Abstract
Electrolytes have a crucial role in maintaining health and their serum levels are homeostatically maintained within a narrow range by multiple pathways involving the kidneys. Here we use metabolomics profiling (592 fasting serum metabolites) to identify molecular markers and pathways associated with serum electrolyte levels in two independent population-based cohorts. We included 1523 adults from TwinsUK not on blood pressure-lowering therapy and without renal impairment to look for metabolites associated with chloride, sodium, potassium and bicarbonate by running linear mixed models adjusting for covariates and multiple comparisons. For each electrolyte, we further performed pathway enrichment analysis (PAGE algorithm). Results were replicated in an independent cohort. Chloride, potassium, bicarbonate and sodium associated with 10, 58, 36 and 17 metabolites respectively (each P < 2.1 × 10−5), mainly lipids. Of all the electrolytes, serum potassium showed the most significant associations with individual fatty acid metabolites and specific enrichment of fatty acid pathways. In contrast, serum sodium and bicarbonate showed associations predominantly with amino-acid related species. In the first study to examine systematically associations between serum electrolytes and small circulating molecules, we identified novel metabolites and metabolic pathways associated with serum electrolyte levels. The role of these metabolic pathways on electrolyte homeostasis merits further studies.
Similar content being viewed by others
Introduction
Electrolytes have a crucial role in maintaining health and their serum levels are homeostatically maintained within a narrow range by multiple mechanisms usually involving the kidneys1. Sodium is the principal cation and osmotic agent in the extracellular fluid (ECF) compartment, while potassium is the main cation in the intracellular fluid (ICF) compartment with the two compartments separated by the cell membrane. The ECF volume is essentially preserved by the factors controlling the body sodium content mainly by the kidneys1. Of the total body sodium, 44% is in the ECF, 9% in the ICF and the remaining 47% in bone2. Only about half of the bone sodium is exchangeable and the rest are osmotically inactive. The ICF contains almost 98% of potassium1,3 and 75% of the body potassium stores is in skeletal muscle1. The sodium-potassium ATPase pump is the most important determinant of potassium distribution and the activity of the pump itself is increased by catecholamines and insulin4,5,6,7. The major anions balancing the cations are chloride and bicarbonate in the ECF while it is proteins and phosphates in the ICF. Regulation of the internal distribution of potassium is efficiently maintained by the kidneys as the movement of as little as 2% of the ICF potassium to the ECF can result in a potentially fatal increase in the serum potassium concentration1. The homeostatic maintenance of the controlled partition of fluid and electrolytes in different body compartments is essential for health, and in clinical medicine, most measurements of electrolyte concentration are performed on the extracellular fluid compartment, notably the blood serum. At a cellular level, metabolic processes are crucial for the regulation of fluid and electrolytes, the maintenance of serum protein level, and control of the amounts of sugar (glucose) and fats (lipids) in the blood the milieu interieur8.
Understanding the relationship between serum electrolytes and small molecules in circulation may generate insights into homeostatic mechanisms that are orthogonal to kidney functional status as proteins, phospholipids, cholesterol, and neutral fats account for 90% of the mass of dissolved solutes in the serum9. The aim of this study is to use metabolomics profiling to discover molecular markers and pathways associated with serum electrolyte levels.
Results
A total of 1523 adults from the TwinsUK cohort not on BP-lowering therapy and without renal impairment were included in the analysis of 592 fasting serum metabolites. The demographic characteristics of the study population are presented in Table 1. Chloride, potassium, bicarbonate, and sodium were found to correlate with 10, 58, 36 and 17 metabolites respectively (each P < 2.11 × 10−5 after multivariate adjustment; Table S1 in the Supplementary Material).
