Abstract
Recent studies have implicated B cells in atherosclerosis and have verified the atheroprotective effect of rituximab. Rituximab is widely used for desensitization in ABO-incompatible or crossmatch-positive kidney transplantation (KT). Using a single-center KT database, we performed propensity-matched analysis to investigate the association between rituximab and posttransplant atherosclerotic cardiovascular disease (ASCVD). Among 1299 eligible patients, 239 given rituximab induction were matched with 401 controls in a 1:2 propensity score matching process. The cumulative rate of ASCVD during 8 years of follow-up was significantly lower in rituximab-treated patients, compared with matched controls (3.7% vs. 11.2%; P = 0.012). However, all-cause mortality did not differ by group (2.9% vs. 4%; P = 0.943). In multivariable Cox analysis, rituximab proved independently protective of ASCVD (hazard ratio = 0.34, 95% confidence interval: 0.14–0.83). The lower risk of ASCVD seen with rituximab induction reached significance only in patient subsets of diabetes mellitus, pretransplant dialysis, or older age (>50 years). Rituximab induction confers a lower risk of ASCVD during the posttransplant period. This atheroprotective effect appears particularly beneficial in patients whose risk of ASCVD is heightened.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the leading cause of death after kidney transplantation (KT)1, and the incidence of CVD is higher in KT recipients than in the general population2. Numerous studies reported that the risk of CVD is elevated after KT because of non-traditional risk factors such as immunologic alteration and the various medications related to the transplantation surgery3,4,5,6,7,8,9. Atherosclerosis is an underlying chronic inflammatory condition in CVD, and the role of the immune system in the development of atherosclerosis is well descried in the literature10. B lymphocytes were considered to have a protective effect against atherosclerosis until nearly 201011. However, recent experimental studies revealed opposing roles of different B cell subsets, atheroprotective B1 cells and atherogenic B2 cells12,13,14,15,16. Those studies also demonstrated reduced atherosclerotic plaques in atherosclerosis-prone mice treated with rituximab, a B cell-depleting anti-CD20 monoclonal antibody. The potential of B cell targeting as a treatment for atherogenesis has been suggested17, although heretofore there was no comparative human study of B cell depletion and atherosclerotic CVD (ASCVD).
Rituximab was introduced as an effective alternative to splenectomy for desensitization of patients requiring KT18. Given the excellent outcomes reported in ABO-incompatible and crossmatch-positive KT19,20, rituximab use in the realm of KT is increasing. Herein, we sought to explore the effects of rituximab induction on ASCVD in the aftermath of KT through propensity-matched analysis.
Results
Descriptions of entire cohort
For the 1299 eligible patients, mean age was 45.3 ± 11.3 years, and there were 761 men (58.6%). Nine hundred forty-five patients (72.7%) underwent living donor kidney transplantation (LDKT), and in 126 (9.7%), retransplantation took place. One hundred ten grafts were lost including 46 deaths (7 as cardiovascular deaths) and 77 ASCVDs occurred during a mean follow-up of 82.3 ± 35.5 months. Rituximab was administered preoperatively to 245 patients (18.9%; 117 ABO incompatibility, 43 crossmatch positivity, 13 simultaneous ABO incompatibility and crossmatch positivity, and 72 high reactivity to panel antibodies). Among patients treated with rituximab, 167 (68.1%) were given standard doses (375 mg/m2), and 78 (31.9%) received reduced doses (200 mg).
Baseline characteristics before and after matching
Table 1 shows baseline characteristics of rituximab and control group patients. Before matching, there were differences in some variables, such as sex, deceased donor, duration of dialysis, retransplantation, tacrolimus use, pretransplant diabetes melitus (DM), baseline Low-density lipoprotein (LDL), biopsy-proven acute rejection (BPAR) within 1 year, and estimated glomerular filtration rate (eGFR) at 1 month. In 1:2 propensity score matching, 239 patients given rituximab were matched with 401 controls. Most variables (except BPAR within 1 year) were then balanced within the two groups (see Statistical analysis).
