Abstract
Resveratrol (RSV) and nicotinamide (NAM) have garnered considerable attention due to their anti-inflammatory and anti-aging properties. NAM is a transient inhibitor of class III histone deacetylase SIRTs (silent mating type information regulation 2 homologs) and SIRT1 is an inhibitor of poly-ADP-ribose polymerase-1 (PARP1). The debate on the relationship between RSV and SIRT1 has precluded the use of RSV as a therapeutic drug. Recent work demonstrated that RSV facilitates tyrosyl-tRNA synthetase (TyrRS)-dependent activation of PARP1. Moreover, treatment with NAM is sufficient to facilitate the nuclear localization of TyrRS that activates PARP1. RSV and NAM have emerged as potent agonists of PARP1 through inhibition of SIRT1. In this study, we evaluated the effects of RSV and NAM on pro-inflammatory macrophages. Our results demonstrate that treatment with either RSV or NAM attenuates the expression of pro-inflammatory markers. Strikingly, the combination of RSV with NAM, exerts additive effects on PARP1 activation. Consistently, treatment with PARP1 inhibitor antagonized the anti-inflammatory effect of both RSV and NAM. For the first time, we report the ability of NAM to augment PARP1 activation, induced by RSV, and its associated anti-inflammatory effects mediated through the induction of BCL6 with the concomitant down regulation of COX-2.
Similar content being viewed by others
Introduction
Inflammation is a process by which our body responds to injuries and attacks pathogens. This process involves various cell types (e.g., neutrophils, T-cells, B-cells, and macrophages), cytokines, and chemokines to restore tissue homeostasis1,2. In normal conditions, the process begins with acute inflammation. However, if the immune system fails to resolve the acute inflammatory phase, it can progress to chronic inflammation3, which is associated with various degenerative diseases.
Inflammation is commonly treated with non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, antibodies, and biological agents. Despite significant progress, negative side effects such as damage to the gastrointestinal track, cardiotoxicity, and hepatotoxicity compromises the effectiveness of these therapeutic strategies4,5. The limited clinical benefits of these strategies is rooted in their inability to simultaneously target multiple signaling pathways that regulate inflammation. To this end, there is a critical need for the development of novel multiple-targeting approaches that enable us to modulate the interdependencies of active mediators and ultimately modulate inflammation at a higher clinical efficacy.
Natural products have been a major focus of drug discovery6,7,8,9. The main inspiration behind their use is the extraordinary ability of natural compounds to target multiple molecular mechanisms in immune, redox, and metabolic regulatory pathways10,11. For this reason, resveratrol (RSV), a polyphenolic phytoestrogen found in grapes, nuts, and berries, is increasing in prominence, with reports demonstrating its remarkable potential to induce survival genes that impart cardio-neuro-protective, anti-diabetic, and anti-cancer effects12,13,14,15,16,17,18,19. Several published reports have shown that RSV activates SIRT1 and inhibits transcriptional activation of nuclear factor kappa B (NF-κB) resulting in suppression of inflammatory mediators including IL-1β, matrix metallopeptidase 13 (MMP-13), COX-2, IL-6, and TNF-α13,15,20. However, two seminal studies published by Sajish and Schimmel, and Park et al. have established a SIRT1 independent mechanism of action for RSV through other potential targets, such as mammalian phosphodiesterase-4 (PDE4) and poly-ADP-ribose polymerase-1 (PARP1)21,22. Interestingly, PDE4 degrades poly(ADP-ribose) polymer and recycles the ADP-ribose into adenosine monophosphate (AMP) units, resulting in a transient competitive inhibition of cAMP hydrolysis23,24,25,26. cAMPs regulate the localization, duration, and amplitude of cyclic nucleotide signaling. Consistently, RSV facilitates the activation of PARP1 and accumulation of poly(ADP-ribose) that would lead to ADP-ribose-driven modulation of PDE and subsequent upregulation of cAMP and associated Ca2+ signaling21,22. These two studies21,22 thus suggest that PARP1 and PDE4 act in tandem to exert the metabolic benefits of resveratrol. Because both PARP1 and SIRTuins use NAD+, they antagonistically regulate each other, i.e; PARP1 activation inhibits SIRTuins through generation of nicotinamide22,27,28 and vice versa the metabolic byproducts of nicotinamide (1-Methylnicotinamide)29,30 or treatment with nicotinamide riboside (NR) result in the inhibition of PARP128,30 through SIRT1 activation28,29,31. Most significantly, inhibition of PARP1 leads to induction of DNA damage and cytotoxicity32,33 and mitochondrial dysfunction34, which are implicated in the etiology of various metabolic disorders. Although pro-inflammatory interferon gamma (IFN-γ) is known to activate PARP135, prolonged inflammation triggers DNA damage through the inhibition of PARP136,37. Further, PARP1 depletion leads to the sustained induction of a large number of interferon-stimulated genes (ISGs)38 and induces senescence39. Consistently, emerging works suggest that inhibition of PARP1 induces DNA damage-dependent pro-inflammatory response36,37,40,41,42. These observations suggested that activation of PARP-1 not only enhances DNA repair43 but also triggers an anti-inflammatory signaling cascade36,37,38,39.
