Abstract
In this study, we explored the role of biochar (BC) and/or urease inhibitor (UI) in mitigating ammonia (NH3) and nitrous oxide (N2O) discharge from urea fertilized wheat cultivated fields in Pakistan (34.01°N, 71.71°E). The experiment included five treatments [control, urea (150 kg N ha−1), BC (10 Mg ha−1), urea + BC and urea + BC + UI (1 L ton−1)], which were all repeated four times and were carried out in a randomized complete block design. Urea supplementation along with BC and BC + UI reduced soil NH3 emissions by 27% and 69%, respectively, compared to sole urea application. Nitrous oxide emissions from urea fertilized plots were also reduced by 24% and 53% applying BC and BC + UI, respectively, compared to urea alone. Application of BC with urea improved the grain yield, shoot biomass, and total N uptake of wheat by 13%, 24%, and 12%, respectively, compared to urea alone. Moreover, UI further promoted biomass and grain yield, and N assimilation in wheat by 38%, 22% and 27%, respectively, over sole urea application. In conclusion, application of BC and/or UI can mitigate NH3 and N2O emissions from urea fertilized soil, improve N use efficiency (NUE) and overall crop productivity.
Similar content being viewed by others
Introduction
In terrestrial ecosystems, nitrogen (N) deficiency is the main growth limiting factor, thereby adversely affecting crop productivity1,2,3,4. Therefore, an exogenous supply of N along with phosphorus as fertilizers is key farming practice for improving crop yield3. Conferring to5, a huge quantity of chemically synthetized N compounds will be required to fulfill the food requirements of 9 billion people by 2050. However, the higher land application of N compounds as chemical fertilizers has a negative influence on the environment through the release of N containing gasses such as NH3 and N2O, and its losses to the ground and surface water by nitrate leaching and runoff6,7,8. At current, the N use efficiency of synthetic N fertilizers is too low, only amounting approximately 50%9,10, which adds to the adverse environmental, agronomic and economic impact11,12.
Ammonia volatilization is a major source for N losses and is considered to be the main cause of low N use efficiency9,13. Ammonia is a major alkaline atmospheric pollutant that plays an important role in the formation of aerosols14,15, which badly affect human health16, reduce visibility17,18, alter Earth’s radiative balance, and contribute to a global redistribution of N through atmospheric deposition19,20. Although ammonia is not considered a potential greenhouse gas (GHS), its emission and re-deposition can adversely affect the environment21, and it may act as a secondary cause of N2O emission in soil22,23.
Nitrous oxide is a potential (150 years lifetime) GHS, which is 298 times more efficient than CO2 for its heat-trapping capacity with a 7% contribution to the total GHS emission and 0.26% annual growth rate24. Agricultural soils annually emit 4.1 Tg N to the global atmospheric N2O emission of 14 Tg N24,25. In agricultural soils, N2O is emitted mainly by biological and chemical processes such as nitrification and denitrification26,27,28. Additionally, N2O emissions may also happen via nitrification–denitrification through autotrophic NH3-oxidizing bacteria, where ammonia is oxidized to NO2−, followed by its reduction into NO, N2O and N226,29,30. It can also be produced during a hybrid reaction by co-denitrification where two N atoms are released one each from organic N by mineralization and NO2− by denitrification31. In soil containing high organic matter, it can be released by heterotopic reduction of nitrite by the oxidation of organic N32,33.
Various studies have been aiming to develop sustainable and eco-friendly management practices for reducing N losses, improving NUE and crop yield34,35. Such practices include application of nitrification inhibitors, urease inhibitor (UI), elemental S and polymers36, removal of crop residues from the land in various ratios37,38 and application of biochar39,40. Biochar, a carbon-rich pyrolitic product of organic waste, has received ample attention for its environmental benefits41. Utilization of biochar as a soil amendment/conditioner not only provides a way to recycle environmental waste but also optimizes soil health, crop yield and stimulates soil C sequestration42,43,44,45,46,47,48,49,50. Studies have demonstrated that biochar may be used as a compound fertilizer in conjunction with mineral sources51,52, as a slow-release fertilizer53,54,55, which could enhance fertilizer56 and may increase N use efficiency under different soil conditions51,52. Biochar may have the capacity to sorb NH4+ or NH3 gas released during composting, thereby lowering NH3 emission. Biochar may also adsorb organic N compounds, thus decreasing their mineralisation57 and consequently NH3 emission. This gradual availability of N may be partially explained by lower atmospheric N2O emissions from N fertilizer applied in the presence of biochar58,59.
The application of UI [N-(n-butyl)] and thiophosphoric triamide (nBTPT or NBPT for short) have been reported to reduce urease activity in soil and slow down NH4+ release from urea, thus reducing NH3 emission34,35,60. Urease inhibitor significantly decreases urea hydrolysis, reduced NH4+ concentrations and, thereby limited NO3− supply and nitrification rate thus reducing N2O emission and improves crop yield61,62,63,64.