ASCVD and all-cause death in matched cohort
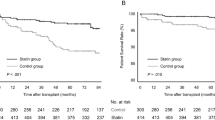
In the matched cohort, patients undergoing rituximab induction experienced only 6 ASCVD events within 8 years after KT (1 fatal myocardial infarction [MI] and 5 percutaneous coronary revascularizations). However, 35 ASCD events were recorded in the control group (3 fatal MIs, 1 nonfatal MI, 8 percutaneous coronary revascularizations, 4 coronary artery bypass surgeries, 8 acute cerebral infarctions, and 11 peripheral artery revascularizations). The cumulative rate of ASCVD was significantly lower in rituximab-treated patients than in controls (3.7% vs. 11.2%, P = 0.012; Fig. 1a). Seven deaths occurred in the rituximab group, including one cardiovascular death. Among 13 posttransplant deaths in the control group, three were from cardiovascular causes. During the 8-year follow-up period, rituximab-treated patients and controls showed no significant difference in all-cause death (2.9% vs. 4%, P = 0.943; Fig. 1b). ASCVD events and causes of death in both groups are detailed in Table 2.
Serial comparison of blood pressure, renal function, and LDL
Figure 2 depicts changes over time in several variables known to be CVD risk factors. Although systolic blood pressure (SBP) and diastolic blood pressure (DBP) were higher in treated patients (vs controls) 1 month after transplantation, both levels appeared similar thereafter at most points in time. eGFR did not differ by group up to 1 year posttransplantation, surging higher in controls than in rituximab-treated patients at Years 2 and 3. LDL was higher in controls at 6 months only, approaching levels similar to treated patients for the duration of follow-up.
Cox proportional hazard analysis for ASCVD
In multivariable Cox analysis of matched cohort data (Table 3), rituximab emerged as independently protective of ASCVD developing after KT (hazard ratio [HR] = 0.34, 95% confidence interval [CI]: 0.14–0.83). Other risk factors for ASCVD were maleness (HR = 2.14, 95% CI: 1.01–4.52), duration of dialysis (HR = 1.07, 95% CI: 1.03–1.11 [per 6 months]), retransplantation (HR = 2.90, 95% CI: 1.34–6.27), pretransplant DM (HR = 3.68, 95% CI: 1.71–7.88), and pretransplant ASCVD history (HR = 4.92, 95% CI: 2.42–10.03). BPAR within 1 year, which differed in rituximab and control groups, was not a risk factor (HR = 0.95, 95% CI: 0.40–2.25). LDL cholesterol, statin use, blood pressure, and eGFR at 1 month were not associated with posttransplant ASCVD.
Subgroup analysis
Subjects of the matched cohort were stratified by pretransplant DM, sex, pretransplant dialysis, and age ≥ 50 years, followed by analyzing HRs of rituximab treatment for ASCVD in each subgroup (Fig. 3). There was no apparent interaction between any subgroup and rituximab treatment. HRs of patients with and without DM were 0.34 (95% CI: 0.12–0.99; P = 0.048) and 0.32 (95% CI: 0.07–1.15; P = 0.140), respectively. Respective HRs of male and female patients were 0.45 (95% CI: 0.17–1.18; P = 0.103) and 0.20 (95% CI: 0.03–1.57; P = 0.125), neither showing significance. In the presence and absence of dialysis prior to KT, HRs were 0.25 (95% CI: 0.07–0.82; P = 0.023) and 0.81 (95% CI: 0.15–4.31; P = 0.805), respectively; and HRs of patients beyond or less than 50 years old were 0.61 (95% CI: 0.20–1.88; P = 0.020) and 0.61 (95% CI: 0.20–1.88; P = 0.390), respectively.
Hazard ratios for posttransplant ASCVD in clinical subgroups treated with rituximab. From matched cohort, subjects were stratified by DM, sex, pretransplant dialysis and 50 years of age. CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; KT, kidney transplantation; ASCVD, atherosclerotic cardiovascular disease.
Discussion
In the propensity-matched analysis herein, rituximab induction significantly reduced the incidence of ASCVD developing after KT. Prior in vivo studies demonstrated that the use of anti-B-cell agents could be an effective therapeutic approach for the treatment and prevention of atherosclerosis15. Few studies have investigated the atheroprotective effect of rituximab using clinical data. Hsue et al.21 reported that rituximab improved endothelial function and reduced inflammation in patients with rheumatoid arthritis; however, they did not show a difference in ASCVD based on rituximab treatment.
