Abstract
Telomere length (TL) is linked to various age-related diseases, but little is known about telomeres in gestational diabetes mellitus (GDM). We surveyed 509 subjects (113 GDM patients and 396 frequency matched controls) in Nanjing Drum Tower Hospital, Jiangsu province of eastern China. Relative telomere length (RTL) of genomic DNA extracted from peripheral blood leukocytes was measured using quantitative polymerase chain reaction (qPCR). Odds ratios (OR) and 95% confidence interval (CI) of GDM risk were calculated across tertiles of RTL using logistic regression model. Lipid parameters during the third trimesters of gestation (after 32 weeks) were collected from medical records. The general linear correlation test was used to explore the associations of lipid parameters with RTL. Our results showed that the RTL in GDM patients were significantly shorter than controls (0.302 ± 0.112 vs. 0.336 ± 0.164, P = 0.046). However, the GDM risk was significantly increased in subjects with median RTL (adjusted OR [aOR]: 1.936, 95% CI: 1.086, 3.453, P = 0.025) and the shortest RTL (aOR: 1.795, 95% CI: 1.004, 3.207, P = 0.048), compared to subjects with longest RTL. We also demonstrated that the lipid ratios (TC/TG, LDL/TG, HDL/TG, LDL/TC, TC/LDL) were significantly associated with RTL among controls. Overall, the present study indicated that attrition of telomeres would increase GDM risk among pregnant women, and the altered lipid levels may play an important role in RTL related GDM risk and pathogenesis.
Similar content being viewed by others
Introduction
Gestational diabetes mellitus (GDM), defined as a disease of glucose intolerance that leads to hyper-glycaemia with the first recognition during pregnancy1, is the most common metabolic abnormality and is increasing in prevalence worldwide2. Women with GDM are confronted with a great threat of diabetes, cardiovascular disease or hypertension later in life3. In addition, GDM also causes both immediate and long-term adverse consequences in the offspring4. Although several risk factors such as advanced maternal age, obesity5, family history of diabetes and specific racial backgrounds6 have been proved to be associated with GDM but the actual causes are still under investigation. It is widely accepted that the main pathological mechanisms of GDM involve the biochemical pathways resulting in insulin resistance and low-grade inflammation, in which oxidative stress plays a key role7,8.
Telomere length, an established biomarker of oxidative stress, has been associated with various age-related disorders (e.g. malignant tumor9, and infections10). Therefore, it has been proposed as a systemic marker for the development and progression of those biological aging diseases11. There are mounting evidences supporting associations between short telomeres and diabetes12,13. Hyperglycemia in diabetes status is known to increase oxidative stress, and then accelerates the telomere length shortening14. However, the studies involved in association between telomere length and gestational diabetes mellitus (GDM) are scarce15.
Circulating lipid patterns in GDM have been extensively studied, dyslipidemia is widely reported to be associated with the risk of developing GDM16. But the results of dyslipidemia in diabetic pregnancy are various due to different trimesters. Recently, a cross-sectional study suggested that metabolic syndrome and its components (e.g. lipid profiles) have also been associated with short telomere length17. To our knowledge, no studies have been carried out on the associations between GDM risk, lipid parameters, and telomere length. Hence, we hypothesize that altered lipid patterns may impose an influence on telomere length related GDM risk modification.
The prevalence of GDM in China is also alarming. A recent review systematically suggested the high incidence rate of GDM among women in mainland China18. In short, given the inconclusive evidence, we design a case-control study with 113 gestational diabetes mellitus (GDM) cases and 396 controls in Chinese population to evaluate the association between telomere length and GDM risk, and further to examine whether lipid metabolism plays a role in the relations between telomere length and GDM development.
Results
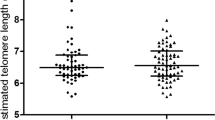
The baseline characteristics of 113 GDM cases and 396 controls were shown in Table 1. There were no differences in maternal age at delivery, BMI (body mass index, Kg/m2), secondhand smoking status, pregnant experience and physical activity, total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides (TG) and fasting blood glucose (FBG) between these two groups (P > 0.05). In contrast, the significant differences were observed both in 1-hour and 2-hour postprandial blood glucose among groups (P < 0.001). Participants within GDM cases exhibited shorter RTL compared to those in control cases (0.302 ± 0.112 vs. 0.336 ± 0.164, P = 0.046). Strikingly, in subgroups divided by different characteristics, we also detected a similar trend (Table 2).