Under stress conditions, tyrosyl-tRNA synthetase (TyrRS), which activates L-tyrosine for protein synthesis, translocates to the nucleus and activates PARP122, a major modulator of nicotinamide adenine dinucleotide (NAD+) metabolism and signaling. RSV-mediated activation of PARP1 also results in the generation of nicotinamide (NAM)22. This leads us to hypothesize that the anti-inflammatory effects of RSV can be mimicked and enhanced upon treatment with NAM. Also known as niacinamide, NAM is a form of vitamin B3 and acts as a classical inhibitor of SIRT127,28. In most mammalian cells, NAM is the main source of NAD+, which is necessary for cellular function and energy metabolism44,45. NAM has been studied in the context of neuronal diseases, oxidative stress, diabetes, and inflammation45,46,47,48,49,50.
For the first time, we report the ability of NAM to activate PARP1 and its associated anti-inflammatory effects mediated through the induction of BCL6 with the concomitant down regulation of COX-2. Due to their critical role of in immunity and homeostasis, we used macrophages as a testbed for determining the anti-inflammatory signaling of RSV and NAM. The phenotype of macrophages, generally classified as pro-inflammatory (M1 macrophages) and wound healing (M2 macrophages), appears to be a key player in immune response51,52,53,54. This designation, although conceptually useful, is an oversimplification of the phenotypic diversity of macrophages55,56,57,58. In reality, macrophages exhibit a complex progression of phenotypes that are affected by local signals as well as the stage of tissue remodeling59,60,61. Significant effort has been dedicated to modulate the phenotypic adaptation of macrophages as a therapeutic target for pathologies62,63,64,65. To this end, we demonstrate that synergistic activation of PARP1 mediated by RSV and NAM can be exploited as a strategy to tune inflammatory response.
Results
Pro-inflammatory macrophage proliferation in the presence of NAM and RSV
The in vitro studies aimed at obtaining a better understanding of the mechanisms that govern the immunomodulatory effects of RSV and NAM on macrophages. We launched our studies by determining the dose response of macrophages to NAM and RSV. Cell viability was determined using a colorimetric assay after the cells were treated with NAM, RSV, their combination (RSV + NAM) at two time points (2 and 5 days). Figure 1A shows the cell viability as compared to non-treated cells (control). We did not observe any adverse effect on macrophage viability when exposed to NAM, RSV, or the combination.
(A) Effects of NAM, RSV, and NAM + RSV on cell viability as percentage of untreated control and (B–D) change in cytokine secretion by ELISA assay. Pro-inflammatory macrophages were incubated with NAM, RSV, or NAM + RSV. (B) TNF-α, (C) IL-6, and (D) VEGF. Data was plotted as mean ± SD (N = 3). *Denotates the (p ≤ 0.05) difference compared to the control group in the same time point. The results demonstrated the potential of NAM and RSV to modulate pro-inflammatory cytokines.
Pro-inflammatory macrophage cytokine secretion in the presence of NAM and RSV and PARP1 activation
To characterize cytokine secretion of inflammatory macrophages, we assessed the cytokine levels in the supernatants of the treated cells at different time points (2 and 5 days). As expected, LPS-induced inflammatory macrophages produced high levels of TNF-α, IL-6, and VEGF (Fig. 1B–D). The presence of NAM, RSV, and RSV + NAM decreased the LPS dependent induction of pro-inflammatory cytokines TNF-α and IL-6, where the lowest pro-inflammatory cytokine expression level was observed in the NAM treated group (Fig. 1B,C). VEGF expression was also suppressed when pro-inflammatory macrophages were treated with RSV or NAM + RSV, while the NAM-treated macrophages exhibited an increased VEGF expression at day 5 (Fig. 1D).
We assessed cytokine production at the mRNA level using quantitative RT-PCR. The analysis of pro-inflammatory genes (IL-6, TNF-α, and VEGF) and anti-inflammatory genes (MRC-1 and IL-10) demonstrated that NAM, RSV, and RSV + NAM promote anti-inflammatory cytokine expression (Fig. 2A–E). TNF-α was significantly reduced at 5 days in all treated groups (Fig. 2A). Consistently, the inflammatory marker IL-6 was lowered with the addition of 500 μM NAM or 500 μM NAM + 10 μM RSV (Fig. 2B). We observed statistically higher levels of VEGF gene expression for the group containing NAM at day 5 (Fig. 2C). MRC-1 levels increased in all treated groups (Fig. 2D). In addition, IL-10 levels were significantly higher for the inflammatory macrophages cultured with RSV + NAM, as compared to the control group with no compounds (Fig. 2E). And at day 5, all treated groups demonstrated higher IL-10 as compared to the control non-treated inflammatory macrophages. The highest level of IL-10 expression was observed in macrophages exposed to RSV (Fig. 2E). Further, we measured the activation of PARP1 in pro-inflammatory macrophages treated with RSV and NAM by monitoring the induction of poly-ADP-ribose (PAR) levels. Figure 2F demonstrates that NAM activates PARP1 more robustly than RSV. The results all together suggested that NAM and RSV facilitates the activation of PARP1 and stimulates a PARP1-dependent anti-inflammatory signaling cascade22,36,38.