There has been concern about cardiac complications after the use of rituximab to treat hematologic or rheumatologic diseases22,23,24. However, acute or delayed cardiac toxicity was suggested to be a result of cytokine release related to the infusion-related reaction rather than an aggravation of atherosclerosis. In addition, several randomized controlled trials showed that there was no difference in cardiac adverse events between patients with or without rituximab treatment for lymphoma and rheumatoid arthritis25,26.
In the field of renal transplantation, there was only one report on rituximab and CVD. Tyden et al.27 reported higher mortality from MI in patients treated with rituximab than in controls. That result came from a 3-year follow-up of a randomized control trial, but the number of participants was small, and there was no information about CVD risk factors. Also, the incidence of fatal MI was higher (about 10% during just 3 years of follow-up) compared with previous reports4,5,28. The high rate of cardiac mortality might be attributable to other CVD-related patient factors rather than to an adverse effect of rituximab. In fact, another recently conducted randomized controlled trial has established a safety profile for rituximab in terms of serious adverse events in KT29.
Rituximab is a monoclonal antibody that globally destroys CD 20-positive B cells. However, the aforementioned experimental evidence demonstrated that rituximab reduced atherosclerosis. That atheroprotective effect might come from the fact that atherogenic B2 cells are a major population of the adult human B cell repertoire30. Another hypothesis is that because the peritoneal cavity provides rituximab resistance, resident B1 cells may be spared31. Although the mechanism of the atheroprotective effect of rituximab is not yet known in humans, our results provide an important hint for investigating the role of B cell subsets in atherogenesis.
We found that the incidence of ASCVD was relatively low in our study population. Previous large-volume studies in Western populations have cited CVD rates of ~15% during 4–5 years of follow-up after KT8,32, whereas that rate was only 6% during entire follow-up in our study. The difference might be due to dietary and lifestyle differences as well as the lower incidence of pretransplant ASCVD observed in Korea, compared to Western countries. Furthermore, more than 20% of patients were using statin before KT, so the mean LDL level (84.4 mg/dL) in our study exceeded by the mean (100 mg/dL) determined in Western studies. The relatively low incidence of CVD in Korean KT patients was also observed in another Korean study33 and in a previous international cohort4.
Anti-B-cell agents, including rituximab and B cell activating factor receptor antibody (belimumab), have been suggested as possible anti-atherogenic treatments17, but there has not yet been a clinical study. In a situation where the number of KTs with prior desensitization is increasing34, especially in Korea where LDKT prevails35,36, our results provide clinical evidence to help understand the role of rituximab in ASCVD development.
Through subgroup analysis, we extended our investigation to various CVD risk factors and their influence on rituximab atheroprotection. Rituximab proved significantly protective in specific clinical subsets, namely DM, pretransplant dialysis, and older age ( ≥ 50 years); but no HRs proved significant in the absence of these factors. In addition, gender of KT recipients had no apparent impact on ASCVD in rituximab-treated patients. Adjusted HRs were unobtainable for subgroups, given the low event totals. However, assessment of matched subgroups revealed greater rituximab atheroprotection in patients at higher risk of ASCVD. A broad multicenter study, perhaps utilizing a national registry, would likely yield more conclusive results.
Despite our propensity-matched analysis, retrospective feature is still limitation of our study for which the results should be cautiously interpreted. Also, single center design is another major limitation. Prospective and multicenter study should be needed.
In conclusion, this study has demonstrated that rituximab induction reduces the rate of ASCVD during posttransplant periods. Furthermore, the atheroprotective effect of rituximab appears more advantageous for some KT recipients with heightened risks of ASCVD.
Methods
Patient selection
We conducted a retrospective study, accessing a database of 1,462 patients who underwent KT at Severance Hospital, Seoul, Korea, between January 2006 and December 2015. Exclusion criteria were as follows: (1) age < 18 years at time of KT, (2) multiorgan transplantation, (3) rituximab use in treating antibody-mediated rejection after KT, and (4) acute coronary disease or patient death within 1 month after KT (2 fatal MIs, 2 nonfatal MIs, 2 death from cerebrovascular accidents, 1 non-cardiovascular death, 1 peripheral artery revascularization). Ultimately, 1299 patients qualified for analysis (Fig. 4).