Logistic regression was used to estimate adjusted OR (aOR) and 95% confidence interval(95% CI) for GDM risk with RTL. When compared with subjects in the highest (longest) tertile group, significant elevated risk of GDM was observed in the subjects from the median tertile (aOR: 1.936, 95% confidence interval [95% CI]: 1.086–3.453, P = 0.025) and the lowest (shortest) tertile (aOR: 1.795, 95% CI: 1.004–3.207, P = 0.048) groups, with adjustment for maternal age at delivery (Table 3).
Given the observed reverse correlation between GDM risk and RTL, further retrospective analysis was conducted to investigate the associations between altered lipid parameters in the third trimesters of gestation and RTL. As a result, no significant correlations were observed (Supplementary Table 1). Considered about the changes in lipid ratios have been shown as a better indicator of disease risk than changes in absolute levels of lipids or lipoproteins, we examined the general linear correlations between lipid ratios and RTL. We observed significant positive correlations between TC/TG ratio (r = 0.215, P = 0.002), LDL/TG ratio (r = 0.283, P < 0.001), HDL/TG ratio (r = 0.162, P = 0.021), LDL/TC ratio (r = 0.214, P = 0.002), but an inverse correlation was found between TC/LDL ratio (r = −0.185, P = 0.008) (Table 4). The correlations between lipid ratios and RTL were also shown in (Supplementary Fig. 1).
Discussion
The present case-control analysis was conducted to evaluate the associations of GDM with telomere length from peripheral blood leukocytes and lipid profiles on the ground of Chinese women population. We found that participants within GDM cases showed shorter RTL compared to those in control cases, which also suggested that shorter telomere length was related to the increase of GDM risk. In view of the effects of lipid on RTL, we also observed significantly correlations between TC/TG, LDL/TG, HDL/TG, LDL/TC ratios and RTL.
A previous study was conducted with 25 cases of GDM and 50 controls in Washington State. Harville et al.15 showed a trend of shorter telomeres in women with GDM, although the association was not statistically significant, which may be explained by the limited sample size, or the variability of leukocyte between individuals. Several previous studies also reported that reduced leukocyte telomere length was associated with incident diabetes risk12,13. In addition, shorten telomere length in GDM subjects has been shown in cord blood19 or in placentas20. While inflammation and oxidative stress have been postulated as crucial contributors to diabetes including GDM, telomere attrition can aggravate the chronic inflammation21 or oxidative stress22 of individuals. Higher plasma glucose status, which in turn can increase oxidative stress to accelerate the telomere length shortening and good glycemic control was associated with more favorable telomere dynamics23. Therefore, the relationship between decreased telomere length and increased GDM risk in our current study is biologically eligible. Meanwhile, since the blood samples were collected within 1 week before delivery, the possibility of GDM-induced telomere length shortening should not be excluded. Therefore, a well-designed study is necessary for further confirming the association between GDM risk and telomere length.
As a biomarker of aging, telomeres are also recognized to be influenced by the subject’s chronological age11,24. We observed a trend of negative relation between RTL and maternal age at delivery, but the result was not significant, which may be due to the narrow range of maternal age in our study. The most persuasive evidence indicated that impacts on telomere attrition with aging is relatively rapid during childhood and adolescence, while stable in adulthood25,26. In addition, pregnant women may be more likely to keep a healthy lifestyle and escape from adverse exposures. An experimental study with 5-year follow-up has suggested that comprehensive lifestyle intervention may promote the lengthening of telomere length27. The above interpretations may underestimate the adverse effects of aging on telomere length in our study.
Recently, metabolic syndrome was confirmed as a precursor to GDM, with abnormal lipid metabolism being associated with high risk of GDM16,28. In the present study, the comparisons of baseline TC, TG, LDL, HDL between cases and controls were not significant. Existing study also showed that there are no significant differences in any of lipid profiles between GDM and controls29. However, in diabetic or normal pregnancy, the results of lipid patterns are not universal. For instance, the cholesterol level in the third trimester of gestation were lower, unchanged or even higher in different studies29,30. These discrepant results may be owing to some confounders such as lifestyle habits, ethnicity, different collection protocols and genetic risks for GDM, as those factors are important sources of heterogeneity in the relationship between lipid levels and GDM. In the present study, all the lipid profiles were retrospectively collected following the criteria: the third trimesters (after 32 weeks) based on the medical records, regularly visiting the clinic, and healthier lifestyle, which could exclude the effects of lipid profiles on GDM. Lipid metabolism could be stable while telomere length manifested shortening in GDM cases, which was in accordance with the results from type 2 diabetes31, indicating that telomere length shortening may be sensitive and potentially could be a biomarker in the early stage of GDM development.