So far, many experiments have focused the effect of biochar and urease inhibitor sole application on gaseous N emissions, N-transformation processes and crop yield in variable agro-climatic circumstances. However, limited work is documented regarding the influence of urea treated with urease inhibitor and biochar or alone on N2O and NH3 emission from arable agricultural system under hot climate of Pakistan. Therefore, main objectives of current study were to explore the effect of urea together with urease inhibitor and biochar or alone on crop productivity, N2O and NH3 emission and N efficiency.
Results
Soil mineral N dynamics
The NH4+-N concentration observed in urea treated plots on day 1 was significantly (p < 0.05) higher (5–25 mg N kg−1 soil) compared to the control (Fig. 1). In urea treatment, the NH4+-N concentration increased because of the applied urea's fast hydrolysis during the first 3 days after first fertilization. Thereafter, a sharp decrease was observed (Fig. 1). The plots treated with biochar + urea showed maximum soil NH4+-N concentration 28 days after fertilization, which was significantly higher than the plots treated with sole urea (Fig. 1). Furthermore, the application of UI considerably decreased the average soil NO3−-N content. After the first fertilization, soil NO3–-N content slightly increased, and most mineral N was observed as NH4+-N. Soil NO3–-N concentration peaked just after the second urea application in the urea treatment and then displayed a rapidly decreasing trend within 14 days in after fertilizer application (Fig. 1). Additionally, biochar supplementation significantly decreased NO3–-N concentration in the soil.
Ammonia volatilization
The results concerning NH3 emission are presented in Fig. 2 and Table 1. A maximum NH3 discharge was detected between days 1 and 3 after every application. The application of BC and UI with urea significantly affected daily and total cumulative NH3 discharge during the initial 10 to 12 days. Total NH3 losses as kg N ha−1 amounted 14.4 with BC and 6.2 with BC + UI compared to sole urea (19.7), representing 27% and 69% reduction by BC and BC + UI, respectively, relative to sole urea application.
Nitrous oxide emissions
The application of urea, BC, and UI significantly affected the daily and cumulative N2O emissions (Fig. 3 and Table 2). The highest and lowest cumulative emissions of N2O were measured for urea and urea + BC + UI treatments, respectively (1.7 and 0.8 kg N2O-N ha−1) (Table 2). Biochar + urea significantly reduced N2O emissions by 24% compared to urea alone. Urea applied in combination with BC and UI reduced N2O emission by 53% over urea alone treatment.
N-use efficiency, yield and N uptake by wheat
Wheat N uptake, yield and NUE were significantly improved due to biochar and/or UI application (Table 3). Wheat biomass (24%) and grain yields (13%) were improved by BC over sole urea. In contrast, urea's combined application with BC and UI increased wheat biomass and grain yields by 38% and 22% over the sole urea treatment. The highest total N uptake, above ground wheat biomass, contained 101 and 114 kg N ha−1 from urea + BC and urea + BC + UI treated plots, respectively (Table 3). The BC and UI application increased total N uptake by 12% and 27%, respectively, over sole urea (Table 3). Similarly, the highest N uptake and NUE were observed in plots treated with urea + BC + UI, followed by urea + BC (Table 3). The NUE observed for sole urea, urea + BC and urea + BC + UI were 27%, 35%, and 43%, respectively (Table 3).
Discussion
Ammonia volatilization as affected by biochar and urease inhibitor
A 27% decrease was observed in NH3 volatilization with BC compared to sole urea (Table 1), which can be attributed to its highly porous structure, surface area and high sorption capacities60,61. Furthermore, BC may absorb NH4+-N62 due to the presence of acidic functional groups63, hence decreasing the NH3 volatilization64. Biochar application with N fertilizers could avoid soil alkalization65,66 and decrease organic N mineralization through the adsorption of organic N compounds32,57.
On day 1 after application, a rapid increase in NH4+-N content was observed with sole application of urea (Fig. 1), which could be ascribed to the fast hydrolysis of urea. As a result, more NH4+-N and OH− ions are produced and allowing significant NH3 losses (Fig. 1). Urease inhibitor reduce the effect of pH increasing by delaying urea hydrolysis, as demonstrated by lower NH4+-N (Fig. 1) which could significantly reduce NH3 emissions. Slower urea hydrolysis due to UI application could also be associated with increasing pH around urea particles, which prevents hydrolytic action on urea via the enzyme urease10,35,67. It has also been reported that UI slows down the microbial decomposition of ammonia68,69,70,71. Additionally, slower urea hydrolysis due to application of UI could also provide more time for rainfall or irrigation to move the applied urea from the soil surface to the sub-soil layers vertically as well as and laterally consequently protects the applied N from volatilization72,73.