Data collection
We collected data on baseline characteristics of recipients and donors, lipid profiles, pretransplant use of statins, and BPAR within 1 year posttransplantation. SBP, DBP and eGFR were checked every 3 months. LDL concentrations were checked every 6 months for 1 year posttransplantation and annually thereafter. During posttransplant periods, we checked electrocardiography at least yearly. Patients with cardiac symptoms or electrocardiographic abnormalities were referred to a cardiologist for further evaluation. Those undergoing coronary revascularization were routinely followed by coronary angiography every 1–2 years, screening for restenosis or newly developed lesions. If suspected, patients were evaluated for acute cerebral infarction and peripheral arterial occlusive disease. We defined ASCVD as any of the following: fatal or nonfatal MI, coronary revascularization (by percutaneous intervention or surgery), acute cerebral infarction, or revascularization of peripheral arterial occlusion (by percutaneous intervention or surgery).
Rituximab use in desensitization protocol
Indications for rituximab induction were ABO-incompatible or crossmatch-positive KT (living donors, both instances) and ≥ 50% panel reactive antibodies prior to KT (living or deceased donors). In the event of LDKT, rituximab was given within 7–14 days in advance, delivering a single dose of 375 mg/m2. As of August 2013, rituximab dosing for ABO-incompatible LDKT was reduced to 200 mg if baseline anti-donor isoagglutinin IgG titers were ≤1:12837. For deceased donor kidney transplantation, 200 mg of rituximab was given prior to operations as indicated. Standard regimens used at our institution for preoperative desensitization and postoperative immunosuppression are well described elsewhere37,38,39.
Study outcomes
We compared composite ASCVD and all-cause mortality by rituximab induction in the matched cohort. Because the longest follow-up period in rituximab-treated patients was 102 months, we limited such comparisons to 8 years after KT. We also conducted subgroup analyses, examining various patient characteristics (DM, sex, required pretransplant dialysis, and age ≥ 50 years) to assess the potential influence of each in relation to ASCVD and rituximab use.
Statistical analysis
Propensity matching was undertaken to coordinate baseline characteristics of the two groups (with or without rituximab induction). Subsequent scores generated via logistic regression analysis included the following covariates, which differed for above groups or were known risk factors for CVD in KT recipients4,28: age, sex, deceased donor, duration of dialysis, retransplantation, tacrolimus use, smoking, pretransplant DM, pretransplant ASCVD history, baseline LDL, and eGFR at 1 month. BPAR was not matched, because rituximab-treated patients had high risk for rejection by nature, and because BPAR had no impact on development of ASCVD in our entire cohort. Using the nearest neighbor method, we matched patients given rituximab to controls at 1:2 without replacement, setting the caliper at 0.25 width. All assessments were achieved using R freeware v3.5.0 (R Foundation for Statistical Computing, Vienna, Austria) with MatchIt package40.
In comparing baseline characteristics of both groups before and after matching, data were expressed as mean ± standard deviation or median (interquartile range) for continuous variables and numbers (%) for categorical variables. Student’s t-test, Mann-Whitney U test, or chi square test was applied as needed for group comparisons. We used Kaplan-Meier analysis to estimate differences in probabilities of ASCVD and all-cause mortality according to rituximab induction and calculated P-values by log-rank test. For estimates of ASCVD, death from causes other than ASCVD served for censoring.
In the matched cohort, we invoked Cox proportional-hazards regression analysis to assess the relation between rituximab induction and posttransplant ASCVD. Multivariable Cox regression entailed backward stepwise selection, based on covariates with P-values < 0.05 in univariable analysis. Patients were further stratified by known CVD risk factors such as DM, sex, pretransplant dialysis, and age ≥ 50. To retain the homogeneity of rituximab and control groups, only matched pairs with or without specified variables qualified for subgroup analysis. Small ASCVD events in each subgroup prohibited multivariable Cox regression, so we presented only unadjusted HR of rituximab induction in subgroup analysis. All comparative and survival analyses relied on standard software (SPSS v23.0; IBM, Armonk, NY, USA), setting statistical significance at P < 0.05.
Ethics statement
This study was conducted according to the principles of the Declaration of Helsinki and the Declaration of Istanbul. It was also approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (IRB No.: 4-2018-0355) with exemption for the informed consent due to the retrospective feature of this study.
