Abstract
Here, we developed a new approach to synthesize NiCo2S4 thin films for supercapacitor application using the successive ionic layer adsorption and reaction (SILAR) method on Ni mesh with different molar ratios of Ni and Co precursors. The five different NiCo2S4 electrodes affect the electrochemical performance of the supercapacitor. The NiCo2S4 thin films demonstrate superior supercapacitance performance with a significantly higher specific capacitance of 1427 F g−1 at a scan rate of 20 mV s−1. These results indicate that ternary NiCo2S4 thin films are more effective electrodes compared to binary metal oxides and metal sulfides.
Similar content being viewed by others
Introduction
The development of sustainable electrochemical energy conversion methods and storage has sparked the interest of researchers aiming to produce devices that offer high power output, a long lifetime, and a short charging time to meet the increasing demand for power in daily life1,2. Supercapacitors have emerged as a promising energy storage device in this respect, with outstanding properties that include a high power density, a long cycle life, short response, rapid charging times, moderate energy density, modest maintenance requirements, and safe operation2,3,4,5. However, existing supercapacitor electrodes are mainly composed of activated carbon, binders, and conductivity enhancers, thus it is difficult to develop simple, lightweight supercapacitors. In general, supercapacitor performance depends mainly on the properties of the materials and synthesis methods used. Besides, carbon-based materials such as activated carbon, carbon nanotubes, and graphene exhibit low capacitance due to their surface dominant electrochemical double-layer storage mechanism2.
In recent years, significant research progress has been made on improving supercapacitor performance via the fabrication of nickel-cobalt-sulfide nanostructured electrode materials due to their higher electronic conductivity, strong redox reactions, high theoretical capacity, high cycling stability, variable oxidation states, environmental benign nature, easy and low preparation cost6. Nanomaterial composed of ternary metal sulfides with various structural morphologies have been applied as high performance pseudo supercapacitor electrodes, such as nanosheets arrays2, nanotubes7, nanorods8, urchins9, nanosheets10, hallow spheres11,12, nano-buds13, and flowers14. Ternary sulfides such as nickel cobalt sulfides have unique physical, chemical, and electrochemical properties, such as high specific capacitance, good electrochemical stability, and higher electronic and electrical conductivity compared to their oxide counterparts and binary sulfides15,16,17. In addition, the combination of Co and Ni in bimetallic sulfides leads to a higher redox potential and enhanced electrochemical energy storage performance compared with monometallic sulfides9,18.
Recently, Wei et al.11 synthesized hierarchically porous NiCo2S4 core-shell hollow spheres using the self-template method, which represents a one-pot solvothermal approach to the synthesis of hierarchical Ni-Co solid sphere precursors, followed by conversion to hierarchically porous NiCo2S4 core-shell hollow spheres via sulfidation treatment. Their core-shell hollow spheres depicted a specific capacitance of 1870.2 F g−1 at 2.0 A g−1 and excellent long-duration cycling. Shahrokhian et al.17 also reported a simple and efficient method for fabricating ternary metal sulfide electrodes based on the electrodeposition of nickel cobalt iron sulfide (Ni-Co-Fe-S) ultrathin nanosheets on the surface of 3D nickel nanocone arrays. The ternary metal sulfide electrode exhibited a high specific capacitance of 2159.7 F g−1 at 7 A g−1 with excellent rate capabilities. Lei et al.19 prepared NiCo2S4 nanosheets on carbon sponge using a hydrothermal method, leading to enhanced conductivity and ideal structural integrity. Their composite electrode delivered a specific capacitance of 1093 F g−1 at 0.5 A g−1 in a three-electrode system. Tao et al.20 developed a hierarchical Ni-Co-S nanosheets array based on a metal–organic framework on an Ni-foam electrode for supercapacitor applications. This nanosheets array system delivered rapid electron transportation, a short ion diffusion path, abundant active sites, and rich redox reactions. The electrode exhibited an electrochemical capacitance of 1406.9 F g−1 at 0.5 A g−1. Sun et al.21 reported the hydrothermal synthesis of hierarchical Ni-Co-S@Ni-W-O core–shell nanosheets arrays on nickel foam, producing a high specific capacitance of 1988 F g−1 at 2 A g−1.