N2O emission as affected by biochar and urease inhibitor
Nitrous oxide emissions are directly related to the amount of mineral N available in the soil. A two-way ANOVA indicated that seasonal N2O emissions during the wheat growing season were significantly affected by biochar application (Fig. 2), in agreement with previous results Zhang et al.33 and Schirrmann et al.74. Our results confirm that the application of biochar reduced N2O emissions, because biochar resulted in lower NO3− contents and higher NH4+ concentrations after N addition (Fig. 2) which affect N availability in the soil, either physically by sorption to surfaces or microbiologically75. These results are in agreement with the findings of Lehmann et al.76 and Kammann et al.77 who reported that after application of N fertilizer, the adsorption of soil NH4+ by biochar on its surface, especially by biochar with a maximum C/N ratio reduce N2O emissions, subsequently resulting a decrease in the processes of ammonification and nitrification.
Nitrous oxide is generated by both nitrification and denitrification processes; therefore, it may be closely related to soil NH4+-N and NO3–-N concentrations78. Under high nitrification activity, when a lot of NO3− is produced in urea-treated soils during the first week of treatment application (Fig. 1) the observed N2O may actually be related to nitrification which produces N2O as a byproduct10. Apart from high water content also a high microbial activity can cause O2 depletion and therefore anoxic conditions that might promote denitrification. Due to the strong nitrification usually taking place during aerobic conditions, NO3− predominates in soils. As a result, denitrification would be prompted by the large amounts of NO3− accumulated in soil, especially under high soil moisture conditions. For a given soil, whether nitrification or denitrification contributed more to N2O emission may be closely related to soil NH4+-N and NO3−-N concentrations when the initial physicochemical factors of the tested soil are almost identical79.
The results showed lower NH4+-N concentrations in plots treated with urea + BC + UI than sole urea treated plots, while NO3-N concentrations were higher in urea treated plots than in urea + BC + UI (Fig. 1 and Fig. 3). It is likely that the combination of biochar and UI significantly decreased NO3− supply and thus reduced the activity of denitrifiers. Here, it is evident that UI application reduced the activity of ammonia oxidase and nitrification process and played a significant role in converting NH4+-N to NO3–-N, which is significantly related to N2O discharge from soil10,36,67. These findings imply that applying either BC or BC + UI plays a significant role in reducing NH3 and N2O emissions from urea fertilized soils. Additional investigations are recommended to elucidate the role of BC and BC + UI in reducing NH3 and N2O emissions at varying soil and climatic conditions.
Wheat yield and N uptake as affected by biochar and urease inhibitor
Both BC and UI significantly improved total N uptake and yield of wheat (Table 3). Application of BC and/or UI with N fertilizers can reduce N losses as GHS and may prevent NO3− leaching, thus enhancing the bioavailability of N36,48,60. Dawar et al.13 reported that the retention of mineral N in the form of NH4+, rather than NO3−, for several days after urea application increasing N uptake and thus improved crop yield. The results also show that BC improved soil WHC, allowing the wheat to retain a proper moisture level between irrigation periods, with a subsequent positive impact on final grain yields80. Furthermore, BC + UI could significantly improve soil conditions such as soil organic content, pH, and total N content81, improving N availability by reducing NH3 and N2O from the soil. Urease inhibitor and BC retain NH4+ in the soil for a longer time and improve its subsequent uptake by wheat, thus may improve crop yield. This not only offers environmental benefits by preventing and NO3− leaching and sinking NH3 and N2O release13,36, but also includes economic benefits using an increase in NUE. The plant uses relatively less energy to absorb NH4+ than NO3− as the transformation of NO3− into NH4+ via amides, amines, and amino acids is energy-consuming. Thus, higher availability of NH4+ facilitates improved crop yield and nutrition10.
Conclusions
It was observed that BC and UI have the highest potential to reduced NH3 and N2O emissions in urea fertilized soils. Furthermore, applying BC and/or UI with urea significantly improved wheat biomass and grain yield compared to sole urea. Therefore, the application of BC and/or UI with urea plays a significant role in mitigating NH3 and N2O emissions from cultivable land and improving crop yield under the hot and semi-arid agro-climatic conditions of Pakistan.
Materials and methods
Location
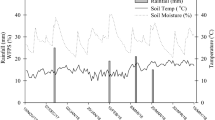
This experiment was executed at the National Institute of Food and Agriculture (NIFA), Peshawar (34.01°N, 71.71°E), Pakistan. The study area is semi-arid to arid and humid in the north to dry in the southern parts with a mean yearly precipitation of 384 mm and temperature of 22.7 °C (Fig. 4). The field had been conventionally cultivated with maize-wheat cropping system for approximately 10 years. The main soil (0–15 cm) properties of the composite sample are provided in Table 4.