Gene expression changes in pro-inflammatory macrophages over time incubated with NAM, RSV, or NAM + RSV, as analyzed by RT-PCR. Measured genes included (A) TNF-α, (B) IL-6, (C) VEGF, (D) MRC-1, and (E) IL-10. Gene expression was normalized over the control group and the housekeeping gene GAPDH (2−ΔΔC). *Denotes the (p ≤ 0.05) difference compared to the control group in the same time point. (F) RSV and NAM synergistically activate PARP1. PARP1 was immunoprecipitated (IP) from activated pro-inflammatory macrophages in the presence of NAM, RSV, or RSV + NAM and immunoblotted (IB) for the presence of poly-ADP-ribose (PAR) using anti-PAR antibody (Millipore). Total PARP1 levels were assessed by anti-PARP1 antibody both by immunoblot and by western blot (WB) in the cell lysate. The cropped blots are displayed in (F) and the full-length of blots are presented in Supplementary Fig. 1S.
PARP1 inhibition in pro-inflammatory macrophages in response to NAM and RSV
To further establish the role of PARP1 in RSV- and NAM-mediated anti-inflammatory effects, we added a chemical inhibitor of PARP1 (AG14361) to the culture media of the LPS-induced inflammatory macrophages. Supernatant and RNA was collected after 2 days of treatment. Cytokine expression in the supernatant was determined by ELISA. Levels of pro-inflammatory cytokines in the cell culture media were lower for all groups except those treated with PARP1 inhibitor, as compared to the non-knockdown group. We observed that AG14361 reversed the anti-inflammatory effects of NAM, RSV, or RSV + NAM as demonstrated by higher expression levels of TNF-α, IL-6 (Fig. 3A–C). We performed RT-PCR to confirm the cytokine expression via mRNA levels when the LPS-induced inflammatory macrophages were exposed to AG14361 PARP1 inhibitor. As demonstrated in Fig. 4A, TNF-α expression was consistently higher in all groups when compared to the group treated with pro-inflammatory LPS media free of AG14361. The same effect was observed for IL-6 (Fig. 4B). VEGF expression was the same for all groups (Fig. 4C). The anti-inflammatory cytokine expression of MRC-1 was not as enhanced for the NAM, RSV, or RSV + NAM treated samples, which were exposed to AG14361 (Fig. 4D) when compared to those same groups in the absence of AG14361, while the IL-10 cytokine expression was enhanced for almost all the groups treated with AG14361 with the exception of the NAM + RSV group (Fig. 4E). These findings revealed that the pro-inflammatory and anti-inflammatory effects of these mediators (IL-6, TNF-α, VEGF, MRC-1, and IL-10) are regulated by PARP1.
Change in cytokine secretion over time. The PARP1 pathway was blocked by the addition of AG14361 inhibitor to the pro-inflammatory macrophages. Then the cells were treated with NAM, RSV, or NAM + RSV, and the supernatant was collected 48 hours later for analysis by ELISA. (A) TNF-α, (B) IL-6, and (C) VEGF. *Denotes the (p ≤ 0.05) difference compared to the control group in the same time point. #Denotes the (p ≤ 0.05) difference for all AG14361 treated groups compared to the control + AG14361 group. & denotes the (p ≤ 0.05) difference between the same treatment in the presence or absence of AG14361 at the same time point.
Gene expression of pro-inflammatory macrophages incubated with NAM, RSV, or NAM + RSV in the presence (+) or absence (−) of AG14361 (PARP1 inhibitor). RNA was extracted after 2 days and analyzed by RT-PCR. (A) TNF-α, (B) IL-6, (C) VEGF, (D) MRC-1, and (E) IL-10. Gene expression was normalized over the control group and the housekeeping gene GAPDH (2−ΔΔC). *Denotes the (p ≤ 0.05) difference compared to the control group in the same time point. #Denotes the (p ≤ 0.05) difference for all AG14361 treated groups compared to the control + AG14361 group. & denotes the (p ≤ 0.05) difference between the same treatment in the presence or absence of AG14361 at the same time point.
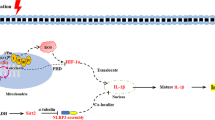
B-cell lymphoma-6 protein (BCL6) is a corepressor for inflammatory mediators that recruits monocytes to vascular endothelial cells upon inflammation. Because PARP1 mediated expression of BCL6 is known to play an anti-inflammatory role66, we checked the induction of BCL6 after RSV and NAM treatment and observed a strong induction of BCL6 (Fig. 5). More interestingly, we observed that similar to RSV/PARP1-dependent AMPK activation22,67, NAM also triggered a profound activation of 5′ AMP-activated protein kinase (AMPK) which is also a well-known negative regulator of NF-kB68. Further, consistent with its known anti-inflammatory effect48 and PARP1-mediated inhibition of COX-269, treatment with NAM also resulted in the profound down regulation of COX-2, a well-known mediator of inflammation. Finally, treatment with AG14361- a potent PARP1 inhibitor, abrogated NAM + RSV mediated AMPK activation, Bcl-6 induction and COX-2 down regulation (Fig. 5). Therefore, this work demonstrated that PARP-1 is critical for the RSV and NAM-mediated anti-inflammatory effects.