The electrochemical performance of Ni-Co-S is influenced by several factors. For example, Jiang et al.22 synthesized porous NixCo3−xS4 (x = 0, 1, 1.5, 2, and 3) nanoparticles with various compositions of Ni–Co–S. The Ni1.5Co1.5S4 sample produced the highest specific capacitance (1093 F g−1 at 1 A g−1) in a three-electrode system. In contrast, Gao et al.23 reported the preparation of Ni3−xCoxS4 (x = 1.5, 2, 2.25, and 2.5) nanotube arrays on carbon cloth with different Co/Ni molar ratios. Ni0.75Co2.25S4 demonstrated a capacitance of 1856 F g−1 at 1 A g−1. Similarly, Chen et al.24 reported the fabrication of sea urchin-like Ni–Co sulfides with different ratios of Ni/Co, in which Ni0.25Co0.75S delivered the highest specific capacitance (676 C g−1 at 1 A g−1) in a three-electrode system.
Though the fabrication of NiCo2S4 nanostructures has been well researched to date, the synthesis of NiCo2S4 with specific hierarchical structures requires further investigation. In this sense, the development of Ni-Co-S electrode materials with varying compositions of Ni and Co is crucial to achieve optimal supercapacitor properties, such as high electrical conductivity, a porous structure, large capacitance, and excellent electrochemical stability. In the present study, we report the facile synthesis of Ni-Co-S (NCS) flake-like nanostructures on Ni mesh for supercapacitor applications. The effect of varying the composition of Ni and Co in the NCS nanostructures was also studied because the electrochemical performance of NCS electrodes can be enhanced by altering the molar ratio of Ni to Co precursors.
Experimental Details
Materials
Nickel nitrate hexahydrate (Ni(NO3)2.6H2O), cobalt nitrate hexahydrate (Co(NO3)2.6H2O), sodium sulfide nonahydrate (Na2S.9H2O), pottasium hydroxide (KOH) and ammonium hydroxide (NH4OH) were procured from Sigma Aldrich and used without futher purification.
Synthesis of NiCo2S4 thin films
The fabrication process for nanoflakes-like NiCo2S4 thin films using the successive ionic layer adsorption and reaction (SILAR) method on Ni mesh is represented in Fig. S1a. SILAR is simplistic, economically feasible and most useful method in order to growth of material directly on the conducting and non-conducting thin films. Furthermore, SILAR method holds a potential to improve different surface morphologies by monitoring simple preparative parameters. To fabricate the basic form of this NCS nanostructure with a Ni to Co molar ratios of 40:10 (NCS:40), 0.05 M Ni(NO3)2.6H2O, 0.1 M Co(NO3)2.6H2O and 0.1 M Na2S.9H2O were each dissolved in 100 mL of double-distilled water (DDW) and pH was adjusted to 12 by addition ammonia solution. First, the flexible Ni mesh was immersed in a Ni(NO3)2 bath for 20 s to allow the Ni2+ ions to be adsorbed on the surface of the mesh. The Ni mesh was then cleaned in DDW for 5 s to remove any loosely bound Ni2+ ions. Following this, the Ni2+ adsorbed Ni mesh was placed in a Co(NO3)2.6H2O bath for 20 s to allow the adsorption of Co2+ ions onto the surface. The mesh was then washed with DDW to remove loosely bound Co2+ ions. In the final step, the Ni2+/Co2+ deposited flexible Ni mesh was immersed in the Na2S precursor solution where S2− ions from the solution reacts with Ni2+ and Co2+ ions to form mixed metal sulfide film. Furthermore, it is rinsed in the DDW for 5 s to remove loosely bound S2− ions. This process was repeated for 6 SILAR cycles to achieve appropriate film thickness25,26. In this work, different molar ratios between Ni to Co precursors have been considered (40:10, 30:20, 25:25, 20:30, and 10:40, referred to as NCS:40, NCS:30, NCS:25, NCS:20, and NCS:10, respectively).
Characterization techniques
X-ray diffraction analysis of the prepared thin films was performed using a Rigaku Ultima III diffractometer operated at 40 kV and 40 mA with Cu Kα radiation (1.54 A°) as a source, with a scanning range of 2θ over 20–80°. Step-scan mode was applied with a step width of 0.02°, a sampling time of 1 s, and a measurement temperature of 25 °C. The chemical states of the elements present in the thin films were investigated by X-ray photoelectron spectroscopy (XPS; ULVAC-PHI Quantera SXM). The morphology of the samples was investigated via field emission scanning electron microscopy (FE-SEM) using a JEOL JSM-7100. The nanostructures of the prepared samples were visualized by high-resolution transmission electron microscopy (TEM; JEOL, Model JEM-2100).
Electrode preparation and electrochemical measurements
Electrochemical performance was evaluated using a Versa Stat 3 (Princeton Applied Research) workstation by measuring cyclic voltammetry (CV), galvanostatic charge/discharge, and electrochemical impedance. A reference electrode probe was connected to an Ag/AgCl electrode and a counter-electrode probe was connected to thin platinum foil. A working electrode probe attached to the NCS/Ni mesh electrode and immersed in a 5 M KOH electrolyte solution. A cyclic potential sweep was applied with initial and final voltages of −0.4 and 0.6 V, respectively. Electrochemical impedance measurements were taken between 1 Hz and 100 kHz with an AC amplitude of 10 mV and a bias potential of 0.4 V.