Metabolites-electrolytes associations
Backward linear regressions identified 6 metabolites independently associated with sodium (R2 = 0.07), 4 with chloride (R2 = 0.08), 8 with potassium (R2 = 0.16), and 13 with bicarbonate (R2 = 0.27), (Table 2). Serum sodium and chloride levels show significant direct association predominantly with amino-acid metabolites. In the methionine-cysteine pathway, serum sodium is significantly associated with cystine (Beta(SE) = 0.31(0.07), P = 6.78 × 10−6) and cystathionine (0.39(0.06), P = 2.67 × 10−10) and serum chloride with N-acetyl methionine (0.33(0.07), P = 7.65 × 10−7) (Table S1). In the urea cycle pathway, serum sodium directly associated with pro-hydroxy-pro (0.29(0.05), P = 1.00 × 10−7) and N-acetylcitrulline (0.31(0.06), P = 3.01 × 10−8). N-acetylputrescine (polyamine pathway) is inversely associated with serum sodium (−0.23(0.05), P = 5.61 × 10−6), while aspartate is inversely associated with serum chloride (−0.40(0.09), P = 7.34 × 10−6). Other significant associations are DHEA-S for serum sodium (0.25(0.06), P = 1.61 × 10−5) and the inverse association of γ-glutamyl-ε-lysine (−0.30(0.07), P = 9.11 × 10−6) and oxalate (−0.42(0.08), P = 1.37 × 10−7) for serum chloride (Table S1).
Serum potassium exhibited significant associations with tricarboxylic acid (TCA) cycle intermediates and metabolites involved in lipid metabolism pathways, including medium-chain fatty acids, long-chain and polyunsaturated fatty acids, dicarboxylic acids, acylcarnitines, and glycerol. Each of these metabolites were inversely correlated with serum potassium; only sphingomyelin showed a direct correlation. Associations between non-lipid-related metabolites and serum potassium were also observed (Table S1).
Serum bicarbonate concentrations significantly associated with amino-acid as well as lipid species. Most of the amino-acids were positively associated, including suberate, N-acetylphenylalanine, glycerophosphorylcholine (GPC), and citrulline, while lysine, threonine, and phenylpyruvate were inversely associated. All the lipids, 4-hydroxychlorothalonil, methyl indole-3-acetate, and 1-docosapentaenoyl-GPC (22:5n3) were positively associated.
Metabolite association by physicochemical patterns
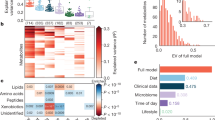
The results of metabolite association by physicochemical patterns are presented in Fig. 1. Hypochloremic alkalosis pattern is evident with metabolites both in the amino-acid and lipid groups, while volume contraction alkalosis pattern is predominantly seen with amino-acids and only one lipid (deoxycarnitine) metabolite. The amino-acid associations for hypochloremic alkalosis show highly significant associations with serum bicarbonate while the associations with potassium were only minor. In contrast, the lipid associations showed nominally significant bicarbonate and highly significant potassium associations. Nearly all the amino-acids in the volume contraction alkalosis group showed significant sodium and bicarbonate associations with borderline to no potassium associations. Aspartate was solely associated with hypochloremia with no associations with bicarbonate or other electrolytes. Interestingly, lipid metabolites were consistently significantly associated with hypokalemia either as a solo association or as part of a physicochemical pathway, while other metabolites showed variable association with potassium as part of physicochemical pathways. This contrasts with sodium or bicarbonate associations which showed the significant metabolites distributed across amino-acids and lipids.
Replication
We replicated the metabolites independently associated with either serum potassium or serum sodium in the SHIP-TREND cohort. Out of the 8 metabolites independently associated with serum potassium levels, 4 (indolelactate, myristoleate, 10-heptadecenoate, and linolenate [alpha or gamma]) were also measured in SHIP-TREND and were significantly replicated (Table 2), while out of the 6 metabolites independently associated to serum sodium, two (pro-hydroxy-pro and DHEAS) were also present in SHIP-TREND and pro-hydroxy-pro was significantly replicated.
Pathway enrichment analyses (Fig. 2) show long chain fatty acid, PUFA, monohydroxy fatty acid and acylcarnitine pathways specifically associated with just serum potassium. Dicarboxylic fatty acid pathway is enriched for potassium, chloride and bicarbonate while the TCA cycle pathway is associated with serum potassium and serum bicarbonate. Arginine-proline pathway is associated with only sodium and chloride.
