Research 1, 105–110 (2003).
Kaul, R.-U. R., Masoodi, M. A., Wani, K. A., Hamdy, G. & Qureshi, K. A. Prevalence of Rheumatic Heart Disease in School Children (5-15 years) in a Rural Block of Srinagar. JK Practitioner 12, 160–162 (2005).
Gautam, S. Prevalence and pattern of Rheumatic Heart Disease in School children from Eastern part of underdeveloped, poor country Nepal, A cross-sectional, observational study. Global heart 9, e159, https://doi.org/10.1016/j.gheart.2014.03.1793 (2014).
Farrag, A. A. M., El Aroussy, W. A., El Hagracy, N., Fawzy, H. & Taha, N. Echocardiographic screening for Rheumatic Valvular Heart Disease among Egyptian school children. Global heart 9, e158–e159, https://doi.org/10.1016/j.gheart.2014.03.1792 (2014).
Abdel-Moula, A. M. et al. Prevalence of rheumatic heart disease among school children in Alexandria, Egypt: a prospective epidemiological study. The Journal of the Egyptian Public Health Association 73, 233–254 (1998).
Uener, A. et al. The ratio of congenital heart disease and innocent murmur in children in Van city, the Eastern Turkey. Anadolu Kardiyoloji Dergisi-the Anatolian Journal of Cardiology 9, 29–34 (2009).
Schaffer, W. L. et al. Prevalence and correlates of rheumatic heart disease in American Indians (The Strong Heart Study). American Journal of Cardiology 91, 1379–1382, https://doi.org/10.1016/s0002-9149(03)00338-2 (2003).
Sadiq, M. et al. Prevalence of rheumatic heart disease in school children of urban Lahore. Heart (British Cardiac Society) 95, 353–357, https://doi.org/10.1136/hrt.2008.143982 (2009).
Oli, K. & Porteous, J. Prevalence of rheumatic heart disease among school children in Addis Ababa. East African medical journal 76, 601–605 (1999).
Misra, M. et al. Prevalence of rheumatic heart disease in school-going children of Eastern Uttar Pradesh. Indian heart journal 59, 42–43 (2007).
Anabwani, G. M. & Bonhoeffer, P. Prevalence of heart disease in school children in rural Kenya using colour-flow echocardiography. East African medical journal 73, 215–217 (1996).
Zuhlke, L. J., Engel, M. E., Nkepu, S. & Mayosi, B. M. Evaluation of a focussed protocol for hand-held echocardiography and computer-assisted auscultation in detecting latent rheumatic heart disease in scholars. Cardiology in the young 26, 1097–1106, https://doi.org/10.1017/s1047951115001857 (2016).
Remenyi, B. et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease–an evidence-based guideline. Nature reviews. Cardiology 9, 297–309, https://doi.org/10.1038/nrcardio.2012.7 (2012).
Okello, E. et al. Socioeconomic and environmental risk factors among rheumatic heart disease patients in Uganda. PloS one 7, e43917, https://doi.org/10.1371/journal.pone.0043917 (2012).
Marijon, E., Mirabel, M., Celermajer, D. S. & Jouven, X. Rheumatic heart disease. Lancet (London, England) 379, 953–964, https://doi.org/10.1016/s0140-6736(11)61171-9 (2012).
Saxena, A. et al. Prevalence and outcome of subclinical rheumatic heart disease in India: the RHEUMATIC (Rheumatic Heart Echo Utilisation and Monitoring Actuarial Trends in Indian Children) study. Heart (British Cardiac Society) 97, 2018–2022, https://doi.org/10.1136/heartjnl-2011-300792 (2011).
Zuhlke, L. et al. The natural history of latent rheumatic heart disease in a 5 year follow-up study: a prospective observational study. BMC cardiovascular disorders 16, 46, https://doi.org/10.1186/s12872-016-0225-3 (2016).
WHO. Rheumatic Fever and Rheumatic Heart Disease: report of a WHO Expert Consultation. (WHO, Geneva, Switzerland, 2001).