Results and Discussion
Formation of NiCo2S4 thin films
NiCo2S4 thin films were synthesized by dipping a substrate into aqueous solutions of Ni(NO3)2.6H2O, Co(NO3)2.6H2O, and Na2S.9H2O separately. SILAR process is mainly based on ion by ion deposition, which exhibits the deposition at nucleation places on the immersed surfaces of Ni mesh. The growth mechanism of NiCo2S4 thin films by SILAR method is depicted as follows. When Ni(NO3)2.6H2O, Co(NO3)2.6H2O, and Na2S.9H2O independently dissolved in DDW water, following three reactions occur, respectively.
When Ni mesh is immersed in the above solution 1, Ni2+ ions start adsorbing on the Ni mesh due to attraction between Ni2+ ions and the surface of Ni mesh. These forces may be cohesive or van der Waals forces or chemical attractive forces. Similarly, Ni2+ adsorbed Ni mesh is immersed in the above solution 2, Co2+ ions are adsorbed on Ni mesh. Final step of reaction process was followed by the immersion of Ni2+/Co2+ coated Ni mesh into Na2S anionic solution. During this process, Ni2+ and Co2+ ions reacts with S2− ions from the Na2S anionic solution. Possible reactions are shown below,
In the aqueous solution, Na2S dissolves to form S2− ions that are simultaneously hydrolyzed to generate HS− and H2S species. These species serve as the sulfur sources for the ion-exchange reaction that converts Ni and Co precursors to form NiCo2S4. Previously, Dubal et al.25 have reported similar reaction mechanism for the deposition of Co–Ni mixed hydroxide thin films.
X-ray diffraction (XRD) analysis
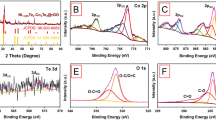
The XRD patterns for the NiCo2S4 thin films prepared with various molar ratios of Ni to Co source, such as 40:10, 30:20, 25:25, 20:30, 10:40 are shown in Fig. 1. The peaks at 17.54°, 26.60°, 31.85°, 33.06°, 48.05°, 50.53°, 65.42°, and 74.19° are attributed to the (111), (220), (311), (222), (422), (511), (533), and (642) planes of the metallic nickel cobalt sulfide, respectively27. For sample NCS:20, two main peaks are observed at 48.05° and 65.42°, which are ascribed to the (422) and (533) planes, corresponding to the ternary phase of the nickel cobalt sulfide. In sample NCS:25 (shown in Fig. 1), one peak was observed at 33.06°, which is in good agreement with the Ni-Co-S28. Furthermore, one strong peak at 44.4°, which is originating from the Ni foam29. All peaks and peak positions closely match JCPDS card 020-0782, with the synthesized composites exhibiting a cubic crystal structure with lattice parameters a = b = c = 9.30, which closely follows standard results30,31. Therefore, it can be concluded that the NiCo2S4 was successfully deposited on the flexible Ni mesh31.
X-ray photoelectron spectroscopy (XPS) analysis
XPS was used to further examine the chemical state, elemental valency, and chemical composition of the NiCo2S4 thin films prepared at optimized molar ratio of Ni and Co. Figure 2a displays the survey spectrum of the NCS:25 sample, which show the presence of Ni, Co, and S elements. Figure 2(a–d) presents the core level spectra for Ni 2p, Co 2p, and S 2p, respectively. The peaks at 168.65 eV correspond to S 2p1/2, while the peaks at 857.50 eV and 875.19 eV are related to Ni 2p3/2 and Ni 2p1/2, respectively31. The peak at 533.41 eV is related to oxygen due to exposure to the air30. The Co2p spectra exhibited peaks at 783.19 and 798.37 eV, which are attributed to Co 2p3/2 and Co 2p1/2, respectively. The energy difference between Ni 2p and Co 2p was 17.69 and 15.19 eV, which reflects differences in Ni and Co valence (e.g., Ni2+, Ni3+, Co2+, and Co3+)9,32,33. For the core-level spectra of S 2p, the peaks at 168.65 eV is related to S 2p1/2 (Fig. 2d). From Fig. 2, it can be concluded that Ni2+, Co2+, Ni3+, Co3+ and S2− are present in the NiCo2S4 thin films. The XPS results closely agree with previously reported data for NiCo2S4 thin films30,34.
