Abstract
Excess nitrogen (N) fertilizer applied to crops, which discharges to the environment, principally through denitrification, runoff, leaching, and volatilization, results in a waste of resources and pollution. Here, a high-performance loss control urea (LCU) was prepared by adding a loss control agent (LCA) with high thermal stability, large specific surface area, and good water retention capacity complex (6%) to traditional urea (94%). The existence of hydrogen bonds between LCA and N source for LCU in the presence of water enhanced N source adsorption capacity, where adsorption between LCA and NH4+-N was strongest, for urea and NO3−-N was weakest. In a laboratory experiment, cumulative losses of NH3 volatilization from soils treated with N application rates of 80, 160 and 240 kg N ha−1 were 14.8, 18.1, and 24.2% for urea, respectively, and 10.1, 12.7, and 17.5% for LCU. Simulated rapid and long-term leaching experiments showed that, compared with urea, LCU reduced N leaching loss within 30 d, and delayed long-term N leaching loss. Hydrogen bonds in LCU effectively controlled NH3 volatilization and N leaching loss. This type of LCU may optimize supply of N in soils and increase adsorption and utilization of N in crops.
Similar content being viewed by others
Introduction
Over 100 million tonnes of N fertilizer is applied annually to agricultural crops worldwide; however, in the last 30 years, the N use efficiency in cereals of China is c. 27–40%1,2,3, and much of the surplus N that is lost to the environment results in atmospheric and freshwater pollution4. Therefore, sustainable agriculture depends on achieving reductions in N inputs, while maintaining high levels of crop yield. Approaches that address this challenge tend to be at the agro-economic scale and include optimization of crop rotations, environment of sensor-based fertilizer applications, integrated crop management that uses livestock systems5,6, and improvement of crop N use efficiency7. Among these, improvement of N use efficiency is key and has been widely studied. N fertilizer not utilized by crops tends to discharge to the environment through a range of processes that include nitrification-denitrification, ammonia (NH3) volatilization, leaching, and runoff8. In addition to traditional approaches designed to improve N use efficiency, such as use of appropriate rates and deep applications of N, current techniques include site-specific and real-time N and nutrient management, non-destructive quick testing of plant N status, and application of controlled/slow release fertilizers9; that latter approach is considered to be the quickest and most convenient for reducing fertilizer losses and increasing fertilizer use efficiency10.
Reduction of NH3 volatilization and leaching losses is key in the improvement of N use efficiency, and the employment of controlled/slow release N fertilizer mainly tends to delay its availability for plant uptake and use after application, or extend its availability to the plant significantly longer than a reference ‘rapidly available nutrient fertilizer’ through a specific control mechanism11. For example, the coated N fertilizer control N interactions between crops and soils by delaying availability release; the urease inhibitor which inhibits hydrolytic action on urea by the enzyme urease; and the nitrification inhibitor which inhibits the biological oxidation of ammoniacal-N to nitrate-N. In addition, other methods tend to enhance N adsorption through the addition of high adsorption capacity substances. Moreover, The effect of controlling NH3 volatilization after using them, as stated earlier12 that the use of non-urea based fertilizers (ammonium nitrate and ammonium sulfate), urease inhibitors, controlled release fertilizers, deep placement of fertilizers, irrigation, and addition of mixed amendments (pyrite, zeolite, and organic acids) decreased NH3 volatilization by 75, 54, 68, 55, 35 and 35%, respectively. Use of ammonium nitrate or ammonium sulfate may contribute to soil acidification and lead to subsequent decreases in NH3 volatilization13,14,15, but long-term application may result in significant damage to the soil; therefore, the use of controlled release fertilizers may represent an effective and convenient approach to reduce NH3 volatilization. Sanchita Mandal16 and Chen et al.17 found that addition of biochar to soils reduced NH3 volatilization by approximately 70%18,19,20, where mitigation of N leaching losses following biochar addition is mainly attributed to NH4+ adsorption and enhanced N immobilization21,22,23, whereas the high financial cost of biochar limits its extensive use. While application of nitrification inhibitors mitigates NO3−-N leaching loss24,25,26, it may increase the loss of NH3 volatilization, influence yield and quality of partial crops and alter physicochemical properties and biological activity in soils27. Although N leaching losses from soils have been found to be prevented by trees as ecosystem service providers28, optimization of timing of tillage29, combination of low quality maize stover residue with N fertilizer30, and reductions in N application rates31,32, the efficacy of these measures is production system-dependent.