To the best of our knowledge, lipid ratios have been shown as better indicators for risk assessment and progression of certain disease than individual lipid profiles themselves32,33. Herein, we hypothesized that altered lipid ratios could indicate the alteration of telomere length. In the present study, there are positive correlations between RTL and TC/TG, LDL/TG, HDL/TG, LDL/TC ratios, an inverse correlation between RTL and TC/LDL ratio. Lipid ratios were more comprehensive than assessing the association between single lipid parameter and RTL, which may avoid the fluctuation of single parameter among individuals, and reflect the disease or health status more objectively. Previous studies demonstrated that HDL exerts anti-oxidant and anti-inflammatory effects and TG reflects the accruing burden of oxidative stress and inflammation17. They found that higher TG and lower HDL were associated with shorter telomere length. Furthermore, the elevated TC levels may cause augmented cell turnover and increased production of reactive oxygen species (ROS)34 and increased LDL levels were closely associated with decreased serum total antioxidant status (TAS)35,36. Thus, our results of correlations between lipid ratios and RTL were partially in consistency with these previous studies. Nevertheless, we firstly reported that correlations between RTL and each lipid ratio were weak in GDM cases. Further studies should focus on validating the mechanism of lipid parameters on telomeres in GDM.
This study has important strengths and limitations. The advantage is that we firstly observed the contribution of telomere length to the risk of GDM among Chinese women and revealed that shorten telomere length was significantly related to higher GDM risk, and ratios for lipid parameter may be better for evaluating on its association with telomere length than single lipid parameter. Nonetheless, there were still some potential limitations required discussion. Firstly, lipid parameters in this study were obtained during late pregnancy while blood samples for telomere length measuring were collected before childbirth, this unsynchronized data may cause potential bias on our analysis. Secondly, as any of case-control study, we could not confirm the exact causation between shorter telomere length and GDM. Thirdly, since we tested samples during the last trimester of gestation, the effect of GDM on telomere length and lipid profiles was hard to evaluate. Moreover, there is substantial evidence that it is the shortest telomeres that leads to telomere dysfunction. However, although the qPCR method is widely used in population study, which can only provide a mean relative telomere length. Further studies with more accurate telomere length measuring method may provide more reliable evidences37.
In conclusion, our study provided some valuable clues for further exploration on GDM risk and pathogenesis.
Methods
Study recruitment
GDM cases and controls were recruited from Nanjing Drum Tower Hospital in Nanjing, Jiangsu province of eastern China between April 2014 and April 2015. According to the guidelines from the International Association of Diabetes and Pregnancy Study Groups (IADPSG), GDM is diagnosed by specialist doctors38. During the same period, the women who also presented for routine GDM screening but in normoglycemic status were selected as controls. As a result, 113 cases and 396 age frequency matched controls were included in current study. After writing informed consent, each participant was interviewed by a well-trained interviewer with a structured questionnaire. Data available in the questionnaire include demographic data and lifestyle related factors such as cigarette exposure, physical activity, alcohol consumption, tea or coffee drinking, medical and reproductive characteristics etc. Full details of lipid parameters in the last trimesters of gestation were retrospectively extracted from medical records, including total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein (HDL), and fasting blood glucose (FBG). Overall, 509 pregnant women provided venous blood samples within 1 week before delivery. Blood samples were shipped by cold chain equipment to the study laboratories and stored until analysis. Ethical approval for the study was obtained from the ethics committees of Nanjing Medical University and Nanjing Drum Tower Hospital and this study complied with the Declaration of Helsinki.