References
2017 USRDS annual data report, Vol 2 - ESRD in the United States, Chapter 5: Mortality. Am J Kidney Dis 71, S337–S350, https://doi.org/10.1053/j.ajkd.2018.01.018 (2018).
Sarnak, M. J. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108, 2154–2169, https://doi.org/10.1161/01.CIR.0000095676.90936.80 (2003).
Stoumpos, S., Jardine, A. G. & Mark, P. B. Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int 28, 10–21, https://doi.org/10.1111/tri.12413 (2015).
Israni, A. K. et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant 10, 338–353, https://doi.org/10.1111/j.1600-6143.2009.02949.x (2010).
Vanrenterghem, Y. F. et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation 85, 209–216, https://doi.org/10.1097/TP.0b013e318160254f (2008).
Marcen, R. Cardiovascular risk factors in renal transplantation–current controversies. Nephrol Dial Transplant 21(Suppl 3), iii3–8, https://doi.org/10.1093/ndt/gfl298 (2006).
Jardine, A. G. et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis 46, 529–536, https://doi.org/10.1053/j.ajkd.2005.05.014 (2005).
Holdaas, H. et al. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant 5, 2929–2936, https://doi.org/10.1111/j.1600-6143.2005.01105.x (2005).
Holdaas, H. et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 361, 2024–2031, https://doi.org/10.1016/S0140-6736(03)13638-0 (2003).
Moriya, J. Critical roles of inflammation in atherosclerosis. J Cardiol. https://doi.org/10.1016/j.jjcc.2018.05.010 (2018).
Caligiuri, G., Nicoletti, A., Poirier, B. & Hansson, G. K. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest 109, 745–753, https://doi.org/10.1172/jci7272 (2002).
Kyaw, T. et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol 185, 4410–4419, https://doi.org/10.4049/jimmunol.1000033 (2010).
Ait-Oufella, H. et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med 207, 1579–1587, https://doi.org/10.1084/jem.20100155 (2010).
Kyaw, T. et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res 109, 830–840, https://doi.org/10.1161/CIRCRESAHA.111.248542 (2011).
Tsiantoulas, D., Diehl, C. J., Witztum, J. L. & Binder, C. J. B cells and humoral immunity in atherosclerosis. Circ Res 114, 1743–1756, https://doi.org/10.1161/CIRCRESAHA.113.301145 (2014).
Kyaw, T., Tipping, P., Bobik, A. & Toh, B. H. Opposing roles of B lymphocyte subsets in atherosclerosis. Autoimmunity 50, 52–56, https://doi.org/10.1080/08916934.2017.1280669 (2017).
Tsiantoulas, D., Sage, A. P., Mallat, Z. & Binder, C. J. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler Thromb Vasc Biol 35, 296–302, https://doi.org/10.1161/ATVBAHA.114.303569 (2015).
Tanabe, K. Japanese experience of ABO-incompatible living kidney transplantation. Transplantation 84, S4–7, https://doi.org/10.1097/01.tp.0000296008.08452.4c (2007).
Orandi, B. J. et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N Engl J Med 374, 940–950, https://doi.org/10.1056/NEJMoa1508380 (2016).
Opelz, G. et al. Three-year outcomes following 1420 ABO-incompatible living-donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation 99, 400–404, https://doi.org/10.1097/TP.0000000000000312 (2015).
Hsue, P. Y. et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc 3, e001267, https://doi.org/10.1161/JAHA.114.001267 (2014).
Foran, J. M. et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol 18, 317–324, https://doi.org/10.1200/JCO.2000.18.2.317 (2000).
Armitage, J. D., Montero, C., Benner, A., Armitage, J. O. & Bociek, G. Acute coronary syndromes complicating the first infusion of rituximab. Clin Lymphoma Myeloma 8, 253–255, https://doi.org/10.3816/CLM.2008.n.035 (2008).
Mehrpooya, M., Vaseghi, G., Eshraghi, A. & Eslami, N. Delayed Myocardial Infarction Associated With Rituximab Infusion: A Case Report and Literature Review. Am J Ther 23, e283–287, https://doi.org/10.1097/MJT.0000000000000214 (2016).
Emery, P. et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE). Ann Rheum Dis 69, 1629–1635, https://doi.org/10.1136/ard.2009.119933 (2010).