Peng et al.33 reduced N leaching losses through the use of controlled-release N fertilizer that have been developed during the past 20 years using chemical or physical techniques, such as sulfur coated, urease inhibitor, urea formaldehyde and resin coated34,35,36, and among them, the employment of the resin coating type is the most stable and extensive. Most types of controlled release coated fertilizer are encapsulated by a slow degrading polymer37,38,39 or materials that have strong adsorption properties40,41, but although these products effectively enhance N use efficiency, the problem of migration of decomposed N to crops remains unresolved. A recently developed type of fertilizer, known as loss control fertilizer (LCF), effectively reduces NH3 volatilization and leaching loss42,43,44,45 as a result of added attapulgite that is modified by high-energy electron beam irradiation and treated with O3 oxidation and hydrothermal processes46. The lack of need for polymer coating materials in LCFs during the production process facilitates single field applications and confers environmentally sustainable characteristics, such as natural degradation, so that their long-term application may improve soil physical and chemical properties, soil fertility and water retention capacity45, crop biological traits, and crop yield, while reducing N loss that contribute to improved N utilization rates47,48,49,50,51,52,53,54. However, the lack of understanding of underlying mechanisms associated with these positive effects of LCFs55 limits their potential use. In this study, we investigated effects of composition, performance, and associated mechanisms of N losses, measured as NH3 volatilization and nitrate leaching, of a loss control urea (LCU)56 as a type of LCF that contained silica aluminate mineral of montmorillonite as a loss control agent (LCA)57.
Results and Discussion
Adsorption of sources of N
We used XRD to analyze the structure and composition of urea (U), LCA, and LCU, and found LCA was the silica aluminate mineral of montmorillonite, while comparison of LCA and LCU standard peaks showed that LCU comprised 94% urea and 6% LCA (Table 1), moreover, the elemental analyses of LCU obtained by XRF (Supplementary Table S1) are in well agreement with the result from XRD, further revealing the component content of the LCU; comparison of LCU with P-LCU showed that LCU was a simple physical mix of urea and LCA (Fig. 1). These results demonstrate that part of the urea particle enters the channel of P-LCU58. Analysis of the mass ratio of LCA and urea in LCU showed that LCA interacted with type of N source in the LCU in the presence of water, because we showed that a specified amount of LCA adsorbed urea-N and NH4+-N from NH4Cl, and NO3−-N from NaNO3 at a range of concentrations (0.1, 0.01, 0.005, and 0.002 mol L−1). Thus, it is possible to quantify adsorption capacity of LCA to N source types. We used two approaches to calculate the amount of adsorbed N by the LCA: firstly, concentration of N source following adsorption by LCA was assumed to approximate the adsorption capacity of LCA, and secondly, we used elemental analysis of N from dried LCA to directly measure the adsorbed N content by LCA. Results from the two approaches were generally consistent and variation was within the acceptable range (Table 2): LCA mainly adsorbed urea-N and NH4+-N, but adsorbed low levels of NO3−-N, where adsorption of NH4+-N was 1.6-fold greater than urea-N, and 3-fold greater than NO3−-N. Therefore, the adsorption capacity between LCA and NH4+-N was the strongest, while that between urea-N and NO3−-N was weakest under the presence of water.
Mechanisms of N loss
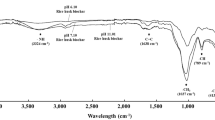
We analyzed the force between LCA and N source using FTIR and 1H MAS NMR, where vibrational bands for commercial urea, LCU, and P-LCU (Fig. 2A, curves a, c, and e, respectively) were in ranges of 3348–3465 and 1672 cm−1, showing they belonged separately to the –CONH2 and –NH2 groups59. Content of LCA was very low in LCU, as shown by obscure peaks that indicate that LCU preparation was a simple physical mix of the LCA and urea. The LCA and P-LCA (Fig. 2A, curves b and d, respectively) showed separate vibrational bands for the –SO2 and –OH groups at 1074 and 3642 cm−1, indicating they are highly susceptible to hydrogen bonds with sources of N and water molecules. We also used 1H MAS NMR spectra of P-LCU to determine the existence of a hydrogen bond and the deconvolution of 1H MAS NMR by fitting with multiple peaks is shown in Fig. 2B. Therein, the peak of 7.28 ppm (35%) was probably attributed to the hydrogen bond formed between LCA and urea or LCA and NH4+-N, while the peak of 7.02 ppm (47%) one without H-bond46,60. Therefore, in addition to van der Waals forces, there was formation of hydrogen bonds between LCA and urea in the presence of water that increased adsorption capacity of LCA.