Experiments
The field experiment including five treatments [control; urea (150 kg N ha−1); BC (10 t ha−1); urea + BC and urea + BC + UI] was designed following a randomized complete block design (RCBD) with 4 replications. The ‘Pirsabak-2013’ variety of wheat was sown in 5 m × 3 m plots containing 10 rows with row-to-row distance was kept 30 cm apart at a seed ratio of 120 kg ha−1 on 15th Nov, 2017. The conventional tillage was implemented using a mouldboard plow (40–50 cm deep) followed by disking (20–25 cm deep) and land leveller operations for seed bed preparation. Before seeding, 90 kg P ha−1 and 60 kg K2O ha−1 as single super phosphate and sulphate of potassium, respectively, were applied as a basal dose. The biochar used was produced from eucalyptus wastes (branches) pyrolyzed at 350 °C and sieved through a 5 mm sieve. Nitrogen was supplemented in the form of urea as a top dressing in two splits, half each a sowing and tillering. Urease inhibitor was supplemented as 0.1% solution. Granular urea with UI containing 25% NBPT was added at 150 kg N ha–1 as a surface application followed by surface incorporation and irrigation (10 mm) to ensure its proper distribution in the root zone.
N2O measurement
The N2O discharge from soil was measured from November, 2017 to May, 2018 using opaque manual spherical static chambers, following the procedure described in Sanz-Cobena et al.34. For sample collection, the cylindrical (radius 12.5 and height 20 cm) chambers were inserted in the soil at 15 cm depth 24 h before sampling. The chambers were properly sealed, and samples were taken after 0, 30 and 60 min once a week in the morning between 7:00 and 10:00 by a butyl septum installed on the upper part of the chamber. The samples were injected in 20 ml glass vials (Agilent Technologies, USA) via syringes (50 ml) equipped with inner three-way stopcocks (0.7-mm). The air and soil temperatures inside the chambers were recorded during gas sampling via a portable digital thermometer. Gas samples were analyzed on gas chromatographs (Varian Aerograph Series 2800 in NZ; Perkin Elmer Auto system XL B5902) equipped with 63Ni electron capture detectors (Pye Unicam) and two manual switching valves (Valco Instruments Co., Inc.)
The mean variation in gas concentration was calculated through linear regression while the ideal gas law was applied for quantification of gas-fluxes as follows:
where F, P, V, A, Δc/Δt and T represent N2O flux (µg m−2 h−1), density (mg m−3), chamber size (m3), base size (m2) of the chamber, mean rate of variation in gas concentration per unit time (mg kg−1 h−1) and chamber inner temperature (°C), respectively. The acceptable range of R2 for N2O fluxes was taken as 0.80 for the static chamber, except where the maximum change in concentration was lower than the gas-specific GC detection limit (< 10 ppb for N2O), where no filtering criterion was adopted (Järveoja et al.82). Using these criteria, 10% of fluxes (N2O) were subtracted from succeeding data analysis.
The following formula was used for the determination of cumulative N2O emissions (Ec, kg N2O-N ha−1):
where Fi and Fi + 1 is the ith and (i + 1)th measured value, respectively, of N2O flux (µg N2O-N m−2 h−1 ); ti and ti + 1 is the day when the ith and (i + 1)th measurement of N2O flux is taken, respectively (d); and n is the total number of the measurements.
Ammonia measurement and analysis
Ammonia volatilization was quantified by a 5 L (plastic bottle) semi-static open chamber, according to Araujo et al.83. In each plot, a single chamber was installed 5 mm above the ground surface and was randomized daily as advised by Jantalia et al.84. A foam strip pre-soaked in 1 molar H2SO4 and 4% (V/V) glycerol was kept moist throughout sampling duration by immersing one end into a polypropylene jar containing 15 mL 1 molar H2SO4 solution suspended inside the chamber. In each plastic bottle, the solution and foam strip were initially replaced by fresh solution after 12 h and then every 24 h and 14 days and processed for NH4+-N concentration via the steam distillation method85.
The following equation was applied for the quantification of NH3 fluxes (kg N ha−1 d−1):
where; C, V, t and r represent the molar concentration of H2SO4, amount of H2SO4 consumed during titration (ml), sampling time (hours) and chamber radius (m). The cumulative NH3 flux was determined by adding the NH3 fluxes for all sampling days for their respective treatment plots.
This method showed 57% NH3 recovery by calibration with 15N isotope equilibrium technique (Araújo et al.83). Therefore, for the accurate estimation of cumulative NH3 emissions and flows, a correction factor of 1.74 was applied (Araújo et al.86). According to Jantalia et al.84, this method for quantification of NH3 emissions is more suitable than the wind tunnel procedure35 for comparison of different treatments.
Soil and plant analysis
From each plot, five soil samples (0–10 cm) were randomly collected, well mixed, sieved (2 mm) and analyzed for key soil properties (Table 4). To determine mineral N (NO3− and NH4+), extraction was done with 1:5, 2 M KCl for 1 h on a rotary shaker10, filtered and analyzed via ultraviolet spectrophotometry (Jenway, 6305 UV/Vise, UK). Soil moisture was measured by oven-drying and was transformed to water-filled pore space (WFPS) using the formula of Li et al.85.