PARP-1 inhibitor antagonizes the anti-inflammatory effects of RSV by down-regulating the expression of BCL6 and upregulating COX-2. (A) AG14361 PARP-1 inhibitor was added to the pro-inflammatory polarization media in the presence or absence of NAM, RSV, or RSV + NAM. “+” indicates that AG14361 was added. Samples were processed and western blotted for the expression level of BCL6 and COX-2. The cropped blots were displayed in (A) and the full-length of blots were presented in Supplementary Fig. 2S. (B) Proposed mechanism of PARP-1-mediated anti-inflammatory effects of RSV and NAM.
Discussion
The imbalance of cytokines, chemokines, and some other mediators can affect cell differentiation and disturb cellular homeostasis. All these changes influence cell metabolism and change tissue functions that may favor disease progression. Various treatments have been applied to treat chronic inflammation, including antibodies, biological agents, and NSAIDs, which target different pro-inflammatory mediators, such as TNF-α, IL-6, IL-1β, and COX, to name a few70. However, these treatments can results in different side effects, like gastritis, ulcers, colitis, bleeding, hepato-renal dysfunction, organ failure, and skin dysfunction71,72. As a result, there has been increasing interest in treatments derived from natural products with minimal side effects.
RSV is among the natural compounds that have demonstrated unique potential suppress the inflammatory process by downregulating pro-inflammatory cytokine secretion and improve health17,73,74,75. Previous studies have shown that RSV acts through NAD+ dependent proteins, and play an important role in PARP1 activation22. NAM is a precursor of NAD+ that prevents the degradation of PARP1 and protects cells by maintaining DNA integrity and preventing acute cellular degradation76. Thus, we hypothesized that the anti-inflammatory effects of RSV can be enhanced upon treatment with NAM.
We began this work by designing a series of in vitro experiments using pro-inflammatory macrophages to confirm the anti-inflammatory effect and the molecular mechanism of action of RSV and NAM. The results obtained in this study provide information on baseline cytokine profiles of LPS-induce pro-inflammatory macrophages, and the anti-inflammatory effects of NAM and RSV in this system. Consistent with a previous published report46, we observed that both NAM and RSV could suppress the expression of pro-inflammatory cytokines, including TNF-α and IL-6 and could up-regulate the expression levels of the anti-inflammatory mediators (IL-10 and MRC-1). Strikingly, the effects of NAM were more profound as compared to RSV alone; while the co-treatment with RSV further modulated the effects of NAM. The results suggest that a combination of RSV and NAM helps trigger the conversion of NAD+ through the salvage pathway and result in a favorable immune response.
We hypothesized that the anti-inflammatory effect of RSV and NAM was related to AMPK and PARP1 activation. We knew that SIRT1 did not play any role in this anti-inflammatory effect because NAM has been used as a potent SIRT1 inhibitor27,50, but we were not sure about the AMPK and PARP1. To test this hypothesis, we used a PARP1 inhibitor (AG14361) with established potency in previous studies22,77 in the presence or absence of RSV and/or NAM to assess. Our results suggest a novel mechanism of action mediated through PARP1 -dependent induction of BCL666 with concomitant inhibition of COX-269,78 that is responsible for the observed anti-inflammatory effects of RSV78 and NAM. Our work is also consistent with the published study by Pantano et al. reporting that in LPS stimulated human macrophages the basal levels of BCL6 disappear right after LPS treatment and was fully restored between 1 and 2 hours post-treatment79. This work thus provides the molecular basis for the newly emerging PARP1-mediated anti-inflammatory signaling cascade36,37,38,39.
Conclusions
A single dose of RSV, NAM, or RSV + NAM attenuated inflammation by suppressing pro-inflammatory cytokines released in LPS-induced mouse and LPS-treated macrophages through PARP1 activation. Therefore, like RSV, NAM is also a potent activator of PARP1. Furthermore, this work demonstrates that beyond facilitating DNA repair, PARP1 activation also triggers anti-inflammatory effects. The present study thus suggests that the synergistic effect of RSV and NAM on PARP1 activation would provide a potential therapeutic strategy to treat inflammatory diseases.
Materials and Methods
Monocyte culture, differentiation to M0, and polarization to pro-inflammatory macrophages
Human monocytic cells (THP-1 cells) were obtained from the American Type Culture Collection (ATCC) and cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin (PS), and 0.05 mM 2-mercaptoethanol. THP-1 cells were differentiated into M0 macrophages by culturing the cells with 100 ng/ml of 12-myristate 13-acetate (PMA) (Sigma) for 24 hours17,80,81. After differentiation, the cells were washed three times with serum free culture media to remove non-differentiated cells. To activate M0 macrophages, the cells were exposed to polarization media, which consisted of culture media supplemented with 100 ng/ml of lipopolysaccharide (LPS) (Sigma) from Escherichia Coli serotype 0111:B4 and 20 ng/ml of interferon (IFN)-γ (Peprotech). To assess the effects of NAM, RSV, and the combination of RSV and NAM (RSV + NAM), pro-inflammatory macrophages were exposed to RSV (10 µM), NAM (500 µM) or RSV (10 µM) plus NAM (500 µM) for durations of 16, 24, 48, and 120 hours.