LCU physicochemical properties
TG curves of urea and LCU (Fig. 3A) showed a shift in mass loss of LCU between c. 240 and 350 °C to between c. 250 and 360 °C, indicating that LCA effectively enhanced the thermal stability of urea46 as a result of greater stability of LCU than urea conferred by the effects of hydrogen bonding. SEM images of urea, LCU, and P-LCU (Fig. 3B) showed a stone-like, smooth surface morphology of urea, a rough surface of LCU, and a contrasting micro/nano network surface of P-LCU. The surface morphologies of LCU and P-LCU are attributed to the addition of LCA that had a morphology of agglomerated particles (Supplementary Fig. S1a); the surface of P-LCA comprised uniformly dispersed pores (Supplementary Fig. S1b). These results suggest that, following hydrothermal pretreatment, LCU creates a diffuse surface with more network channels that facilitates adsorption of urea and reduces its loss.
N2 sorption isotherms of LCA and P-LCA (Fig. 4A) showed they were type I at low relative pressure (P/P0 < 0.1), with an intrinsic microporous structure, while they were type IV at high relative pressure (P/P0 > 0.6) due to a mesoporous structure. We found that surface area and pore diameter of LCA were 101 m2 g−1 and 2.18 nm, respectively, while P-LCA had a surface area of 206 m2 g−1 and pore diameter of 3.72 nm, indicating that hydrothermal pretreatment improved pore formation and associated adsorption performance. This result supports previous indications from SEM characterizations, and shows that when LCA adsorbed water, its surface become viscous and hydrophobic.
We investigated this hydrophilic-hydrophobic property of LCA using contact-angle assessment (Fig. 4B). If a water droplet contacts LCA, it yields a contact angle of up to 151° that indicates super hydrophobicity61, such that small amounts of adsorbed water may saturate LCA. We assessed water retention capacity of LCA (Supplementary Fig. S2), where LCA continuously adsorbed water for 3 h, and after 24 h of filtration, the moisture content of LCA reached 204%; its moisture content remained high (199%) after storage for 7 d in ambient conditions. These results show high water retention performance of LCA, and indicate that when LCA is mixed with urea, sources of N may be stored in the surface pores following contact with water due to an increased hydrophobic status of the surface that prevents further absorption of water.
LCU resistance to N loss
We simulated NH3 volatilization, leaching, and runoff, based on this principal N loss pathway and observed the resistance control performance of LCU to N loss. Fluxes in NH3 volatilization from soil treated with urea reached a maximum 2 d after application at three levels of N (80, 160, and 240 kg N ha−1), where they ranged between 0.69 and 2.91 mg N/(kg·d); however, fluxes from soil treated with LCU reached a maximum 3 d after application, where they were in the range of 0.49–1.99 mg N/(kg·d) (Fig. 5). Fluxes in NH3 volatilization tended to remain stable from the 9 d after treatment, when N was applied at 80 kg N ha−1; when N application rates were 160 and 240 kg N ha−1, fluxes tended to stabilize at 11 and 15 d, respectively. Fluxes in NH3 volatilization were consistently lower for LCU than urea, regardless of N application rate, indicating that application of LCU may reduce NH3 volatilization. Cumulative losses of NH3 volatilization from soils treated with LCU were lower than from urea treatment under the same levels of N application (Supplementary Fig. S3): when N application rates were 80, 160 and 240 kg N ha−1, cumulative losses from soils treated with urea and LCU were 14.8% and 10.1%, 18.1% and 12.7%, and 24.2 and 17.5%, respectively. These results, combined with the previous characterizations, indicate it is likely that the hydrogen bond adsorption force of LCU between LCA and N sources reduced fluxes in NH3 volatilization and cumulative losses from the soils that were also reduced with decreasing N application rate.
Soils in drought areas tend to be sandy, and under these conditions, fertilizer may easily be leached with irrigation and rainwater. We assessed effect of LCU on leaching loss of N, and found that mass percent of retained TN in sand was 62.7% and 75.5% for urea and LCU, respectively, and mass percent of TN leaching loss was 23.1% and 11.6%, respectively, indicating LCU was more effective in leaching loss control than urea (Supplementary Fig. S4). Moreover, the mineral nitrogen content of leachate and sand retention (Supplementary Fig. S5) indicated that N leaching loss was mainly based on NO3−-N both for urea and LCU in the rapid N leaching experiments. And compared with traditional urea, the employment of LCU could obviously retain more NH4+-N and delay nitrification as a result of the presence of hydrogen bonds between LCA and NH4+-N.