Morphological (FE-SEM) analysis
Figures 3(a–d) and S1b present FE-SEM images of NiCo2S4 thin films prepared for different molar ratios of Ni and Co on Ni mesh, and corresponding high magnification images are shown in the inset. The high magnified image of NCS:25 sample is shown in the Fig. S1b. It can be seen that all of the NiCo2S4-coated Ni mesh samples were uniformly covered with different types of nanostructure, including petal-shaped structures similar to the top view of a rose, spherical nanoparticles, and interconnected nanoflakes35,36,37. We clearly observed that the nanostructure, depth, thickness, and length of the vertical interconnected nanoflakes were affected by the ratio of Ni to Co. The films fabricated with lower levels of both Ni and Co (i.e., Ni:Co ratios of 40:10 and 10:40) are shown in Figs 3a and S1b. Both NiCo2S4 thin film samples (Fig. 3(a–d)) exhibited an equal covering of larger-sized nanoplates-like structures and comparatively fewer porous nanostructures9,38. In contrast, for a Ni/Co ratio of 25:25, the Ni mesh was completely covered with vertically interconnected nanoflakes in a barrier-wall-like structure (as shown in Fig. 3c). This type of nanostructure provides a large active surface area and faster ion transfer during the electrochemical reaction between the NiCo2S4 electrode and KOH electrolyte. High scale FE-SEM images of the NiCo2S4 thin film are also presented in Fig. S1b31,38. Compared with the other composites, sample NCS:25 exhibited more hierarchical flake-like inter-network structures, indicating that this sample provides a higher porous surface area for the NiCo2S4 thin film and higher electrical conductivity31,39. The preparation and schematics growth formation of hierarchical NiCo2S4 thin films on Ni mesh is schematically presented in Fig. S1c40,41,42. In the formation thin films is related to the main four steps, such as nucleation, aggregation, coalescence and growth of nanostructure. Figure 4a shows the transmission electron microscopy (TEM) images of optimized NCS:25 sample. This structure provides a higher surface area because the vertically interconnected nanoflakes were more porous with a thickness and length of 15–20 nm and 80–120 nm, respectively. Vertically interconnected plates are greatly beneficial because they supply both sides of a nanoplates and the ion exchange process is prominently facilitated during cell testing41,42. The composition of the nanocomposites was determined using the EDS analysis. The representative EDS spectra of NCS:25 composite is shown in Fig. 4b. The sample shows the presence of Ni, Co and S elements. Figure 4b shows EDS spectrum and inset shows the elemental mapping of NCS:25 sample which confirms formation of a porous nanostructure, respectively40. Elemental mapping shows that all the elements are present and homogenously distributed over the film surface for the optimized NCS:25 sample, which was prepared with an equal molar ratios of Ni and Co. Quantitative elemental analysis of nanocomposite revealed that the composition ratio of Ni:Co was in good agreement with stoichiometric ratio. The EDS spectrum indicates that samples are consistent with their elemental signals and stoichiometry is as expected. These results are in good agreement with the XPS results.
Electrochemical studies
Figures 5a and S2 (supporting information) present the CV curves for the NiCo2S4 composite thin films for various Ni and Co ratios (NCS:40, NCS:30, NCS:25, NCS:20, and NCS:10) at scan rates from 20 to 100 mV s−1 with a 5 M KOH electrolyte. Two redox peaks can be observed in all composite NiCo2S4 thin films due to their pseudocapacitor behavior and the faradaic reaction32,43. The NCS:25 composite (Fig. 5a) demonstrated a higher current density and areal capacitance compared with the other composites due to the vertically interconnected nanoflakes providing a higher surface area and faster ion exchange and faradaic reactions31,32,39,44. The higher current density indicates that the NiCo2S4 thin films shows a higher specific capacitance compared with other composites. The NCS:25 electrodes exhibited the best electrical properties compared with the other four samples because it promoted higher electrical conductivity and faster electron transport and was more porous30,31,45. The redox reaction showed the most intense peaks of the NiCo2S4 thin films due to the presence of different Ni2+ and Co2+ valencies in the KOH electrolyte7.
(a) CV curves for the optimized NiCo2S4 composite thin films for various Ni and Co ratios (NCS:25) at scan rate from 20 to 100 mV s−1 with a 5 M KOH electrolyte, (b) Specific capacitance of NCS:30, NCS:25, and NCS:20 composite thin films, (c) Charge-discharge curves for NCS:30, NCS:25, and NCS:20 at 10 mA cm−2 current density in 5 M KOH with −0.4 to 0.6 V potential window, (d) Specific capacitance for NCS:40, NCS:30, NCS:25, NCS:20 and NCS:10 electrodes, (e) Charge-discharge curves for NCS:25 at 1–10 mA cm−2 current densities in a 5 M KOH electrolyte, (f) specific capacitance of NCS:25 electrode at various current densities from 1–10 mA cm−2.