Relative telomere length measurement
Genomic DNA was extracted from leukocytes of peripheral blood. Telomere length was measured on a modified quantitative polymerase chain reaction (qPCR) by ABI PRISM 7900HT Sequence Detection System (Applied Biosystems), which was previously described in our study39. This method expressed telomere length as a ratio of telomere repeat length (T) to single-gene copy number (S)(T/S). A lower T/S ratio reflects shorter RTL. The reference DNA (pooled from 5 healthy controls) was used to generate a standard curve for quantification. After exclusion of outliers, average cycle threshold (Ct) values of the remaining samples were calculated. Telomere PCR systems included primer TEL1,5′-GGTTTTTGA[GGGTGA]4GGGT-3′ and primer TEL2,5′-TCCCGACTAT[CCCTAT]4CCCTA-3′. The single-copy gene(36B4) primers included 36B4u, 5′-CAGCAAGTGGGAAGGTGTAATCC-3′, and 36B4d, 5′-CCCATTCTATCATCAACGGGTACAA-3′. Samples were performed in duplicate in 384-well plate and mean was used for calculations. Each reaction system contains 10 μl SYBR ® Green PCR Master Mix (Applied Biosystems) and a 5 ng/µl of DNA. Personnel of the laboratory that measured LTL were blinded to characteristics of the participants. RTL was calculated on the basis of Cawthon’s formula40,41:
Statistical analysis
Analyses were all performed with Stata version 9.2 (Stata Corp, College Station, TX). Sample characteristics were described as Means and SDs, or percentages. Pairwise comparisons for categorical variables (maternal age at delivery, secondhand smoking status, physical activity) were performed using Pearson Chi-square test. Continuous variables were analyzed by Paired t-test. To assess the associations of RTL on the risk of GDM, Logistic regression model was used to estimate odds ratios (ORs) and 95% confidence interval (CI) for GDM risk with RTL (categorized as RTL of <0.264, 0.264–0.355, ≥0.355), adjusting for maternal age at delivery. In addition, Linear correlation test was used to test the association of lipid profiles with RTL among controls. All statistical tests were two-sided. P value < 0.05 considered significant.
Data Availability
The datasets employed to support this study are available from the corresponding author on reasonable request.
References
Ferrara, A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 30(Suppl 2), S141–146, https://doi.org/10.2337/dc07-s206 (2007).
Egan, A. M. et al. Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe. Diabetologia 60, 1913–1921, https://doi.org/10.1007/s00125-017-4353-9 (2017).
Daly, B. et al. Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS Med 15, e1002488, https://doi.org/10.1371/journal.pmed.1002488 (2018).
Group, H. S. C. R. et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358, 1991–2002, https://doi.org/10.1056/NEJMoa0707943 (2008).
White, S. L. et al. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia 60, 1903–1912, https://doi.org/10.1007/s00125-017-4380-6 (2017).
Bardenheier, B. H. et al. Variation in prevalence of gestational diabetes mellitus among hospital discharges for obstetric delivery across 23 states in the United States. Diabetes Care 36, 1209–1214, https://doi.org/10.2337/dc12-0901 (2013).
Lekva, T., Norwitz, E. R., Aukrust, P. & Ueland, T. Impact of Systemic Inflammation on the Progression of Gestational Diabetes Mellitus. Curr Diab Rep 16, 26, https://doi.org/10.1007/s11892-016-0715-9 (2016).
Pickup, J. C. & Crook, M. A. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41, 1241–1248, https://doi.org/10.1007/s001250051058 (1998).
Suraweera, N. et al. Relative telomere lengths in tumor and normal mucosa are related to disease progression and chromosome instability profiles in colorectal cancer. Oncotarget 7, 36474–36488, https://doi.org/10.18632/oncotarget.9015 (2016).
Helby, J., Nordestgaard, B. G., Benfield, T. & Bojesen, S. E. Shorter leukocyte telomere length is associated with higher risk of infections: a prospective study of 75,309 individuals from the general population. Haematologica 102, 1457–1465, https://doi.org/10.3324/haematol.2016.161943 (2017).
Bojesen, S. E. Telomeres and human health. J Intern Med 274, 399–413, https://doi.org/10.1111/joim.12083 (2013).
Zhao, J. et al. Short leukocyte telomere length predicts risk of diabetes in american indians: the strong heart family study. Diabetes 63, 354–362, https://doi.org/10.2337/db13-0744 (2014).
Zee, R. Y., Castonguay, A. J., Barton, N. S., Germer, S. & Martin, M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res 155, 166–169, https://doi.org/10.1016/j.trsl.2009.09.012 (2010).
Olivieri, F. et al. Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis 206, 588–593, https://doi.org/10.1016/j.atherosclerosis.2009.03.034 (2009).
Harville, E. W., Williams, M. A., Qiu, C. F., Mejia, J. & Risques, R. A. Telomere length, pre-eclampsia, and gestational diabetes. BMC Res Notes 3, 113, https://doi.org/10.1186/1756-0500-3-113 (2010).
Herrera, E. & Ortega-Senovilla, H. Disturbances in lipid metabolism in diabetic pregnancy - Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 24, 515–525, https://doi.org/10.1016/j.beem.2010.05.006 (2010).