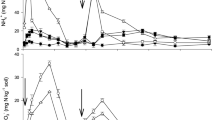
We assessed the long-term resistance control performance of LCU to N loss, by measuring the time course of N (NO3−-N, NH4+-N, Organic N and Total N) concentration of leachate from soil column over 110 d (Fig. 6). We found that when urea was applied with one of three levels of N (80, 160, and 240 kg N ha−1), TN concentration of leachate peaked 30 d after application, before reaching levels associated with no treatment after 80 d. In contrast, TN concentration in LCU was lower than for conventional urea up to 30 d after treatment, and higher between 40 and 70 d after treatment. Leachate NO3−-N concentration curves were similar to the corresponding TN at the three levels of N for LCU and urea, indicating that N leaching loss was mainly based on NO3−-N. Leaching loss of urea was greater than that of LCU in the first 30 d after treatment; this result corresponds to those for the rapid leaching test reported above. However, after 40 d, leaching loss of LCU was greater than that of urea, indicating that LCU may delay longer term leaching loss of N. However, for both LCU and urea, NH4+-N concentration of leachate was generally low, and there was little difference between the two types of fertilizer. However, the organic N concentration curves of the leachate were different between urea and LCU treatment. As the figure showed that the peak time of the latter (LCU) is obviously 10–20 days later than the former (urea). Moreover, We found there was no significant difference (except that LCU treatment had slightly higher nitrogen retention than urea treatment) in the amount of leaching and retention of fertilizer N at the end of experiment from 110 days incubation between conventional urea and LCU treatment among all levels of N addition (Supplementary Fig. S6), indicating that under strong leaching conditions without crop growth, applied LCU had no effects on N leaching loss. However, Supplementary Fig. S7 give the amounts of retention of fertilizer nitrogen (N) respectively within the top, middle and bottom of the soil column at the end of strong leaching experiment from 110 days incubation. We could get that the soil column with LCU treatment obviously retained more fertilizer N amount in the middle and upper layers than using urea, and the N amount in the soil column for employing urea was mainly left in the bottom layer. The results indicated that the application of LCU could mitigate the increase of N concentration in the bottom layer of the soil column to some extent.
The time course of nitrogen (N) concentration in leachates from a 110-day soil leaching incubation treated with urea or LCU at N rates of 0, 80 (a), 160 (b) and 240 (c) kg N ha−1 under 30 °C and 65% soil water-holding capacity. Organic N (ON) was calculated as the differences between total N, NO3−-N and NH4+-N. Error bars indicate standard deviation of replicates (n = 3).
Overall, our results show that LCU may retain nutrients shortly after application of fertilizer that may delay losses of fertilizer N caused by excessive applications; this may optimize supply of N in the soil and increase adsorption and utilization of N in crops.
Conclusions
A high-performance loss control urea (LCU) prepared by mechanically mixing 6% of loss control agent (LCA), which mainly comprised montmorillonite, with 94% of urea. The LCU exhibited higher thermal stability, larger specific surface area, and better water retention capacity compared to traditional urea following hydrothermal treatment. For hydrothermal treated LCU, hydrogen bonds between LCA and N source (especially NH4+-N) reduced loss of N. Cumulative losses of NH3 volatilization from soils treated with LCU decreased by 4.7%, 5.4%, and 6.7% compared with traditional urea at N application rates of 80, 160 and 240 kg N ha−1, respectively. Compared with traditional urea, LCU reduced short-term N leaching following application of N fertilizer and delayed long-term nitrification. Our study shows that use of LCU may effectively control NH3 volatilization and N leaching loss, principally as a result of the presence of hydrogen bonds between LCA and NH4+-N. We conclude that the use of LCU may optimize supply of N in soil and increase adsorption of N in crops to improve fertilizer use efficiency and reduce environmental pollution. However, further field tests under contrasting soil types and crop rotations are required to confirm this potential use of LCU. Currently, the long-term LCU field tests are arranged to investigate the feasibility of LCU on enhancing crop productivity and mitigating N losses in cropland use, and the relevant results will be reported in our follow-up work after systematic scientific field experiments.