Total N in soil and plant samples was determined by the Kjekdhal method of Keeney and Nelson86. In this method, 0.2 g of finely ground samples of dry materials were digested with 3 ml of concentrated H2SO4 in the presence of 1.1 g digestion mixture containing CuSO4, K2SO4 and Se on a heating mantle for about 1 h. The digest was transferred quantitatively to the distillation flask and distilled in the presence of 10 ml of 10 MNaOH solutions. The distillate was collected in 5 ml boric acid mixed indicator solution and then titrated against 0.01 MHCl solution by adding 5 ml boric acid mix Indicator. Using the follow formula total N was calculated.
For obtaining the data on grain and biological yield, the central four rows were harvested from each plot at physiological maturity and data were recorded on various agronomical traits (biomass, grain yield and straw yield) and total N uptake in crop. Biomass yield was separated into grain and above ground plant tissue (i.e. shoot and leaves) and record their fresh bulk weight immediately. Five randomly chosen plant tissue sub-samples (ca. 1000 g fresh weight) from each sub-plot; were transferred to sealable plastic bags, and transferred to lab in container with ice to ensure no water losses occur from collected plant tissue. After transporting the plant tissue samples to the lab, fresh weight was immediately recorded. After recording the fresh weight, harvested material was placed in pre-weighed paper bags and dried at 65 °C for 7 days. Dry weights of the plant tissue after 7 days were recorded in order to calculate its moisture content or fraction. The grain yield was adjusted for moisture fraction, prior to obtaining its dry weight, using a moisture tester. For N uptake by above ground plant tissues (i.e. shoot and leaves) and by the grain, the two tissues separately samples were ground to a fine powder (for determination of the total N.
Grains yield was recorded after threshing of wheat plants taken from central four rows of each treatment and then converted into kg ha−1 by using the following formula.
Biological yield was recorded by harvesting 4 central rows in each plot, dried and weighed and then weight was converted into kg ha−1 using the following formula;
Nitrogen uptake and NUE were then calculated as follows:
Statistical analysis
Standard error (SE) of mean (n = 4) was quantified via descriptive statistics. The replicated data were processed using analysis of variance (ANOVA) followed by least significant difference (LSD) test (P < 0.05) using general linear model (GLM)87.
Plant material collection and use permission
No permission is required for plant material as it was purchased from certified dealer of local area.
Ethics approval and consent to participate
We all declare that manuscripts reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Complies with international, national and/or institutional guidelines
Experimental research and field studies on plants (either cultivated or wild), comply with relevant institutional, national, and international guidelines and legislation.
References
Battaglia, M. L., Lee, C. & Thomason, W. Corn yield components and yield responses to defoliation at different row widths. Agron. J. 110, 210–225 (2018).
Liu, X.Y. et al. Nitrate is an important nitrogen source for Arctic tundra plants. Proc. Natl. Acad. Sci. 115, 3398–3403 (2018).
Kumar, P. et al. Impacts of nitrogen fertilization rate and landscape position on select soil properties in switchgrass field at four sites in the USA. CATENA 180, 183–193 (2019).
Diatta, A. A. et al. Assessment of nitrogen fixation by mungbean genotypes in different soil textures using 15N natural abundance method. J. Soil Sci. Plant Nutr. https://doi.org/10.1007/s42729-020-00290-2 (2020).
Müller, C., Laughlin, R. J., Spott, O. & Rütting, T. Quantification of N2O emission pathways via a 15N tracing model. Soil Biol. Biochem. 72, 44–54 (2014).
Yi, Q. et al. Effects of nitrogen application rate, nitrogen synergist and biochar on nitrous oxide emissions from vegetable field in south China. PLoS ONE 12, e0175325 (2017).
Adnan, M. et al. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 9, 900 (2020).
Dawar, K. et al. Effects of the nitrification inhibitor nitrapyrin and the plant growth regulator gibberellic acid on yield-scale nitrous oxide emission in maize fields under hot climatic conditions. Pedosphere 31, 323–331 (2021).
Li, H., Liang, X., Chen, Y., Tian, G. & Zhang, Z. Ammonia volatilization from urea in rice fields with zero-drainage water management. Agric. Water Manag. 95, 887–894 (2008).
Dawar, K., Zaman, M., Rowarth, J. S., Blennerhassett, J. & Turnbull, M. H. Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions. Agric. Ecosyst. Environ. 144, 41–50 (2011).
Galloway, J. N. et al. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320, 889–892 (2008).
Schlesinger, W. H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA. 106, 203–208 (2009).
Dawar, K. et al. The effect of biochar and nitrogen inhibitor on ammonia and nitrous oxide emissions and wheat productivity. J. Plant Growth Regul. https://doi.org/10.1007/s00344-020-10283-1 (2021).