Zuehlke, L. et al. The natural history of latent rheumatic heart disease in a 5 year follow-up study: a prospective observational study. BMC cardiovascular disorders 16, https://doi.org/10.1186/s12872-016-0225-3 (2016).
Saxena, A. Increasing detection of rheumatic heart disease with echocardiography. Expert review of medical devices 11, 491–497, https://doi.org/10.1586/17434440.2014.930661 (2014).
Mayosi, B. M. The challenge of silent rheumatic heart disease. The Lancet. Global health 2, e677–678, https://doi.org/10.1016/s2214-109x(14)70331-6 (2014).
Bhaya, M., Beniwal, R., Panwar, S. & Panwar, R. B. Two years of follow-up validates the echocardiographic criteria for the diagnosis and screening of rheumatic heart disease in asymptomatic populations. Echocardiography (Mount Kisco, N.Y.) 28, 929–933, https://doi.org/10.1111/j.1540-8175.2011.01487.x (2011).
Beaton, A. et al. Latent Rheumatic Heart Disease: Identifying the Children at Highest Risk of Unfavorable Outcome. Circulation, https://doi.org/10.1161/circulationaha.117.029936 (2017).
Engelman, D. et al. Screening-detected rheumatic heart disease can progress to severe disease. Heart Asia 8, 67–73, https://doi.org/10.1136/heartasia-2016-010847 (2016).
Mayosi, B. M. Screening for Rheumatic Heart Disease in Eastern Nepal. JAMA cardiology 1, 96–97, https://doi.org/10.1001/jamacardio.2015.0303 (2016).
Carapetis, J. R. et al. Acute rheumatic fever and rheumatic heart disease. Nature Reviews Disease Primers 2, https://doi.org/10.1038/nrdp.2015.84 (2016).
Tompkins, D. G., Boxerbaum, B. & Liebman, J. Long-term prognosis of rheumatic fever patients receiving regular intramuscular benzathine penicillin. Circulation 45, 543–551, https://doi.org/10.1161/01.cir.45.3.543 (1972).
Watkins, D. A., Mvundura, M., Nordet, P. & Mayosi, B. M. A cost-effectiveness analysis of a program to control rheumatic fever and rheumatic heart disease in Pinar del Rio, Cuba. PloS one 10, e0121363, https://doi.org/10.1371/journal.pone.0121363 (2015).
Dougherty, S., Khorsandi, M. & Herbst, P. Rheumatic heart disease screening: Current concepts and challenges. Annals of pediatric cardiology 10, 39–49, https://doi.org/10.4103/0974-2069.197051 (2017).
Author information
Authors and Affiliations
Contributions
J.J.N. conceived the study. J.J.N., J.J.B. and A.D.K. did the literature search. J.J.N., U.F.N. and V.N.A. selected the studies and extracted the relevant information. J.J.N., A.D.K., J.J.B. and V.N.A. synthesized the data. J.J.N., V.N.A., A.D.K. and J.J.B. wrote the first draft of the paper. J.J.N., A.D.K., J.J.B., U.F.N., V.N.A. and B.M.M. critically revised successive drafts of the paper and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noubiap, J.J., Agbor, V.N., Bigna, J.J. et al. Prevalence and progression of rheumatic heart disease: a global systematic review and meta-analysis of population-based echocardiographic studies. Sci Rep 9, 17022 (2019). https://doi.org/10.1038/s41598-019-53540-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53540-4
This article is cited by
-
2023 World Heart Federation guidelines for the echocardiographic diagnosis of rheumatic heart disease
Nature Reviews Cardiology (2024)
-
The prevalence of rheumatic heart disease in Ethiopia: a systematic review and meta-analysis
Tropical Diseases, Travel Medicine and Vaccines (2023)
-
Analysis and comparison of the trends in burden of rheumatic heart disease in China and worldwide from 1990 to 2019
BMC Cardiovascular Disorders (2023)
-
Systematic review of first-in-human and early phase clinical trials for surgically implantable biological mitral valve substitutes
Journal of Cardiothoracic Surgery (2023)
-
Global burden of rheumatic heart disease: trends from 1990 to 2019
Arthritis Research & Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.