Materials and Methods
Materials
Samples of the upper 15-cm of paddy soil were collected from Yixing Base for Agri-Environment Research, Changshu National Agro–Ecosystem Observation and Research Station, Chinese Academy of Sciences (31°16′N, 119°54′E), near Taihu Lake. The soils are classified as Gleyi-Stagnic Anthrosols, and originate from Lacustrine parent material; samples comprised 8.3% sand, 81.5% silt, and 10.2% clay (v/v), with 15.4 g kg−1 organic C and 1.79 g kg−1 N, and cation exchange capacity and pH (H2O) of 11.8 cmol kg−1 and 5.6, respectively. Soil samples were air-dried and sieved through a 2-mm screen prior to treatment with LCA, LCU, and contrasting rates of N. Analytical reagent grade chemicals used in the experiments were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Samples characterization
Structure of the samples was characterized using X-ray diffraction (XRD) with a SmartLab diffractometer (Rigaku) equipped with a 9 kW rotating anode Cu source at 45 kV and 100 mA, from 5 to 50° with a scan rate of 0.2° s−1. We recorded infra-red spectra using a Nicolet iS10 FT-IR instrument (KBr disks) in the 400–500 cm−1 region, and solid state 1H NMR spectrum analysis was performed at 400 MHz using Hahn echo at room temperature at a spinning rate and contact time of 10 KHz and 2.5 ms, respectively; scan number was 1.2. Thermogravimetric (TG) analysis was carried out using a STA 409 instrument in dry air at a heating rate of 10 °C min−1. Morphology was tested using a field-emission scanning electron microscope (SEM) instrument (Hitachi S-4800), and specific surface area and pore diameter were obtained from N2-adsorption-desorption isotherms using multipoint BET and t-plot methods, where samples were outgassed at 300 °C to a vacuum of 10−3 Torr prior to measurement, and isotherms were obtained at liquid N temperature with a BEL SORP-MAX analyzer. Contact angle was measured using a contact-angle meter (DropMeter A-100P) at 25 °C. The chemical compositions of samples were obtained using ADVANT’XP X-ray fluorescence (XRF) spectrometer (ZSX Primus II), and the elemental analysis was performed on an elemental analyzer Vario EL cube. The concentrations of NH4+-N, NO3−-N, and total N (TN) in deionized water (or KCl) were analyzed using a continuous-flow N analyzer (Skalar San++ System, Netherlands).
Hydrothermal treatment process
The original LCA (Henan Xin Lian Xin Fertilizer Co., Ltd., China) was treated with deionized water to improve its dispersion, and the resulting suspension (10 g L−1) was dried at 80 °C, following hydrothermal treatment at 180 °C for 2 h, with constant stirring, to achieve the final P-LCA powders. For the original LCU (Henan Xin Lian Xin Fertilizer Co., Ltd., China), the resulting suspension (3 g L−1) was dried at 60 °C, following hydrothermal treatment at 30 °C for 30 min, with constant stirring, to achieve the final P-LCU powders.
Assessment of NH3 volatilization
We assessed NH3 volatilization according to previously reported methods62,63,64, where 2 mL of boric acid solution was placed with color reagent (dimethyl red-bromocresol green) in the center of a Petri dish, around which 10 g of soil (65% WHC, 30 °C) was added. Then, the N application rates of 80, 160, and 240 kg N ha−1 were added to three replicates of soil using a sample gun, before the Petri dish was covered and sealed (using Vaseline) and placed in an incubator for 23 d. Volatilized NH3 absorbed by boric acid was assessed at regular intervals using the acid-base titration method.
Assessment of rapid N leaching
N leaching was measured using a previously published method42, where 30 g of dry sand (150–200 mesh) and 5 mL of deionized water to create humid conditions (relative humidity: 30%) were placed in a 50-mL centrifuge tube that had a 2-mm diameter hole at the base. Then, 1 g of LCU or 0.9 g of urea (2-cm diameter × 1-cm long cylinder shaped) was added to the tube and covered with 10 g of dry sand, prior to storage at 30 °C. We sprayed 50 mL of deionized water over the surface of the sand layer, and mass percent of N in the leachate and sand was calculated as: (mass of N in the leachate or sand/the mass of initial N) × 100.
N leaching within a soil column
N leaching from the soil column was tested employing a earlier published method21, where 618 g of soil (65% WHC) was placed in a 30-cm tall soil column that was equally divided into top, middle, and bottom parts, to which urea or LCU with 80, 160, or 240 kg N ha−1 was added and stored at 30 °C for 110 d. Every 10 d, deionized water was added to the top of the column, and leachate was extracted from the base using a pump; soil WHC was maintained at a constant. The leachate was filtered through a 0.45-μm filter membrane and N concentration was quantified using a flow analyzer. After 110 d, about 10 g of soil was removed from each of the three layers of the soil column, to which 50 ml of KCl (2 mol L−1) solution was added, and concentration of extracted N in the filtrate was detected using a flow analyzer. TN in the soil column was calculated as sum of N in three layers.
References
Plett, D., Garnett, T. & Okamoto, M. Molecular genetics to discover and improve nitrogen use efficiency in crop plants. (Adelaide Univ. Press, Australia, 2017).