Provencio, M. et al. A prospective study of left ventricle function after treatment with rapid-infusion rituximab in patients with non-Hodgkin lymphoma. Leuk Lymphoma 50, 1642–1646, https://doi.org/10.1080/10428190903186478 (2009).
Tyden, G., Ekberg, H., Tufveson, G. & Mjornstedt, L. A randomized, double-blind, placebo-controlled study of single dose rituximab as induction in renal transplantation: a 3-year follow-up. Transplantation 94, e21–22, https://doi.org/10.1097/01.tp.0000418580.88642.e1 (2012).
Soveri, I. et al. A cardiovascular risk calculator for renal transplant recipients. Transplantation 94, 57–62, https://doi.org/10.1097/TP.0b013e3182516cdc (2012).
van den Hoogen, M. W. et al. Rituximab as induction therapy after renal transplantation: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant 15, 407–416, https://doi.org/10.1111/ajt.13052 (2015).
Sagaert, X. & De Wolf-Peeters, C. Classification of B-cells according to their differentiation status, their micro-anatomical localisation and their developmental lineage. Immunology Letters 90, 179–186, https://doi.org/10.1016/j.imlet.2003.09.007 (2003).
Hamaguchi, Y. et al. The peritoneal cavity provides a protective niche for B1 and conventional B lymphocytes during anti-CD20 immunotherapy in mice. J Immunol 174, 4389–4399 (2005).
Carpenter, M. A. et al. BP, cardiovascular disease, and death in the Folic Acid for Vascular Outcome Reduction in Transplantation trial. J Am Soc Nephrol 25, 1554–1562, https://doi.org/10.1681/ASN.2013040435 (2014).
Jeong, J. C. et al. Cardiovascular diseases after kidney transplantation in Korea. J Korean Med Sci 25, 1589–1594, https://doi.org/10.3346/jkms.2010.25.11.1589 (2010).
Morath, C., Zeier, M., Dohler, B., Opelz, G. & Susal, C. ABO-Incompatible Kidney Transplantation. Front Immunol 8, 234, https://doi.org/10.3389/fimmu.2017.00234 (2017).
Ko, E. J., Yu, J. H., Yang, C. W. & Chung, B. H., Korean Organ Transplantation Registry Study, G. Clinical outcomes of ABO- and HLA-incompatible kidney transplantation: a nationwide cohort study. Transpl Int 30, 1215–1225, https://doi.org/10.1111/tri.12979 (2017).
Kwon, H. et al. Analysis of 4000 kidney transplantations in a single center: Across immunological barriers. Medicine (Baltimore) 95, e4249, https://doi.org/10.1097/md.0000000000004249 (2016).
Lee, J. et al. The effect of rituximab dose on infectious complications in ABO-incompatible kidney transplantation. Nephrol Dial Transplant 31, 1013–1021, https://doi.org/10.1093/ndt/gfw017 (2016).
Song, S. H. et al. Successful launch of an ABO-incompatible kidney transplantation program to overcome the shortage of compatible living donors: experience at a single center. Clin Nephrol 88, 117–123, https://doi.org/10.5414/CN109114 (2017).
Huh, K. H. et al. Renal transplantation in sensitized recipients with positive luminex and negative CDC (complement-dependent cytotoxicity) crossmatches. Transpl Int 25, 1131–1137, https://doi.org/10.1111/j.1432-2277.2012.01543.x (2012).
Ho, D. E., Imai, K., King, G. & Stuart, E. A. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis 15, 199–236, https://doi.org/10.1093/pan/mpl013 (2007).
Author information
Authors and Affiliations
Contributions
D.G.K., B.S.K., K.H.H. participated in research design. D.G.K., J.L. participated in data acquisition. D.G.K., W.J.S. participated in statistical analysis. J.G.L., K.H.H., M.S.K., S.I.K., Y.S.K. participated in the performance of the research. K.H.H., Y.S.K. supervised the study process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, D.G., Lee, J., Seo, W.J. et al. Rituximab protects against development of atherosclerotic cardiovascular disease after kidney transplantation: a propensity-matched study. Sci Rep 9, 16475 (2019). https://doi.org/10.1038/s41598-019-52942-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52942-8
This article is cited by
-
Cardiovascular Disease in Anti-neutrophil Cytoplasm Antibody-Associated Vasculitis
Current Rheumatology Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.