Finlayson-Pitts, B. J. & Pitts, J. N. Jr. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications (Academic Press Inc. Elsevier, 1999) https://doi.org/10.1016/B978-0-12-257060-5.X5000-X.
Saylor, R., Myles, L., Sibble, D., Caldwell, J. & Xing, J. Recent trends in gas-phase ammonia and PM2.5 ammonium in the Southeast United States. J. Air Waste Manag. Assoc. 65, 347–357 (2015).
Chow, J. C. et al. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 56, 1368–1380 (2006).
Battye, W. H. et al. Evaluating ammonia (NH3) predictions in the NOAA National Air Quality Forecast Capability (NAQFC) using in situ aircraft, ground-level, and satellite measurements from the DISCOVER-AQ Colorado campaign. Atmos. Environ. 140, 342–351 (2016).
Bray, C. D. et al. Evaluating ammonia (NH3) predictions in the NOAA National Air Quality Forecast Capability (NAQFC) using in-situ aircraft and satellite measurements from the CalNex2010 campaign. Atmos. Environ. 163, 65–76 (2017).
Galloway, J. N., Leach, A. M., Bleeker, A. & Erisman, J. W. A chronology of human understanding of the nitrogen cycle. Philos. Trans. R. Soc. B Biol. Sci. 368, 20130120 (2013).
Pinder, G. F. & Gray, W. G. Essentials of Multiphase Flow and Transport in Porous Media (Wiley, 2008).
Megaritis, A. G., Fountoukis, C., Charalampidis, P. E., Pilinis, C. & Pandis, S. N. Response of fine particulate matter concentrations to changes of emissions and temperature in Europe. Atmos. Chem. Phys. 13, 3423–3443 (2013).
Beusen, A. H. W., Bouwman, A. F., Heuberger, P. S. C., Van Drecht, G. & Van Der Hoek, K. W. Bottom-up uncertainty estimates of global ammonia emissions from global agricultural production systems. Atmos. Environ. 42, 6067–6077 (2008).
Del Grosso, S. J., Wirth, T., Ogle, S. M. & Parton, W. J. Estimating agricultural nitrous oxide emissions. EOS Trans. Am. Geophys. Union 89, 529 (2008).
IPCC (Intergovernmental Panel on Climate Change). Climate change 2013: The physical science basis. In Working Group I contribution to the IPCC Fifth Assessment Report. (Cambridge Univ. Press, 2014) https://doi.org/10.1017/cbo9781107415324.023.
Fowler, D. et al. Atmospheric composition change: Ecosystems–atmosphere interactions. Atmos. Environ. 43, 5193–5267 (2009).
Wrage, N., Velthof, G. L., Van Beusichem, M. L. & Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 33, 1723–1732 (2001).
Saggar, S. et al. Denitrification and N2O:N2 production in temperate grasslands: Processes, measurements, modelling and mitigating negative impacts. Sci. Total Environ. 465, 173–195 (2013).
Zhu, T. et al. Nitrogen mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol. Fertil. soils 49, 323–331 (2013).
Mueller, N. D. et al. Closing yield gaps through nutrient and water management. Nature 490, 254–257 (2012).
Wrage-Mönnig, N. et al. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 123, A3–A16 (2018).
Hayatsu, M., Tago, K. & Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 54, 33–45 (2008).
Chen, L., Zheng, H. & Wang, Z. Y. The formation of toxic compounds during biochar production. Appl. Mech. Mater. 361, 867–870 (2013).
Zhang, M. et al. A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agric. Ecosyst. Environ. 201, 43–50 (2015).
Sanz-Cobena, A., Sánchez-Martín, L. & García-Torres, V. A. Gaseous emissions of N2O and NO and NO3− leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agric. Ecosyst. Environ. 149, 64–73 (2012).
Sanz-Cobena, A. et al. Yield-scaled mitigation of ammonia emission from N fertilization: The Spanish case. Environ. Res. Lett. 9, 125005 (2014).
He, T. et al. Effects of application of inhibitors and biochar to fertilizer on gaseous nitrogen emissions from an intensively managed wheat field. Sci. Total Environ. 628–629, 121–130 (2018).
Battaglia, M., Groover, G. & Thomason, W. Harvesting and nutrient replacement costs associated with corn stover removal in Virginia. Virginia Cooperative Extension Publication CSES-229NP https://pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/CSES/cses-229/CSES-229.pdf (2018).
Battaglia, M. et al. The broad impacts of corn stover and wheat straw removal for biofuel production on crop productivity, soil health and greenhouse gas emissions: A review. GCB Bioenergy. 13(1), 45–57 (2021).
Saarnio, S., Heimonen, K. & Kettunen, R. Biochar addition indirectly affects N2O emissions via soil moisture and plant N uptake. Soil Biol. Biochem. 58, 99–106 (2013).
Malińska, K., Zabochnicka-Świątek, M. & Dach, J. Effects of biochar amendment on ammonia emission during composting of sewage sludge. Ecol. Eng. 71, 474–478 (2014).