Figures 5b and S3 shows the specific capacitance of NCS:40, NCS:30, NCS:25, NCS:20, and NCS:10 for various scan rates from 20–100 mV s−1. The specific capacitance was calculated using the following Eq. 1046,47:
where, CS is the specific capacitance (F g−1), (Vc-Va) is the potential window, m is the mass of the electrode, and v is the scan rate. The specific capacitance were found 570, 896, 1427, 1106, and 790 F g−1 for NCS:40, NCS:30, NCS:25, NCS:20, and NCS:10, respectively, indicating that the NCS:25 electrode was most suitable for supercapacitor applications due to its superior electrochemical performance. Figures 5a and S2(b,c) shows CV curves of NCS:30, NCS:25 and NCS:20 electrodes with different scan rates from 20–100 mV s−1. The sample NCS:25 shows the highest specific capacitance values with 20 mV s−1 scan rate. The values of specific capacitance decreased with an increase in the scan rate: 1427, 1202, 1118, 1046, and 976 F g−1 for a scan rate of 20, 30, 40, 50 and 100 mV s−1, respectively. Several previously reported NiCo2S4 based electrodes and their supercapacitor performance are tabulated in the Table 1. It indicates that the NiCo2S4 thin films synthesized by chemical SILAR method and vertical interconnected flakes like nanostructure shows the better performance.
Figures 5c and S4 present the charge-discharge curves for NCS:40, NCS:30, NCS:25, NCS:20, and NCS:10 at current density of 10 mA cm−2 in a 5 M KOH electrolyte, respectively. The specific capacitance was calculated using the following Eq. 1148:
where, CS is the specific capacitance (F g−1), Td is the discharge time (s), m is the active mass of electrode, I is the current (mA), and ΔV is the potential window. From Fig. 5d, we observed that sample NCS:25 shows the higher specific capacitance at 10 mA cm−2, due to the interconnected and highly porous nanostructures and fast ions transferred from electrodes and electrolyte. Figure 5e displays the charge-discharge curves of NCS:25 electrode at different current densities vary from 1–10 mA cm−2 at 5 M KOH electrolyte. The specific capacitance of NCS:25 were calculated as 1146, 985, 922, 776, 764, 745 and 640 F g−1 at current densities of 1–10 mA cm−2 (shown in Fig. 5f).
Figure S5 represents the capacitance retentions of the NCS:25 as a function of the number of GCD cycles at 10 mA cm−2 in 5 M KOH electrolyte solution. The cycling test shows an increase in specific capacitance after initial 500 cycles which is due to activation followed by a gradual decrement in next 1000 cycles. More than 90% capacity retention after 2000 cycles, demonstrate high stability and high performance of the electrode. In summary, after the CV, charge-discharge analyses, and capacitance retention test, the NCS:25 composite was demonstrated to be the superior electrode material when compared with binary sulfides26,39.
Electrochemical impedance spectroscopy (EIS)
EIS was used to determine the electrical properties of the NiCo2S4 samples prepared at different Ni/Co ratios. Figures 6 and S6 show the Nyquist plots for the NCS:40, NCS:30, NCS:25, NCS:20, and NCS:10 electrodes. All of the samples were considered to determine the solution resistance (RS), charge transfer resistance (Rct), Warburg impedance (W), and double-layer capacitance (C). The RS are 15.4, 14.65, 10.54, 11.78, and 24.59 Ω and Rct are 83.2, 38.02, 20.00, 31.82, and 322.26 Ω for NCS:40, NCS:30, NCS:25, NCS:20, and NCS:10, respectively. The proposed equivalent circuit is shown in inset of Fig. 6. The measured values of solution resistance indicate that the NCS:25 shows the lower value as compared to the other electrodes, it means NCS:25 electrode shows high electrical conductivity as compared to the other electrodes. The NCS:25 composite thin film also depicted a lower charge transfer resistance than the other four electrodes. These results indicate that the NiCo2S4 thin films had a lower solution and charge transfer resistance, which may be due to their high electrical conductivity and highly porous nanoflakes-like nanostructure46,47,48,49,50.
Conclusion
In summary, 3D nanoflakes-like NiCo2S4 thin films were successfully synthesized using the SILAR method on Ni mesh with different molar ratios of Ni and Co. The specific capacitance results demonstrated lower performance by NCS:40 and NCS:10 due to their compact morphology, while NCS:25, with its vertically interconnected nanoflakes morphology, produced the highest specific capacitance (1427 F g−1 at 20 mV s−1). We thus successfully developed a new approach for the fabrication of Ni thin-film architecture and demonstrated that NiCo2S4 electrodes are promising materials for supercapacitor applications.