Analysis of pro-inflammatory macrophage proliferation in the presence of NAM and RSV
Cell proliferation was assessed using the CellTitter 96 Aqueous Non-Radioactive Cell Proliferation assay (MTS assay, Promega)82, which is based on the redox conversion of a tetrazolium salt into formazan product. Briefly, THP-1 cells were seeded, differentiated to M0 macrophages, and polarized to pro-inflammatory macrophages in 96-well plates (5 × 104 cells/well). Five groups were considered: (i) activated macrophages in polarization media (control), and pro-inflammatory macrophages in (ii) polarization media, (iii) polarization media including 500 µM of NAM, (iv) polarization media including 10 µM of RSV, or (v) polarization media including 500 µM NAM plus 10 µM of RSV. We monitored the cells at time points of 2 and 5 days. At the end of each exposure time point, MTS solution was added to the cells following the kit’s instruction and the samples were incubated for 2 hours at 37 °C. The absorbance was determined using a Spectramax 190 microplate spectrophotometer at 490 nm. The results were expressed as percent cell viability compared to the control.
Analysis of pro-inflammatory macrophage cytokine secretion in the presence of NAM and RSV
THP-1 cells were seeded, differentiated from monocytes to M0 macrophages, and polarized to pro-inflammatory macrophages in 12-well plates (5 × 105 cells/well). Inflammatory macrophages were exposed to polarization media containing either (i) 500 µM of NAM, (ii) 10 µM of RSV, or (iii) 500 µM NAM plus 10 µM of RSV for time points of 2 and 5 days. Next, the supernatant was collected, centrifuged, and stored at −20 °C. Cytokine secretion (TNF-α, IL-6, and VEGF) in the culture media was assessed using an enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s protocols (Peprotech). To quantify, calibration curves for TNF-α, IL-6, and VEGF standards were generated. Colorimetric changes were measured using a SpectraMax 190 microplate spectrophotometer at 450 nm with a wavelength correction set at 620 nm.
Analysis of pro-inflammatory macrophage phenotypic response to NAM and RSV
We assessed the expression of pro- and anti-inflammatory genes expressed by macrophages, including TNF-α, IL-6, VEGF, MRC-1, and IL-10, using quantitative reverse transcription polymerase chain reaction (RT-PCR). Inflammatory macrophages were exposed to polarization media containing either (i) 500 µM of NAM, (ii) 10 µM of RSV, or (iii) 500 µM NAM plus 10 µM of RSV for time durations of 2 and 5 days. Total RNA was isolated using the Gene Jet RNA Purification kit (Thermo Scientific). The quantification total of RNA was performed using a Nanodrop 2000c spectrometer and considered pure if the ratio of the absorbance at 260 nm/280 nm was ≥2. The RNA was prepared as a template for cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad). Quantitative RT-PCR was performed using 10.4 ng of cDNA per reaction and SYBER® Green PCR Supermix (Bio-Rad). Gene expression was normalized to the housekeeping gene GAPDH and the control group (pro-inflammatory macrophages in polarization media). Gene expression values were calculated using the mean Ct values of the samples. All primers (Table 1) were synthetized by Integrated DNA Technologies.
Analysis of PARP1 activation in pro-inflammatory macrophages in response to NAM and RSV
The activation of PARP1 in pro-inflammatory macrophages treated with RSV, NAM, or both was monitored by measuring downstream signaling markers associated with the NAD+ (substrate for sirtuins and PARP signaling) metabolic flux. Targets were selected if they were activated and/or inhibited under conditions of RSV and NAM treatment stress or NAD+ metabolic byproduct-treatment. For example, the induction of NAMPT (a major regulator of NAD+ levels in cells) is a downstream signaling event of PARP1 activation22. Similarly, PARP1 activation induces phosphorylation of H2B (substrate for AMPK, which upregulates NAMPT expression)83.
Inflammatory macrophages were exposed to polarization media containing either (i) 500 µM of NAM, (ii) 10 µM of RSV, or (iii) 500 µM NAM plus 10 µM of RSV for 24 hours. Cells were rinsed with ice-cold PBS and ice-cold lysis buffer. Next, cells were scrapped off from the tissue culture well-plates, collected, and processed in order to generate whole cell lysate. The whole cell lysate was processed further for western blot analysis using an iBLOT system (Invitrogen). Blots were developed using antibodies and detected using a BioRad Chemi-Doc imaging system. For immunoprecipitation, the supernatants were pre-cleared by incubation with protein G beads. The pre-cleared cell lysates were incubated at 4 °C for 1 hour with either α-PARP1, α-TyrRS, or non-immune immunoglobulin-G (IgG) at a concentration of 5 mg/ml followed by incubation in 30 ml of Protein G beads (pretreated with 10 mg/ml BSA) at 4 °C for 1 hour. Immunoprecipitates were washed three times, subjected to SDS-PAGE, and immunoblotted with antibodies.