Discussion
This is the first study to examine systematically associations between serum electrolytes and small circulating molecules. We identified yet unknown dependencies between metabolites and metabolic pathways associating with serum electrolyte levels, and replicated some of the top associations in an independent cohort. Of all the electrolytes, serum potassium showed the most significant associations with individual fatty acid metabolites and specific enrichment in fatty acid pathways. In contrast, serum sodium and bicarbonate showed associations predominantly with amino-acids. Mapping electrolyte and bicarbonate patterns based on the Stewart physicochemical model of acid-base chemistry10,11, we identify novel correlations between amino-acid and lipid metabolite associations with homeostatic patterns and further refine the electrolyte associations that are truly independent. These associations may be the consequences or the causes of these physicochemical patterns and they warrant replication and further research.
The major finding from this work is an inverse association between serum potassium and a range of long-chain and medium-chain fatty acids. A previous study of male Wistar rats showed induced potassium deficiency was associated with decreased levels of total lipids, phospholipids, and glycerol and increased levels of free fatty acids, implying that potassium-deficient rats use fatty acids as a substrate for oxidative metabolism12. Consistently, our findings indicate that low potassium is associated with increased fatty acids, glycerol, ketoacids, acylcarnitines and TCA cycle intermediates supporting an independent effect of serum potassium on lipolysis and fatty acid production. Furthermore, an increase in free fatty acids (FFA) is known to be a risk factor for insulin resistance and diabetes13. Two observational studies that have examined the association between serum potassium and risk of diabetes independent of antihypertensive use showed increased risk of type 2 diabetes with low potassium levels14,15. Our findings link low potassium with an increase in fatty acid suggesting a possible mechanism for increased risk of type 2 diabetes in relation to serum potassium, although this needs to be validated.
Serum potassium showed a direct association with sphingomyelin levels. A similar relationship was seen with serum sodium, but it did not attain statistical significance. In humans, sphingomyelins comprise nearly 85% of all sphingolipids and 10–20 mol% of the total serum membrane lipids16. Interestingly, the sphingolipids, sphingomyelin and ceramide have been shown to mediate loss of insulin sensitivity, to promote the characteristic diabetic proinflammatory state, and to induce cell death and dysfunction in important organs such as the pancreas and heart17.
Serum potassium also showed a significant inverse association with dihydroorotate (DHO) levels. Dihydroorotate dehydrogenase (DHODs) are flavin mononucleotide-containing enzymes that convert dihydroorotate to orotate in the only redox step in the de novo synthesis of pyrimidines18. The arthritis treatment drug leflunomide is a potent inhibitor of DHOD and a decrease in potassium levels is a known side-effect of this drug19. This indicates a possible role of DHOD in the regulation of serum potassium levels.
Here, serum sodium exhibited a predominantly significant direct association with several amino-acids and derivatives. The relationship between serum sodium and amino-acids may be related to the significant role played by the kidney in maintaining serum amino-acid concentrations; specifically, nearly 99% of all amino-acids filtered by the kidneys are reabsorbed in the proximal renal tubules and returned to serum20,21. The Na+ gradient is established across the cell by Na+-K+ ATPase, and inhibition of this pump by ouabain diminishes amino-acid uptake22. Among the amino-acids and related molecules, the most significant direct associations were with cysteine and its immediate upstream precursor cystathionine, which are components of the methionine-cysteine-taurine pathway. Our data show a direct relationship between circulating levels of cysteine and cystathionine and the electrolyte sodium. Cysteine may contribute to blood pressure control through multiple mechanisms including antioxidant effects that protect nitric oxide from the oxidative effects of reactive oxygen species (ROS), through modulation of the renin-angiotensin system23. Thus, the direct association between blood sodium and cysteine (and cystathionine) may reflect a compensatory mechanism by the body to modulate sodium-induced hypertension. Additionally, we also see two metabolites (21-hydroxypregnenolone and DHEA-S) from the adrenal-steroid synthesis pathway to feature within the top 20 associations with serum sodium, though given this should be taken with caution because of the sex imbalance of our discovery cohort.