References
Jiang, Y., Qian, X., Zhu, C., Liu, H. & Hou, L. Nickel Cobalt Sulfide Double-Shelled Hollow Nanospheres as Superior Bifunctional Electrocatalysts for Photovoltaics and Alkaline Hydrogen Evolution. ACS Appl. Mater. Interfaces 10, 9379–9389 (2018).
Chen, W., Xia, C. & Alshareef, H. N. One-Step Electrodeposited Nickel Cobalt Sulfide Nanosheet Arrays for High-Performance Asymmetric Supercapacitors. ACS Nano 8, 9531–9541 (2014).
Zhang, C. et al. Electrochemically Synthesis of Nickel Cobalt Sulfide for High-Performance Flexible Asymmetric Supercapacitors. Adv. Sci. 5, 1700375 (2018).
Ahuja, P., Ujjain, S. K. & Kanojia, R. Electrochemical behaviour of manganese & ruthenium mixed oxide@reduced graphene oxide nanoribbon composite in symmetric and asymmetric supercapacitor. Appl. Surf. Sci. 427, 102–111 (2018).
Ujjain, S. K., Sahu, V., Sharma, R. K. & Singh, G. High performance, All solid state, flexible Supercapacitor based on Ionic liquid functionalized Graphene. Electrochim. Acta 157, 245–251 (2015).
Rajesh, J. A. et al. Rambutan-like cobalt nickel sulfide (CoNi2S4) hierarchitecture for high-performance symmetric aqueous supercapacitors. J. Ind. Eng. Chem. 63, 73–83 (2018).
Pu, J. et al. Direct Growth of NiCo2S4 Nanotube Arrays on Nickel Foam as High-Performance Binder-Free Electrodes for Supercapacitors. Chempluschem 79, 577–583 (2014).
Xiao, T. et al. Wide potential window and high specific capacitance triggered via rough NiCo2S4 nanorod arrays with open top for symmetric supercapacitors. Electrochim. Acta 269, 397–404 (2018).
Chen, H. et al. Highly conductive NiCo2S4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 5, 8879–8883 (2013).
Li, X., Li, Q., Wu, Y., Rui, M. & Zeng, H. Two-Dimensional, Porous Nickel–Cobalt Sulfide for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 7, 19316–19323 (2015).
Wei, C. et al. Synthesis of hierarchically porous NiCo2S4 core-shell hollow spheres via self-template route for high performance supercapacitors. Appl. Surf. Sci. 453, 288–296 (2018).
Shen, L. et al. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 6, 6694 (2015).
Tie, J. et al. Shape-controlled synthesis of nickel–cobalt–sulfide with enhanced electrochemical activity. J. Mater. Sci. Mater. Electron. 29, 2251–2258 (2018).
Wang, T. et al. Electro-deposition of CoNi2S4 flower-like nanosheets on 3D hierarchically porous nickel skeletons with high electrochemical capacitive performance. J. Mater. Chem. A 3, 23035–23041 (2015).
Zhang, G. & (David) Lou, X. W. Controlled Growth of NiCo2O4 Nanorods and Ultrathin Nanosheets on Carbon Nanofibers for High-performance Supercapacitors. Sci. Rep. 3, 1470 (2013).
Xiao, J., Zeng, X., Chen, W., Xiao, F. & Wang, S. High electrocatalytic activity of self-standing hollow NiCo2S4 single crystalline nanorod arrays towards sulfide redox shuttles in quantum dot-sensitized solar cells. Chem. Commun. 49, 11734–11736 (2013).
Rahimi, S., Shahrokhian, S. & Hosseini, H. Ternary nickel cobalt iron sulfides ultrathin nanosheets grown on 3-D nickel nanocone arrays-nickel plate current collector as a binder free electrode for fabrication of highly performance supercapacitors. J. Electroanal. Chem. 810, 78–85 (2018).
Liu, Q., Jin, J. & Zhang, J. NiCo2S4 @graphene as a Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 5, 5002–5008 (2013).
Liang, X. et al. Highly Compressible Carbon Sponge Supercapacitor Electrode with Enhanced Performance by Growing Nickel–Cobalt Sulfide Nanosheets. ACS Appl. Mater. Interfaces 10, 10087–10095 (2018).
Tao, K., Han, X., Ma, Q. & Han, L. A metal–organic framework derived hierarchical nickel–cobalt sulfide nanosheet array on Ni foam with enhanced electrochemical performance for supercapacitors. Dalt. Trans. 47, 3496–3502 (2018).