Lehmann, J., Gaunt, J. & Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 11, 395–419 (2006).
Scheer, C., Grace, P. R., Rowlings, D. W., Kimber, S. & Van Zwieten, L. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant Soil 345, 47–58 (2011).
Taghizadeh-Toosi, A., Clough, T. J., Sherlock, R. R. & Condron, L. M. Biochar adsorbed ammonia is bioavailable. Plant Soil 350, 57–69 (2012).
Ameloot, N. et al. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 57, 401–410 (2013).
Nelissen, V., Saha, B. K., Ruysschaert, G. & Boeckx, P. Effect of different biochar and fertilizer types on N2O and NO emissions. Soil Biol. Biochem. 70, 244–255 (2014).
Sánchez-García, M., Roig, A., Sánchez-Monedero, M. A. & Cayuela, M. L. Biochar increases soil N2O emissions produced by nitrification-mediated pathways. Front. Environ. Sci. 2, 25 (2014).
Sun, H., Zhang, H., Powlson, D., Min, J. & Shi, W. Rice production, nitrous oxide emission and ammonia volatilization as impacted by the nitrification inhibitor 2-chloro-6-(trichloromethyl)-pyridine. Food Crop. Res. 173, 1–7 (2015).
Sun, H., Lu, H., Chu, L., Shao, H. & Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 575, 820–825 (2017).
Idrees, M. et al. Animal manure-derived biochars produced via fast pyrolysis for the removal of divalent copper from aqueous media. J. Environ. Manag. 213, 109–118 (2018).
Diatta, A. A., Fike, J. H., Battaglia, M. L., Galbraith, J. M. & Baig, M. B. Effects of biochar on soil fertility and crop productivity in arid regions: A review. Arab. J. Geosci. 13, 595 (2020).
Joseph, S. et al. Shifting paradigms: Development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag. 4, 323–343 (2013).
Zheng, J. et al. Biochar compound fertilizer increases nitrogen productivity and economic benefits but decreases carbon emission of maize production. Agric. Ecosyst. Environ. 241, 70–78 (2017).
Zhou, Z. et al. Biodegradation of a biochar-modified waterborne polyacrylate membrane coating for controlled-release fertilizer and its effects on soil bacterial community profiles. Environ. Sci. Pollut. Res. 22, 8672–8682 (2015).
Wen, P. et al. Microwave-assisted synthesis of a novel biochar-based slow-release nitrogen fertilizer with enhanced water-retention capacity. ACS Sustain. Chem. Eng. 5, 7374–7382 (2017).
El Sharkawi, H. M., Tojo, S., Chosa, T., Malhat, F. M. & Youssef, A. M. Biochar-ammonium phosphate as an uncoated-slow release fertilizer in sandy soil. Biomass Bioenergy 117, 154–160 (2018).
Chunxue, Y. A. O. et al. Developing more effective enhanced biochar fertilisers for improvement of pepper yield and quality. Pedosphere 25, 703–712 (2015).
Cole, D. P., Smith, E. A. & Lee, Y. J. High-resolution mass spectrometric characterization of molecules on biochar from pyrolysis and gasification of switchgrass. Energy Fuels 26, 3803–3809 (2012).
Clough, T., Condron, L., Kammann, C. & Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 3, 275–293 (2013).
Nelissen, V., Rütting, T., Huygens, D., Ruysschaert, G. & Boeckx, P. Temporal evolution of biochar’s impact on soil nitrogen processes—a 15N tracing study. Gcb Bioenergy 7, 635–645 (2015).
Mandal, S. et al. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 142, 120–127 (2016).
Chen, W. et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 61, 506–515 (2017).
Thies, J. & Rillig, M. C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology (2009).
Thangarajan, R. et al. The potential value of biochar in the mitigation of gaseous emission of nitrogen. Sci. Total Environ. 612, 257–268 (2018).
Agyarko-Mintah, E. et al. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 61, 129–137 (2017).
Feng, Y. et al. Biochar applied at an appropriate rate can avoid increasing NH3 volatilization dramatically in rice paddy soil. Chemosphere 168, 1277–1284 (2017).
Wang, S. et al. Different effects of biochar and a nitrification inhibitor application on paddy soil denitrification: A field experiment over two consecutive rice-growing seasons. Sci. Total Environ. 593–594, 347–356 (2017).
Abalos, D., Sanz-Cobena, A., Misselbrook, T. & Vallejo, A. Effectiveness of urease inhibition on the abatement of ammonia, nitrous oxide and nitric oxide emissions in a non-irrigated Mediterranean barley field. Chemosphere 89, 310–318 (2012).
Cantarella, H., Otto, R., Soares, J. R. & de Brito Silva, A. G. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 13, 19–27 (2018).
Mira, A. B. et al. Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agric. Ecosyst. Environ. 248, 105–112 (2017).