The renal system provides a powerful mechanism to maintain acid-base balance within the body through modulation of bicarbonate levels in the blood, which help to keep the pH of the blood in the narrow range essential for life. Chloride is transported actively and passively with either HCO3− or sodium. It is absorbed in both the small and large intestine1. There is a normal Cl−–HCO3− exchange in the small bowel1. Acid-base balance and amino-acid metabolism are intimately related. Changes in acid-base balance influence the metabolic fate of many amino-acids24. Serum lysine shows significant inverse association with serum chloride and a direct association with serum bicarbonate. Administration of lysine monochloride is known to profoundly inhibit bicarbonate reabsorption and cause severe bicarbonate diuresis while lysine dichloride does not affect bicarbonate transport25. Citrulline malate has been shown to increase renal reabsorption of bicarbonate and we see a significant direct association between citrulline and bicarbonate26. Glutamine is the primary amino-acid involved in renal ammonia-genesis, a process intimately related to acid excretion24. The basic (cationic) amino-acids (lysine, arginine and histidine) yield neutral end-products plus a proton; sulfur (methionine and cysteine) amino-acids are also acidogenic because they generate sulfuric acid when oxidized2. The dicarboxylic (anionic) amino-acids (aspartate and glutamate, but not asparagine and glutamine) consume acid when oxidized and thus reduce the acid load of the diet2.
In this study, direct associations between serum bicarbonate and numerous protein amino-acids were observed, the strongest of which (Beta = 0.50) involved glutamine, which has the highest concentration in blood of all amino-acids. However, the bicarbonate associations all occurred as part of physicochemical patterns and we did not observe any bicarbonate association unaccompanied by association with other electrolytes.
We also note some study limitations. Our discovery sample consisted of females only. We have only replicated a fraction of the associations and we have not validated the physicochemical pathway signals. The metabolic associations observed do not necessarily have to be related to the renal system, but could also be related to metabolic alterations in the pancreas, liver, muscle, fat or other metabolically active organs. Further studies integrating urinary data will help dissect the renal contributions from others. Moreover, our association data provides only a snapshot on whether levels of proteins, phospholipids, cholesterol, and neutral fats have an impact on free circulating electrolyte concentrations and lack a mechanistic component. Notwithstanding, we identify metabolite pathways associated with complex homeostatic mechanisms which maintain electroneutrality and acid-base balance. This brings a common understanding of the physiological implications of shifts in electrolytes even among healthy individuals and may inform or support future physiological studies.
Methods
Study cohorts
Discovery cohort
Study subjects were twins enrolled in the TwinsUK registry, a national register of adult twins recruited as volunteers without selecting for any particular disease or trait27. All recruited twins were of the same sex. Here we analysed data from 1523 individuals, mainly females (96%) with a wide age range (32.8–74.7 years) without renal impairment (estimated glomerular filtration rate (eGFR) >60 mL/min per 1.73 m2) and not on any blood pressure (BP)-lowering medications. All individuals included in the analysis had metabolomics profiling and electrolytes measured in serum at the same time point. Serum sodium, potassium, chloride and bicarbonate were measured at St Thomas Hospital using the Kodak Ektachem dry chemistry analysers (Johnson and Johnson Vitros Ektachem machine which uses thin-film’dry’ chemistry technology to perform colorimetric and potentiometric analyses. Hospital laboratories in the UK are subject to robust external quality control schemes (NEQAS [National External Quality Assessment Service]). Direct potentiometric electrolyte assays were performed using ion-selective electrodes which has a selective membrane in contact with both the test solution (patient’s sample) and an internal filling solution (containing the test ion at a fixed concentration). The coefficients of variation for chloride, bicarbonate, sodium and potassium were 1%, 1.5%, 0.9% and 4.8% respectively.