To further elucidate the role of PARP1 in anti-inflammatory effects of RSV and NAM on pro-inflammatory macrophages, inflammatory macrophages (5 × 105 cells/ml) were treated with 10 µM of AG14361 (a potent inhibitor of PARP1). The experimental design included four conditions: (i) polarization media (control), (ii) polarization media containing 500 µM of NAM, (iii) polarization media containing 10 µM of RSV, and (iv) polarization media containing 500 µM NAM plus 10 µM of RSV. The negative control was carried out using inflammatory macrophages cultured in polarization media without the PARP1 inhibitor. Cytokine (TNF-α, IL-6, and VEGF) as well as gene expression (TNF-α, IL-6, VEGF, MRC-1, and IL-10) were evaluated using an ELISA assay and quantitative RT-PCR, respectively. Immunoblotting was used to evaluate the BCL6 and AMPK activation 24 hours following the treatment.
Statistical analysis
All the experiments were performed in duplicates. A minimum of three samples (N = 3) were analyzed per condition unless otherwise stated. Data are presented as mean ± standard deviation (SD). Statistical analysis was done by using GraphPad Prism 7. Multiple Student’s t test was performed to evaluate differences between treated groups and control groups. Statistical significance was defined as p ≤ 0.05.
Data Availability
All relevant data are within the paper.
References
Robb, C. T., Regan, K. H., Dorward, D. A. & Rossi, A. G. Key mechanisms governing resolution of lung inflammation. Semin Immunopathol 38(4), 425–48 (2016).
Punchard, N. A., Whelan, C. J. & Adcock, I. The. Journal of Inflammation. J Inflamm (Lond) 1(1), 1 (2004).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454(7203), 428–35 (2008).
Li, B. et al. Novel unsaturated glycyrrhetic acids derivatives: Design, synthesis and anti-inflammatory activity. European Journal of Medicinal Chemistry 139, 337–348 (2017).
Dvorakova, M. & Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacological Research (2017).
Lu, J. J., Pan, W., Hu, Y. J. & Wang, Y. T. Multi-target drugs: the trend of drug research and development. Plos One 7(6), e40262 (2012).
Kinghorn, A. D., Pan, L., Fletcher, J. N. & Chai, H. The relevance of higher plants in lead compound discovery programs. J Nat Prod 74(6), 1539–55 (2011).
Prakash Mishra, A. et al. Bioactive compounds and health benefits of edible Rumex species-A review. Cell Mol Biol (Noisy-le-grand) 64(8), 27–34 (2018).
Salehi, B. et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci Tech 80, 104–122 (2018).
Koeberle, A. & Werz, O. Multi-target approach for natural products in inflammation. Drug Discov Today 19(12), 1871–82 (2014).
Moghadam, S. E. et al. Wound Healing Potential of Chlorogenic Acid and Myricetin-3-O-beta-Rhamnoside Isolated from Parrotia persica. Molecules, 22(9) (2017).
Athar, M. et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol 224(3), 274–83 (2007).
Das, S. & Das, D. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 6(3), 168–173 (2007).
Bereswill, S. et al. Anti-Inflammatory Effects of Resveratrol, Curcumin and Simvastatin in Acute Small Intestinal Inflammation. PLos ONE 5(12), 1–11 (2010).
Elmali, M., Baysal, O., Harma, A., Esenkaya, I. & Mizrak, B. Effects of Resveratrol in Inflammatory Arthritis. Inflammation 30(1–2) (2007).
Baur, J. A. & Sinclair, D. A. Therapeutic Potential of Resveratrol: The in vivo evidence. Drug Disvovery 5, 493–506 (2006).
Rutledge, K., Cheng, Q. & Jabbarzadeh, E. Modulation of Inflammatory Response and Induction of Bone Formation Based on Combinatorial Effects of Resveratrol. Journal of Nanomedicine and Nanotechnology 7(1) (2016).
Soto, B. L. et al. Anti-tumor and immunomodulatory activity of resveratrol in vitro and its potential for combining with cancer immunotherapy. Int Immunopharmacol 11(11), 1877–1886 (2011).
Salehi, B. et al. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines, 6(3) (2018).
Browne, S. & Pandit, A. Biomaterial-mediated modification of the local inflammatory environment. Frontiers in Bioengineering and Biotechnology 3(67), 1–14 (2015).
Park, S. J. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148(3), 421–33 (2012).
Sajish, M. & Schimmel, P. A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature 519(7543), 370–3 (2015).
Imschenetzky, M., Morin, V., Carvajal, N., Montecino, M. & Puchi, M. Decreased heterogeneity of CS histone variants after hydrolysis of the ADP-ribose moiety. J Cell Biochem 61(1), 109–17 (1996).
Boulikas, T. Poly(ADP-ribose) synthesis and degradation in mammalian nuclei. Anal Biochem 203(2), 252–8 (1992).
Moss, J., Yost, D. A. & Stanley, S. J. Amino acid-specific ADP-ribosylation. J Biol Chem 258(10), 6466–70 (1983).