Li, Q. et al. Effect of a new urease inhibitor on ammonia volatilization and nitrogen utilization in wheat in north and northwest China. Food Crop. Res. 175, 96–105 (2015).
Marcinkowski, T. & Kierończyk, M. Effectiveness of urease inhibitor NBPT in mitigation ammonia emission from urea and urea ammonium nitrate solutions (UAN) applied in mineral fertilization of plants. J. Civil. Eng. Environ. Architect 62, 271–279 (2015).
Engel, R. E., Williams, E., Wallander, R. & Hilmer, J. Apparent persistence of N-(n-butyl) thiophosphoric triamide is greater in alkaline soils. Soil Sci. Soc. Am. J. 77, 1424–1429 (2013).
Dawar, K., Zaman, M., Rowarth, J. S., Blennerhassett, J. & Turnbull, M. H. Urea hydrolysis and lateral and vertical movement in the soil: Effects of urease inhibitor and irrigation. Biol. Fertil. Soils 47, 139–146 (2011).
Schirrmann, M. et al. Biochar reduces N2O emissions from soils: A meta-analysis. in EGU General Assembly Conference Abstracts 8265 (2017).
Case, S. D. C., McNamara, N. P., Reay, D. S. & Whitaker, J. Can biochar reduce soil greenhouse gas emissions from a Miscanthus bioenergy crop?. GCB Bioenergy 6, 76–89 (2014).
Sharma, S. P. Biochar for carbon sequestration. In Omics Technologies and Bio-Engineering 365–385 https://doi.org/10.1016/b978-0-12-815870-8.00020-6 (2018).
Kammann, C., Ratering, S., Eckhard, C. & Müller, C. Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J. Environ. Qual. 41, 1052 (2012).
Müller, C. & Sherlock, R. R. Nitrous oxide emissions from temperate grassland ecosystems in the Northern and Southern Hemispheres. Glob. Biogeochem. Cycles 18, GB1045 (2004).
Liu, S. et al. A meta-analysis of fertilizer-induced soil NO and combined NO+ N2O emissions. Glob. Chang. Biol. 23, 2520–2532 (2017).
Niu, Y. et al. Yield-scaled N2O emissions were effectively reduced by biochar amendment of sandy loam soil under maize-wheat rotation in the North China Plain. Atmos. Environ. 170, 58–70 (2017).
Zhang, W. et al. Effect of biochar on root morphological and physiological characteristics and yield in rice. Acta Agron. Sin. 39, 1445–1451 (2013).
Järveoja, J. et al. Impact of water table level on annual carbon and greenhouse gas balances of a restored peat extraction area. Biogeosciences 13, 2637–2651 (2016).
Araújo, E. D. S. et al. Calibration of a semi-opened static chamber for the quantification of volatilized ammonia from soil. Pesqui. Agropecuária Bras. 44, 769–776 (2009).
Jantalia, C. P. et al. Nitrogen source effects on ammonia volatilization as measured with semi-static chambers. Agron. J. 104, 1595–1603 (2012).
Li, B., Fan, C. H., Xiong, Z. Q., Li, Q. L. & Zhang, M. The combined effects of nitrification inhibitor and biochar incorporation on yield-scaled N2O emissions from an intensively managed vegetable field in southeastern China. Biogeosciences 12, 2003–2017 (2015).
Keeney, D. R. & Nelson, D. W. Nitrogen-inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2 (ed Page, A. L.) Vol. 9 643–698 (American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, 1983).
Steel, R. G., Torrie, J. H. & Dickey, D. A. Principles and Procedures of Statistics: A Biometrical Approach (McGraw Hill Book International Co., 1997).
Author information
Authors and Affiliations
Contributions
Conceptualization: S.F., K.D.; Data curation: I.M., S.S.A; Formal analysis: I.A.M., S.D., R.D.S.S.; Investigation: S.F., K.D.; Methodology: M.M.R.J.; A.K.; M.A.; resources: S.A.K., M.N.A.; Writing—original draft: K.D.; Writing—review and editing: S.F., R.D., S.D., R.D., J.B., M.H., M.J.A.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dawar, K., Fahad, S., Jahangir, M.M.R. et al. Biochar and urease inhibitor mitigate NH3 and N2O emissions and improve wheat yield in a urea fertilized alkaline soil. Sci Rep 11, 17413 (2021). https://doi.org/10.1038/s41598-021-96771-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-96771-0
This article is cited by
-
Pyrolysis temperature and time of rice husk biochar potentially control ammonia emissions and Chinese cabbage yield from urea-fertilized soils
Scientific Reports (2024)
-
Greenhouse gas emissions, carbon stocks and wheat productivity following biochar, compost and vermicompost amendments: comparison of non-saline and salt-affected soils
Scientific Reports (2024)
-
Effects of incorporating pine-woodchip biochar with reduced NP fertilizer on calcareous soil characteristics, organic carbon, NPK availability, and maize productivity
Journal of Soils and Sediments (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.