The study was approved by St. Thomas’ Hospital Research Ethics Committee. All participants provided informed written consent. The investigation conforms to the principles outlined in the Declaration of Helsinki. TwinsUK metabolomics, expression and phenotypic data are publicly available upon request on the department website (http://www.twinsuk.ac.uk/data-access/accessmanagement/).
Replication cohort
The replication cohort consisted of individuals not on BP-lowering therapy and without renal impairment drawn from the Study of Health in Pomerania (SHIP-TREND), a population-based research project in West Pomerania, a rural region in north-east Germany28. In total, 938 individuals (aged 20–79 years) with fasting serum metabolomic profiles available using the Metabolon, Inc. (Durham, USA) platform (for details see29) and with measure potassium and sodium were analysed.
Metabolomic profiling
Non-targeted metabolite detection and quantification was conducted by the metabolomics provider Metabolon, Inc. (Durham, USA)on fasting serum samples, as described previously30. The metabolomic dataset measured by Metabolon includes 592 known metabolites containing the following broad categories - amino-acids, peptides, carbohydrates, energy intermediates, lipids, nucleotides, cofactors and vitamins, and xenobiotics.
Statistical analysis
Statistical analysis was carried out using Stata version 11 and R. Quality control of metabolomics data was carried out as previously described30. We inverse normalised the data as the metabolite concentrations were not normally distributed. To avoid spurious false-positive associations due to small sample size, we excluded metabolic traits with more than 20% missing values leaving for analysis 592 metabolites of known chemical identity. We imputed the missing values using the minimum run day measures.
We looked for metabolites (outcome) associated with chloride, sodium, potassium and bicarbonate (exposure) by running linear mixed models adjusting for age, body mass index (BMI), gender and family relatedness. We corrected for multiple comparisons using Bonferroni correction, thus giving a significant threshold of P = 2.1 × 10−5 (0.05/(592 metabolites x 4 phenotypes). We then used a stepwise backward regression model to identify a set of metabolites that were significantly associated with each phenotype using P < 0.01 as cut-off threshold.
We replicated the metabolites independently associated with serum sodium or serum potassium in 938 individuals from SHIP using linear regressions and adjusting for age, BMI and gender. Serum chloride and serum bicarbonate measurements were not available for the SHIP-TREND cohort.
Finally, for each electrolyte, we performed pathway enrichment analysis in the TwinsUK data using the PAGE algorithm implemented in the R-package piano31. Enrichment tests were performed for each given pathway while taking into account the sign of the associations. Statistical significances of all enrichment analyses were assessed using empirical p-values estimated from a background distribution of 10,000 random permutations of the variable labels, and multiple testing correction was used to correct for the number of tested pathways.
Physicochemical pathways
Acid–base balance and electrolyte homeostasis are intricately connected to maintain the internal
environment of the body within narrow and rigidly controlled limits. Thus, the study of metabolomic associations of electrolytes need to be considered in the context of these homeostatic processes. We mapped common electrolyte and acid-base patterns, based on the bicarbonate-centric physicochemical model and electroneutrality, to metabolite associations. We classified metabolites showing a hypochloremic alkalosis pattern, if they showed a nominally significant positive association with serum bicarbonate and a negative association with serum chloride and serum potassium. We contrast this from volume contraction alkalosis, which we defined as metabolites showing nominally significant positive association with serum bicarbonate, positive association with serum sodium and variable association with serum potassium and chloride. Hyperchloremic acidosis and proximal renal tubular acidosis patterns were defined by nominally significant negative association with serum bicarbonate along with positive association with serum chloride in the former and negative association with serum chloride in the latter. Pure electrolyte associations were defined as hypo- or hyper- natremia or kalemia when the metabolites showed null association with serum bicarbonate.
References
Youn, J. H. & McDonough, A. A. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71, 381–401, https://doi.org/10.1146/annurev.physiol.010908.163241 (2009).