Ethier, C., Tardif, M., Arul, L. & Poirier, G. G. PARP-1 modulation of mTOR signaling in response to a DNA alkylating agent. PLoS One 7(10), e47978 (2012).
Bitterman, K. J., Anderson, R. M., Cohen, H. Y., Latorre-Esteves, M. & Sinclair, D. A. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 277(47), 45099–107 (2002).
Galloway, A. et al. Dopamine triggers CTCF-dependent morphological and genomic remodeling of astrocytes. J Neurosci (2018).
Hong, S. Y. et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nature Medicine 21(8), 887–894 (2015).
Rutkowski, B., Rutkowski, P., Slominska, E., Smolenski, R. T. & Swierczynski, J. Cellular toxicity of nicotinamide metabolites. J Ren Nutr 22(1), 95–7 (2012).
Rajamohan, S. B. et al. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol 29(15), 4116–29 (2009).
Ito, S., Murphy, C. G., Doubrovina, E., Jasin, M. & Moynahan, M. E. PARP Inhibitors in Clinical Use Induce Genomic Instability in Normal Human Cells. Plos One 11, 7 (2016).
Hopkins, T. A. et al. PARP1 Trapping by PARP Inhibitors Drives Cytotoxicity in Both Cancer Cells and Healthy Bone Marrow. Molecular Cancer Research 17(2), 409–419 (2019).
Lapucci, A. et al. Poly(ADP-ribose) Polymerase-1 Is a Nuclear Epigenetic Regulator of Mitochondrial DNA Repair and Transcription. Mol Pharmacol 79(6), 932–940 (2011).
Sajish, M. et al. Trp-tRNA synthetase bridges DNA-PKcs to PARP-1 to link IFN-gamma and p53 signaling. Nat Chem Biol 8(6), 547–54 (2012).
Liu, H. et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563(7729), 131–136 (2018).
Malireddi, R. K. S., Ippagunta, S., Lamkanfi, M. & Kanneganti, T. D. Cutting Edge: Proteolytic Inactivation of Poly(ADP-Ribose) Polymerase 1 by the Nlrp3 and Nlrc4 Inflammasomes. J Immunol 185(6), 3127–3130 (2010).
Ghosh, R., Roy, S. & Franco, S. PARP1 depletion induces RIG-I-dependent signaling in human cancer cells. Plos One 13(3), e0194611 (2018).
Nassour, J. et al. Defective DNA single-strand break repair is responsible for senescence and neoplastic escape of epithelial cells. Nature Communications 7 (2016).
Pantelidou, C. et al. PARP Inhibitor Efficacy Depends on CD8(+) T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov (2019).
Ding, L. et al. PARP Inhibition Elicits STING-Dependent Antitumor Immunity in Brca1-Deficient Ovarian Cancer. Cell Rep 25(11), 2972–2980 e5 (2018).
Parkes, E. E. et al. Activation of STING-Dependent Innate Immune Signaling By S-Phase-Specific DNA Damage in Breast Cancer. J Natl Cancer Inst 109(1) (2017).
Schiewer, M. J. et al. PARP-1 regulates DNA repair factor availability. EMBO Mol Med, 10(12) (2018).
Collins, P. & Chakyn, S. The Management of Nicotinamide and Nicotinic Acid in the Mouse. The Journal of Biological Chemistry 247(3), 778–783 (1972).
Ieraci, A. & Herrera, D. G. Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain. PLoS Med 3(4), e101 (2006).
Weiss, R. et al. Nicotinamide: a vitamin able to shift macrophage differentiation toward macrophages with restricted inflammatory features. Innate Immun 21(8), 813–26 (2015).
Alenzi, F. Q. Effect of nicotinamide on experimental induced diabetes. Iran J Allergy Asthma Immunol 8(1), 11–8 (2009).
Hashimoto, T. et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487(7408), 477–81 (2012).
Green, K. N. et al. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci 28(45), 11500–10 (2008).
Liu, D., Gharavi, R., Pitta, M., Gleichmann, M. & Mattson, M. P. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med 11(1), 28–42 (2009).
Williams, G. T. & Williams, W. J. Granulomatous inflammation–a review. Journal of clinical pathology 36(7), 723–33 (1983).
Johnston, R. B. Current Concepts - Immunology - Monocytes and Macrophages. New Engl J Med 318(12), 747–752 (1988).
Cotran, R. S, Kumar, V. & Robbins, S. L. Pathologic Basis of Disease. Saunders: Philadelphia, Vol. 6th edition, pp 50–112 (1999).
Gallin, J. I. & S. R., Inflammation: Basic Principles and Clinical Correlates. Raven: New York, Vol. 2nd edition (1999).
Martinez, F. O., Sica, A., Mantovani, A. & Locati, M. Macrophage activation and polarization. Front Biosci-Landmrk 13, 453–461 (2008).
Sica, A., Invernizzi, P. & Mantovani, A. Macrophage Plasticity and Polarization in Liver Homeostasis and Pathology. Hepatology 59(5), 2034–2042 (2014).
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A. & Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229(2), 176–185 (2013).