Zhang, F. S. et al. Nutrient use efficiencies of major cereal crops in China and measures for improvement. Acta Pedologica Sinica. 45, 915–924 (2008).
Yu, F. & Shi, W. M. Nitrogen use efficiencies of major grain crops in China in recent 10 years. Acta Pedologica Sinica. 52, 1311–1324 (2015).
Kronvang, B. et al. Effects of policy measures implemented in Denmark on nitrogen pollution of the aquatic environment. Environ. Sci. Policy. 2, 144–152 (2008).
Bouchet, A. S. et al. Nitrogen use efficiency in rapeseed. Agron. Sustain. Dev. 38, 1–20 (2016).
Lassaletta, L., Billen, G., Grizzetti, B., Anglade, J. & Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 9, 1–9 (2014).
Hirel, B., Gouis, J. L., Ney, B. & Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 58, 2369–2387 (2007).
Vitousek, P. M. et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750 (1997).
Yan, X., Jin, J. Y., He, P. & Liang, M. Z. Recent advances in technology of increasing fertilizer use efficiency. Scientia Agricultura Sinica. 41, 450–459 (2008).
He, X. S., Li, S. X., Li, X. H. & Lv, D. Q. The progress of studies on controlled a valiability fertilizers. Plant Nutr. And Fert. Sci. 4, 97–106 (1998).
Trenkel, M. E. Slow- and controlled-release and stabilized fertilizers: an option for enhancing nutrient use efficiency in agriculture (Second edition, IFA, Paris, France, October 2010).
Pan, B. B., Lam, S. K., Mosier, A., Luo, Y. Q. & Chen, D. L. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agr. Ecosyst. Environ. 232, 283–289 (2016).
Akhtar, M. & Naeem, A. Reduction in ammonia loss by applying urea in combination with phosphate sources. Commun. Soil Sci. Plant. Anal. 43, 2043–2049 (2012).
DU Preez, C. C. & DU Burger, R. T. Ammonia losses from ammonium-containing and -forming fertilizers after surface application at different rates on alkaline soils. Fert. Res. 15, 71–78 (1998).
Fan, M. X. & Mackenzie, A. F. Urea and phosphate interactions in fertilizer microsites: ammonia volatilization and pH changes. Soil Sci. Soc. Am. J. 57, 839–845 (1993).
Mandal, S. et al. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere. 142, 120–127 (2016).
Chen, C. R. et al. Impacts of greenwaste biochar on ammonia volatilisation from bauxite processing residue sand. Plant Soil. 367, 301–312 (2013).
Congreves, K. A. et al. Predicting ammonia volatilization after field application of swine slurry: DNDC model development. Agr. Ecosyst. Environ. 219, 179–189 (2016).
Esfandbod, M. et al. Aged acidic biochar increases nitrogen retention and decreasesammonia volatilization in alkaline bauxite residue sand. Ecol. Eng. 98, 157–165 (2017).
Rochette, P. et al. Ammonia volatilization and nitrogen retention: how deep to incorporate urea. J. Environ. Qual. 42, 1635–1642 (2013).
Kameyama, K., Miyamoto, T., Shiono, T. & Shinogi, Y. Influence of sugarcane bagasse-derived biochar application on nitrate leaching in calcaric dark red soil. J. Environ. Qual. 41, 1131–1137 (2012).
Ding, Y. et al. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut. 213, 47–55 (2010).
Zheng, H., Wang, Z. Y., Deng, X., Herbert, S. & Xing, B. S. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma. 206, 32–39 (2013).
Di, H. J. & Cameron, K. C. Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor —a lysimeter study. Nutr. Cycl. Agroecosyst. 79, 281–290 (2007).
Zaman, M. & Blennerhassett, J. D. Effects of the different rates of urease and nitrification inhibitors on gaseous emissions of ammonia and nitrous oxide, nitrate leaching and pasture production from urine patches in an intensive grazed pasture system. Agr. Ecosyst. Environ. 136, 236–246 (2010).
Sanz-Cobena, A., Sánchez-Martín, L., García-Torres, L. & Vallejo, A. Gaseous emissions of N2O and NO and NO3 − leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agr. Ecosyst. Environ. 149, 64–73 (2012).
Sun, Z. M., Wu, Z. J., Chen, L. J. & Ma, X. Z. Application effect, affecting factors, and evaluation of nitrification inhibitor. Chin. J. Appl. Ecol. 19, 1611–1618 (2008).
Tully, K. L., Lawrence, D. & Scanlon, T. M. More trees less loss: nitrogen leaching losses decrease with increasing biomass in coffee agroforests. Agr. Ecosyst. Environ. 161, 137–144 (2012).