Keyes, J. L. Fluid, Electrolyte, and Acid-Base Regulation. (Jones and Bartlett Publishers, 1999).
Demigne, C., Sabboh, H., Remesy, C. & Meneton, P. Protective effects of high dietary potassium: nutritional and metabolic aspects. The Journal of nutrition 134, 2903–2906, https://doi.org/10.1093/jn/134.11.2903 (2004).
Gavryck, W. A., Moore, R. D. & Thompson, R. C. Effect of insulin upon membrane-bound (Na+ + K+)-ATPase extracted from frog skeletal muscle. J Physiol 252, 43–58 (1975).
Moore, R. D. Effect of insulin upon the sodium pump in frog skeletal muscle. J Physiol 232, 23–45 (1973).
Ewart, H. S. & Klip, A. Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. The American journal of physiology 269, C295–311, https://doi.org/10.1152/ajpcell.1995.269.2.C295 (1995).
Bertorello, A. M. & Katz, A. I. Short-term regulation of renal Na-K-ATPase activity: physiological relevance and cellular mechanisms. The American journal of physiology 265, F743–755, https://doi.org/10.1152/ajprenal.1993.265.6.F743 (1993).
Hoenig, M. P. & Zeidel, M. L. Homeostasis, the milieu interieur, and the wisdom of the nephron. Clin J Am Soc Nephrol 9, 1272–1281, https://doi.org/10.2215/CJN.08860813 (2014).
Hall, J. & Guyton, A. Guyton and Hall textbook of medical physiology. (Elsevier, 2015).
Seifter, J. L. Integration of acid-base and electrolyte disorders. The New England journal of medicine 371, 1821–1831, https://doi.org/10.1056/NEJMra1215672 (2014).
Stewart, P. A. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol 61, 1444–1461 (1983).
Hohenegger, M., Marktl, W. & Rudas, B. Lipid metabolism in the potassium deficient rat. Pflugers Archiv: European journal of physiology 351, 331–338 (1974).
Boden, G. & Shulman, G. I. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. European journal of clinical investigation 32(Suppl 3), 14–23 (2002).
Chatterjee, R. et al. Serum and dietary potassium and risk of incident type 2 diabetes mellitus: The Atherosclerosis Risk in Communities (ARIC) study. Archives of internal medicine 170, 1745–1751, https://doi.org/10.1001/archinternmed.2010.362 (2010).
Heianza, Y. et al. Low serum potassium levels and risk of type 2 diabetes: the Toranomon Hospital Health Management Center Study 1 (TOPICS 1). Diabetologia 54, 762–766, https://doi.org/10.1007/s00125-010-2029-9 (2011).
Moskot, M., Bochenska, K., Jakobkiewicz-Banecka, J., Banecki, B. & Gabig-Ciminska, M. Abnormal Sphingolipid World in Inflammation Specific for Lysosomal Storage Diseases and Skin Disorders. International journal of molecular sciences 19, https://doi.org/10.3390/ijms19010247 (2018).
Russo, S. B., Ross, J. S. & Cowart, L. A. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handbook of experimental pharmacology, 373–401, https://doi.org/10.1007/978-3-7091-1511-4_19 (2013).
Alves, C. N., Silva, J. R. & Roitberg, A. E. Insights into the mechanism of oxidation of dihydroorotate to orotate catalysed by human class 2 dihydroorotate dehydrogenase: a QM/MM free energy study. Physical chemistry chemical physics: PCCP 17, 17790–17796, https://doi.org/10.1039/c5cp02016f (2015).
Kraft, J. et al. Biological effects of the dihydroorotate dehydrogenase inhibitor polyporic acid, a toxic constituent of the mushroom Hapalopilus rutilans, in rats and humans. Arch Toxicol 72, 711–721 (1998).
Silbernagl, S. The renal handling of amino acids and oligopeptides. Physiol Rev 68, 911–1007, https://doi.org/10.1152/physrev.1988.68.3.911 (1988).