Stout, R. D. et al. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175(1), 342–349 (2005).
Mosser, D. M.; Edwards, J. P. Exploring the full spectrum of macrophage activation (vol 8, pg 958, 2008). Nat Rev Immunol, 10(6), 460–460 (2010).
Tedesco, S. et al. Phenotypic activation and pharmacological outcomes of spontaneously differentiated human monocyte-derived macrophages. Immunobiology 220(5), 545–554 (2015).
Giorgio, S. Macrophages: plastic solutions to environmental heterogeneity. Inflamm Res 62(9), 835–843 (2013).
Das, A., Segar, C. E., Hughley, B. B., Bowers, D. T. & Botchwey, E. A. The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages. Biomaterials 34(38), 9853–9862 (2013).
Kim, Y. H., Furuya, H. & Tabata, Y. Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials 35(1), 214–24 (2014).
Spiller, K. L. et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194–207 (2015).
Spiller, K. L. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35(15), 4477–88 (2014).
Gongol, B. et al. AMPK alpha 2 exerts its anti-inflammatory effects through PARP-1 and Bcl-6. P Natl Acad Sci USA 110(8), 3161–3166 (2013).
Shin, S. M., Cho, I. J. & Kim, S. G. Resveratrol Protects Mitochondria against Oxidative Stress through AMP-Activated Protein Kinase-Mediated Glycogen Synthase Kinase-3 beta Inhibition Downstream of Poly(ADP-ribose)polymerase-LKB1 Pathway. Mol Pharmacol 76(4), 884–895 (2009).
Kawahara, T. L. et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136(1), 62–74 (2009).
Lin, Y., Tang, X. Y., Zhu, Y. X., Shu, T. T. & Han, X. A. Identification of PARP-1 as one of the transcription factors binding to the repressor element in the promoter region of COX-2. Arch Biochem Biophys 505(1), 123–129 (2011).
Yanez, M., Blanchette, J. & Jabbarzadeh, E. Modulation of Inflammatory Response to Implanted Biomaterials Using Natural Compounds. Curr Pharm Des (2017).
Bjarnason, I., Hayllar, J., MacPherson, A. J. & Russell, A. S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 104(6), 1832–47 (1993).
Rainsford, K. D. Profile and mechanisms of gastrointestinal and other side effects of nonsteroidal anti-inflammatory drugs (NSAIDs). Am J Med, 107(6A), 27S–35S, discussion 35S–36S (1999).
Csiszar, A. Anti-inflammatory effects of resveratrol: possible role in prevention of age-related cardiovascular disease. Ann N Y Acad Sci 1215, 117–22 (2011).
Svajger, U. & Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol 31(3), 202–22 (2012).
Ma, Z. H. et al. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm Res 54(12), 522–7 (2005).
Maiese, K. & Chong, Z. Z. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci 24(5), 228–32 (2003).
Smith, L. M., Willmore, E., Austin, C. A. & Curtin, N. J. The novel poly(ADP-Ribose) polymerase inhibitor, AG14361, sensitizes cells to topoisomerase I poisons by increasing the persistence of DNA strand breaks. Clin Cancer Res 11(23), 8449–57 (2005).
Ahmad, S. F. et al. Resveratrol Improves Neuroimmune Dysregulation Through the Inhibition of Neuronal Toll-Like Receptors and COX-2 Signaling in BTBR T+ Itpr3(tf)/J Mice. Neuromol Med 20(1), 133–146 (2018).
Pantano, S., Jarrossay, D., Saccani, S., Bosisio, D. & Natoli, G. Plastic downregulation of the transcriptional repressor BCL6 during maturation of human dendritic cells. Exp Cell Res 312(8), 1312–22 (2006).
Park, E. K. et al. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res 56(1), 45–50 (2007).
Freytes, D. O., Kang, J. W., Marcos-Campos, I. & Vunjak-Novakovic, G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem 114(1), 220–9 (2013).
Othman, S. F., Xu, H. & Mao, J. J. Future role of MR elastography in tissue engineering and regenerative medicine. J Tissue Eng Regen Med 9(5), 481–7 (2015).
Bungard, D. et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science 329(5996), 1201–5 (2010).
Acknowledgements
We gratefully acknowledge support from the National Institutes of Health (NIH P20 GM103641, P20GM109091, NIH AR063338, and NIH EB026813) and the National Science Foundation (NSF 1631439).
Author information
Authors and Affiliations
Contributions
E.J. come up with the original research idea. M.Y., E.J. and S.M. designed the experiments. E.J. and M.Y. wrote the manuscript. M.Y. performed MTS, ELISA and RT-PCR assays. M.J. performed western blotting and immunoprecipitation experiments. K.M. and R.M.G. contributed to design and analysis of data on inflammation. E.J. and M.Y. analyzed the data. All authors read, edited, and approved the final manuscript version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yanez, M., Jhanji, M., Murphy, K. et al. Nicotinamide Augments the Anti-Inflammatory Properties of Resveratrol through PARP1 Activation. Sci Rep 9, 10219 (2019). https://doi.org/10.1038/s41598-019-46678-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46678-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.