Stenberg, M., Aronsson, H., Lindén, B., Rydberg, T. & Gustafson, A. Soil mineral nitrogen and nitrate leaching losses in soil tillage systems combined with a catch crop. Soil Till. Res. 50, 115–125 (1999).
Gentile, R., Vanlauwe, B., Van Kessel, C. & Six, J. Managing N availability and losses by combining fertilizer-N with different quality residues in Kenya. Agr. Ecosyst. Environ. 131, 308–314 (2009).
Luo, J., De Klein, C. A. M., Ledgard, S. F. & Saggar, S. Management options to reduce nitrous oxide emissions from intensively grazed pastures: a review. Agr. Ecosyst. Environ. 136, 282–291 (2010).
Oenema, O., Witzke, H. P., Klimont, Z., Lesschen, J. P. & Velthof, G. L. Integrated assessment of promising measures to decrease nitrogen losses from agriculture in EU-27. Agr. Ecosyst. Environ. 133, 280–288 (2009).
Peng, S. Z., Yang, S. H., Xu, J. Z., Luo, Y. F. & Hou, H. J. Nitrogen and phosphorus leaching losses from paddy fields with different water and nitrogen managements. Paddy Water Environ. 9, 333–342 (2011).
Gullett, L. L., Simmons, C. L. & Lee, R. G. Sulfur coating of urea treated with attapulgite clay. Fert. Res. 28, 123–128 (1991).
Ni, B., Liu, M. Z., Lv, S. Y., Xie, L. H. & Wang, Y. F. Environmentally friendly slow-release nitrogen fertilizer. J. Agric. Food Chem. 59, 10169–10175 (2011).
Li, A., Zhang, J. P. & Wang, A. Q. Preparation and slow-release property of a poly(acrylic acid)/attapulgite/sodium humate superabsorbent composite. J. Appl. Polym. Sci. 103, 37–45 (2007).
Xie, L. H., Liu, M. Z., Ni, B. L. & Wang, Y. F. New environment-friendly use of wheat straw in slow-release fertilizer formulations with the function of superabsorbent. Ind. Eng. Chem. Res. 51, 3855–3862 (2012).
Wang, S. Q. et al. Improving grain yield and reducing N loss using polymer-coated urea in southeast China. Agron. Sustain. Dev. 35, 1103–1115 (2015).
Shen, Y. Z., Du, C. W., Zhou, J. M. & Ma, F. Application of nano FeIII—tannic acid complexes in modifying aqueous acrylic latex for controlled-release coated urea. J. Agric. Food Chem. 65, 1030–1036 (2017).
Ma, Z. H. et al. Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw. Chem. Eng. J. 171, 1209–1217 (2011).
Nogueira, F. G. E. et al. Incorporation of mineral phosphorus and potassium on leather waste (collagen): a new NcollagenPK-fertilizer with slow liberation. J. Hazard. Mater. 176, 374–380 (2010).
Zhou, L. L. et al. Fabrication of a high-performance fertilizer to control the loss of water and nutrient using micro/nano networks. ACS Sustainable Chem. Eng. 3, 645–653 (2015).
Liu, R. H. et al. Use of a new controlled-loss-fertilizer to reduce nitrogen losses during winter wheat cultivation in the danjiangkou reservoir area of China. Commun. Soil Sci. Plant. Anal. 47, 1137–1147 (2016).
Zhou, L. P. et al. Comparison of several slow-released nitrogen fertilizers in ammonia volatilization and nitrogen utilization in summer maize field. J. Plant Nutr. And Fert. 22, 1449–1457 (2016).
Wu, Y. J., Yang, H. C. & Yu, Z. L. Summary on application effect and mechanism research of loss-control fertilizer. Anhui Agri. Sci. Bull. 13, 22–24 (2007).
Cai, D. Q. et al. Controlling nitrogen migration through micro-nano networks. Sci. Rep. 4, 3665–3672 (2014).
Wang, L. Effects of loss-control fertilizer on biological character and grain yield of wheat. Journal of Anhui Agri. Sci. 36, 10552–10553 (2008).
Wang, H. Q., Gao, S. C., Dang, Y. Y. & Lv, J. L. Production demonstration of loss-control fertilizer in 2007. Anhui Agri. Sci. Bull. 13, 45–46 (2007).
Yu, L. X. et al. Influence of “loss-control” of fertilizer on nitrogen agronomic utilized efficiency of crop. Anhui Agri. Sci. Bull. 13, 25–26 (2007).