Young, G. A. Amino acids and the kidney. Amino acids 1, 183–192, https://doi.org/10.1007/BF00806915 (1991).
Silva, E. & Soares-da-Silva, P. In International Review of Cell and Molecular Biology (ed Jeon, K. W.) 99–132 (Academic Press, 2012).
Vasdev, S., Singal, P. & Gill, V. The antihypertensive effect of cysteine. Int J Angiol 18, 7–21 (2009).
Patience, J. F. A review of the role of acid-base balance in amino acid nutrition. Journal of animal science 68, 398–408 (1990).
Walker, W. G., Dickerman, H. & Jost, L. J. Mechanism of Lysine-Induced Kaliuresis. The American journal of physiology 206, 409–414, https://doi.org/10.1152/ajplegacy.1964.206.2.409 (1964).
Callis, A., Magnan de Bornier, B., Serrano, J. J., Bellet, H. & Saumade, R. Activity of citrulline malate on acid-base balance and blood ammonia and amino acid levels. Study in the animal and in man. Arzneimittel-Forschung 41, 660–663 (1991).
Moayyeri, A., Hammond, C. J., Valdes, A. M. & Spector, T. D. Cohort Profile: TwinsUK and healthy ageing twin study. International journal of epidemiology 42, 76–85, https://doi.org/10.1093/ije/dyr207 (2013).
Volzke, H. et al. Cohort profile: the study of health in Pomerania. International journal of epidemiology 40, 294–307, https://doi.org/10.1093/ije/dyp394 (2011).
Pietzner, M. et al. Comprehensive metabolic profiling of chronic low-grade inflammation among generally healthy individuals. BMC medicine 15, 210, https://doi.org/10.1186/s12916-017-0974-6 (2017).
Long, T. et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nature genetics 49, 568–578, https://doi.org/10.1038/ng.3809 (2017).
Varemo, L., Nielsen, J. & Nookaew, I. Enriching the gene set analysis of genome-wide data by incorporating directionality of gene expression and combining statistical hypotheses and methods. Nucleic acids research 41, 4378–4391, https://doi.org/10.1093/nar/gkt111 (2013).
Acknowledgements
This work was supported by the MRC AimHy (MR/M016560/1) project grant, by the Wellcome trust programme grant HATS (Genetics of ageing: The genetic and environmental determinants of ageing in women), project No 081878/Z/06/Z and by the CDRF. Twins UK receives funding from the Wellcome Trust European Community’s Seventh Framework Programme (FP7/2007-2013 to TwinsUK); the National Institute for Health Research (NIHR) Clinical Research Facility at Guy’s & St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. HLI collaborated with KCL to produce the metabolomics data from Metabolon Inc. S.P. is funded by the Medical Research Council (MR/M016560/1 and The AIM-HY Study) and the British Heart Foundation (PG/12/85/29925 and CS/16/1/31878). L.M. is funded by a BHF fellowship (FS/14/52/30901). SHIP is funded by the German Federal Ministry of Education and Research (BMBF, grants 01ZZ96030 and 01ZZ0701), the Ministry of Education, Research and Cultural Affairs as well as the Ministry of Social Affairs of the Federal State of Mecklenburg-West Pomerania. K.S. was supported by the ‘Biomedical Research Program’ funds at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. We like to thank all the study participants.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: C.M., L.M.C., S.P. Performed the experiments: R.P.M. Analyzed the data: C.M., J.Z., M.P. Contributed reagents/materials/analysis tools: A.A., K.S., M.M., N.F., T.D.S. Wrote the manuscript: C.M., L.M.C., R.P.M., S.P. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.P.M. is employee of Metabolon, Inc. T.D.S. is co-founder of MapMyGut Ltd. All other authors declare no competing financial and non-financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Menni, C., McCallum, L., Pietzner, M. et al. Metabolomic profiling identifies novel associations with Electrolyte and Acid-Base Homeostatic patterns. Sci Rep 9, 15088 (2019). https://doi.org/10.1038/s41598-019-51492-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51492-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.