He, W. et al. Hierarchical Ni-Co-S@Ni-W-O core–shell nanosheet arrays on nickel foam for high-performance asymmetric supercapacitors. Nano Res. 11, 1415–1425 (2018).
Chen, H. et al. One-pot synthesis of porous nickel cobalt sulphides: tuning the composition for superior pseudocapacitance. J. Mater. Chem. A 3, 428–437 (2015).
Ding, R., Gao, H., Zhang, M., Zhang, J. & Zhang, X. Controllable synthesis of Ni3−xCoxS4 nanotube arrays with different aspect ratios grown on carbon cloth for high-capacity supercapacitors. RSC Adv. 5, 48631–48637 (2015).
Chen, S., Chen, H., Fan, M., Li, C. & Shu, K. Sea urchin-like Ni–Co sulfides with different Ni to Co ratios for superior electrochemical performance. J. Sol-Gel Sci. Technol. 80, 119–125 (2016).
Dubal, D. P., Jagadale, A. D., Patil, S. V. & Lokhande, C. D. Simple route for the synthesis of supercapacitive Co–Ni mixed hydroxide thin films. Mater. Res. Bull. 47, 1239–1245 (2012).
Li, Z. et al. Three-dimensional graphene-like porous carbon nanosheets derived from molecular precursor for high-performance supercapacitor application. Electrochim. Acta 296, 8–17 (2019).
Sui, Y. et al. High Energy Density Asymmetric Supercapacitor Based ZnS/NiCo2S4/Co9S8 Nanotube Composites. Materials. Adv. Mater. Interfaces 5, 1800018 (2018).
Wei, X. et al. Core-shell NiCo2S4@MnMoO4 as an Advanced Electrode Material for High-performance Electrochemical Energy Storage. ChemElectroChem 4, 2634–2642 (2017).
Huang, M. et al. Facile synthesis of single-crystalline NiO nanosheet arrays on Ni foam for high-performance supercapacitors. CrystEngComm 16, 2878–2884 (2014).
Yu, J. et al. Reverse Microemulsion-Assisted Synthesis of NiCo2S4 Nanoflakes Supported on Nickel Foam for Electrochemical Overall Water Splitting. Adv. Mater. Interfaces 5, 1701396 (2018).
Zhang, Z., Wang, Q., Zhao, C., Min, S. & Qian, X. One-Step Hydrothermal Synthesis of 3D Petal-like Co9S8/RGO/Ni3S2 Composite on Nickel Foam for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 7, 4861–4868 (2015).
Wei, W. et al. Partial Ion-Exchange of Nickel-Sulfide-Derived Electrodes for High Performance Supercapacitors. Chem. Mater. 26, 3418–3426 (2014).
Pu, J. et al. Preparation and Electrochemical Characterization of Hollow Hexagonal NiCo2S4 Nanoplates as Pseudocapacitor Materials. ACS Sustain. Chem. Eng. 2, 809–815 (2014).
Yang, X. et al. One-Step Synthesis of NiCo2S4/Graphene Composite for Asymmetric Supercapacitors with Superior Performances. ChemElectroChem 5, 1576–1585 (2018).
Aboelazm, E. A. A. et al. Magnetic Electrodeposition of the Hierarchical Cobalt Oxide Nanostructure from Spent Lithium-Ion Batteries: Its Application as a Supercapacitor Electrode. J. Phys. Chem. C 122, 12200–12206 (2018).
Khoo, S. Y. et al. One-Step Hydrothermal Tailoring of NiCo2S4 Nanostructures on Conducting Oxide Substrates as an Efficient Counter Electrode in Dye-Sensitized Solar Cells. Adv. Mater. Interfaces 2, 1500384 (2015).
Shinde, S. K., Dubal, D. P., Ghodake, G. S., Kim, D. Y. & Fulari, V. J. Nanoflower-like CuO/Cu(OH)2 hybrid thin films: Synthesis and electrochemical supercapacitive properties. J. Electroanal. Chem. 732, 80–85 (2014).
Xiao, J., Wan, L., Yang, S., Xiao, F. & Wang, S. Design Hierarchical Electrodes with Highly Conductive NiCo2S4 Nanotube Arrays Grown on Carbon Fiber Paper for High-Performance Pseudocapacitors. Nano Lett. 14, 831–838 (2014).
Shen, L. et al. NiCo2S4 Nanosheets Grown on Nitrogen-Doped Carbon Foams as an Advanced Electrode for Supercapacitors. Adv. Energy Mater. 5, 1400977 (2015).
Shinde, S. K., Dubal, D. P., Ghodake, G. S., Kim, D. Y. & Fulari, V. J. Morphological tuning of CuO nanostructures by simple preparative parameters in SILAR method and their consequent effect on supercapacitors. Nano-Structures & Nano-Objects 6, 5–13 (2016).