Hu, R. & Liu, C. S. Effects of different proportion of loss-control nitrogen fertilizer on agronomic traits and yield of double cropping rice. Anhui Agri. Sci. Bull. 13, 40–42 (2007).
Shi, T. Comparative experiment of loss-control fertilizer for early rice. Anhui Agri. Sci. Bull. 16, 89–90 (2010).
Wu, M. & Tian, X. Loss-control fertilizer application in rice. Anhui Agri. Sci. Bull. 20, 30–31 (2014).
Yang, W. et al. Effects of several new fertilizers on maize yield and nitrogen and phosphorus uptake. J. Henan Inst. Sci. Technol. 45, 8–15 (2017).
Zhou, L. P., Yang, L. P., Bai, Y. L., Lu, Y. L. & Wang, L. Effects of different slow-released nitrogen fertilizers on summer maize and nitrogen fate in the field. Scientia Agricultura Sinica. 51, 1527–1536 (2018).
Jing, X. C. The nutrient loss-controlling mechanism and features of loss-controlled compound fertilizer. Phosphate and Compound Fertilizer. 24, 57–58 (2009).
Ni, X. Y. et al. A kind of slow release urea and preparation method, CN 102898234. August 13 (2014).
Zhang, H. et al. A quantitative detection method of polyacrylamide in mixture, CN 102914511. January 07 (2015).
Zhu, J. et al. Encapsulation of metal (Au, Ag, Pt) nanoparticles into the mesoporous SBA-15 structure. Langmuir. 19, 4396–4401 (2003).
Grdadolnik, J. & Maréchal, Y. Urea and urea-water solutions-an infrared study. J. Mol. Struct. 615, 177–189 (2002).
Jeffirey, G. A. An introduction of hydrogen bonding. (Oxford Univ. Press, New York, 1997).
Feng, X. J. & Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 18, 3063–3078 (2006).
Sun, K. J., Mao, X. Y., Lu, Q. M., Jia, A. P. & Liao, Z. W. Mitigation effect of several controlled-release N fertilizers on ammonia volatilization and related affecting factors. Chinese Journal of Applied Ecology. 15, 2347–2350 (2004).
Sun, K. N., Yuan, L., Li, X. H., Lin, Z. A. & Zhao, B. Q. Effects of value-added urea on ammonia volatilization and soil urease activity. Shandong Agri. Sci. 6, 60–62 (2010).
Gao, P. C. & Zhang, Y. P. Research on relationship between volatilization of ammonia and evaporation of soil water. Jour. of Northwest Sci-Tech Univ. of Agri and For. 29, 22–26 (2001).
Acknowledgements
This study was financially supported by the R&D project of Henan Xin Lian Xin Fertilizer Co. Ltd, the National Key R&D Program of China (Nos 2017YFD0200104 and 2017YFD0200704), and the 13th Five Year Plan Forefront Project of Institute of Soil Science, Chinese Academy of Sciences (ISSASIP1646). The authors gratefully acknowledge Dr. Yuejin Wu from Hefei Institute of Physical Science, Chinese Academy of Sciences for his valuable comments. We also thank Dr. Shuiqing Zhang, Dr. Shaomin Huang and Mr. Ke Yue from Henan Academy of Agricultural Science, China for their assistance with field monitoring.
Author information
Authors and Affiliations
Contributions
Z.M. and X.Z. designed the research. Z.M. and X.M. performed the experiments. Z.M. and X.Z. wrote the manuscript. Y.Y., M.F., Y.L. and S.W. contributed to scientific discussions and data interpretations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, Z., Yue, Y., Feng, M. et al. Mitigation of ammonia volatilization and nitrate leaching via loss control urea triggered H-bond forces. Sci Rep 9, 15140 (2019). https://doi.org/10.1038/s41598-019-51566-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51566-2
This article is cited by
-
Pyrolysis temperature and time of rice husk biochar potentially control ammonia emissions and Chinese cabbage yield from urea-fertilized soils
Scientific Reports (2024)
-
Soil mineral nitrogen, soil urease activity, nitrogen losses and nitrogen footprint under machine-planted rice with side-deep fertilization
Plant and Soil (2024)
-
Patterns of crop-specific fertilizer-nitrogen losses and opportunities for sustainable mitigation: A quantitative overview of 15N-tracing studies
Soil Ecology Letters (2024)
-
Blending loss-control and normal urea maximizes nitrogen utilization of summer maize by mitigating ammonia volatilization and nitrate leaching
Plant and Soil (2023)
-
Supraparticles as slow-release fertiliser in seedling potential growth of Eucalyptus urograndis and greenhouse gas flux impacts
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.