Shinde, S. K. et al. Chemical synthesis of flower-like hybrid Cu(OH)2/CuO electrode: Application of polyvinyl alcohol and triton X-100 to enhance supercapacitor performance. Colloids Surfaces B Biointerfaces 156, 165–174 (2017).
Zhao, Y. et al. Hierarchical NiCo2S4@CoMoO4 core-shell heterostructures nanowire arrays as advanced electrodes for flexible all-solid-state asymmetric supercapacitors. Appl. Surf. Sci. 453, 73–82 (2018).
Shinde, S. K., Ghodake, G. S., Fulari, V. J. & Kim, D.-Y. High electrochemical performance of nanoflakes like CuO electrode by successive ionic layer adsorption and reaction (SILAR) method. J. Ind. Eng. Chem. 52, 12–17 (2017).
Chou, S.-W. & Lin, J.-Y. Cathodic Deposition of Flaky Nickel Sulfide Nanostructure as an Electroactive Material for High-Performance Supercapacitors. J. Electrochem. Soc. 160, D178–D182 (2013).
Shinde, S. K. et al. Electrochemical synthesis: Monoclinic Cu2Se nano-dendrites with high performance for supercapacitors. J. Taiwan Inst. Chem. Eng. 75, 271–279 (2017).
Chen, W., Fan, Z., Gu, L., Bao, X. & Wang, C. Enhanced capacitance of manganese oxide via confinement inside carbon nanotubes. Chem. Commun. 46, 3905–3907 (2010).
Chen, J. S., Guan, C., Gui, Y. & Blackwood, D. J. Rational Design of Self-Supported Ni3S2 Nanosheets Array for Advanced Asymmetric Supercapacitor with a Superior Energy Density. ACS Appl. Mater. Interfaces 9, 496–504 (2017).
Chen, Y.-Y., Dhaiveegan, P., Michalska, M. & Lin, J.-Y. Morphology-controlled synthesis of nanosphere-like NiCo2S4 as cathode materials for high-rate asymmetric supercapacitors. Electrochim. Acta 274, 208–216 (2018).
Huang, Y. et al. Graphene-quantum-dots induced NiCo2S4 with hierarchical-like hollow nanostructure for supercapacitors with enhanced electrochemical performance. Electrochim. Acta 269, 45–54 (2018).
Kim, D.-Y. et al. Chemical synthesis of hierarchical NiCo2S4 nanosheets like nanostructure on flexible foil for a high performance supercapacitor. Sci. Rep. 7, 9764 (2017).
Beka, L. G., Li, X. & Liu, W. Nickel Cobalt Sulfide core/shell structure on 3D Graphene for supercapacitor application. Sci. Rep. 7, 2105 (2017).
Jagadale, A., Zhou, X., Blaisdell, D. & Yang, S. Carbon nanofibers (CNFs) supported cobalt-nickel sulfide (CoNi2S4) nanoparticles hybrid anode for high performance lithium ion capacitor. Sci. Rep. 8, 1602 (2018).
Chen, Z. et al. Preparation of Nickel Cobalt Sulfide Hollow Nanocolloids with Enhanced Electrochemical Property for Supercapacitors Application. Sci. Rep. 6, 25151 (2016).
Chiu, C.-T. & Chen, D.-H. One-step hydrothermal synthesis of three-dimensional porous Ni–Co sulfide/reduced graphene oxide composite with optimal incorporation of carbon nanotubes for high performance supercapacitors. Nanotechnology 29, 175602 (2018).
Acknowledgements
Analysis of samples was supported by Dongguk University, Seoul, Korea Research Fund 2018-20. This research was supported by the National Research Foundation of Korea (NRF) and funded by the Ministry of Science, ICT, and Future Planning (2018R1A2B6006056), and (2017R1D1A1B03035957).
Author information
Authors and Affiliations
Contributions
S.K.S. and H.M.Y. design the experiment scheme. S.K.S. and H.M.Y. carried out the experiments. All authors, S.K.S., S.R., G.S.G., C.B., D.-Y.K., A.D.J., A.A.K., D.P.W., T.V.M.S., H.-S.K., P.C.N. and H.M.Y. involved to the characterization of the electrode and the discussions prominent up to the writing of the manuscript. H.M.Y. and S.K.S. discussed main part and that led to the final manuscript; all authors read and approved manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shinde, S.K., Ramesh, S., Bathula, C. et al. Novel approach to synthesize NiCo2S4 composite for high-performance supercapacitor application with different molar ratio of Ni and Co. Sci Rep 9, 13717 (2019). https://doi.org/10.1038/s41598-019-50165-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-50165-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.