Rehkopf, D. H. et al. Leukocyte Telomere Length in Relation to 17 Biomarkers of Cardiovascular Disease Risk: A Cross-Sectional Study of US Adults. PLoS Med 13, e1002188, https://doi.org/10.1371/journal.pmed.1002188 (2016).
Gao, C., Sun, X., Lu, L., Liu, F. & Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J Diabetes Investig 10, 154–162, https://doi.org/10.1111/jdi.12854 (2019).
Xu, J. et al. Reduced fetal telomere length in gestational diabetes. PLoS One 9, e86161, https://doi.org/10.1371/journal.pone.0086161 (2014).
Biron-Shental, T. et al. Telomeres are shorter in placentas from pregnancies with uncontrolled diabetes. Placenta 36, 199–203, https://doi.org/10.1016/j.placenta.2014.11.011 (2015).
Calado, R. T. & Young, N. S. Telomere diseases. N Engl J Med 361, 2353–2365, https://doi.org/10.1056/NEJMra0903373 (2009).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem Sci 27, 339–344 (2002).
Ma, D., Zhu, W., Hu, S., Yu, X. & Yang, Y. Association between oxidative stress and telomere length in Type 1 and Type 2 diabetic patients. J Endocrinol Invest 36, 1032–1037, https://doi.org/10.3275/9036 (2013).
Blasco, M. A. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6, 611–622, https://doi.org/10.1038/nrg1656 (2005).
Brummendorf, T. H. et al. Telomere length dynamics in normal individuals and in patients with hematopoietic stem cell-associated disorders. Ann N Y Acad Sci 938, 293–303; discussion 303–294 (2001).
Yamaguchi, H. et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med 352, 1413–1424, https://doi.org/10.1056/NEJMoa042980 (2005).
Ornish, D. et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol 14, 1112–1120, https://doi.org/10.1016/S1470-2045(13)70366-8 (2013).
Chatzi, L. et al. The metabolic syndrome in early pregnancy and risk of gestational diabetes mellitus. Diabetes Metab 35, 490–494, https://doi.org/10.1016/j.diabet.2009.07.003 (2009).
Rizzo, M. et al. Atherogenic lipoprotein phenotype and LDL size and subclasses in women with gestational diabetes. Diabet Med 25, 1406–1411, https://doi.org/10.1111/j.1464-5491.2008.02613.x (2008).
Montelongo, A., Lasuncion, M. A., Pallardo, L. F. & Herrera, E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes 41, 1651–1659 (1992).
Adaikalakoteswari, A., Balasubramanyam, M. & Mohan, V. Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabet Med 22, 1151–1156, https://doi.org/10.1111/j.1464-5491.2005.01574.x (2005).
dos Santos-Weiss, I. C. et al. The plasma logarithm of the triglyceride/HDL-cholesterol ratio is a predictor of low risk gestational diabetes in early pregnancy. Clin Chim Acta 418, 1–4, https://doi.org/10.1016/j.cca.2012.12.004 (2013).
Ridker, P. M., Rifai, N., Cook, N. R., Bradwin, G. & Buring, J. E. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 294, 326–333, https://doi.org/10.1001/jama.294.3.326 (2005).
Ludwig, P. W., Hunninghake, D. B. & Hoidal, J. R. Increased leucocyte oxidative metabolism in hyperlipoproteinaemia. Lancet 2, 348–350 (1982).
Belo, L. et al. LDL size, total antioxidant status and oxidised LDL in normal human pregnancy: a longitudinal study. Atherosclerosis 177, 391–399, https://doi.org/10.1016/j.atherosclerosis.2004.07.023 (2004).
Sanchez-Vera, I. et al. Changes in plasma lipids and increased low-density lipoprotein susceptibility to oxidation in pregnancies complicated by gestational diabetes: consequences of obesity. Metabolism 56, 1527–1533, https://doi.org/10.1016/j.metabol.2007.06.020 (2007).
Lai, T. P., Wright, W. E. & Shay, J. W. Comparison of telomere length measurement methods. Philos Trans R Soc Lond B Biol Sci 373, https://doi.org/10.1098/rstb.2016.0451 (2018).
International Association of, D. et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682, https://doi.org/10.2337/dc09-1848 (2010).
Du, J. et al. Telomere length, genetic variants and gastric cancer risk in a Chinese population. Carcinogenesis 36, 963–970, https://doi.org/10.1093/carcin/bgv075 (2015).
Cawthon, R. M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37, e21, https://doi.org/10.1093/nar/gkn1027 (2009).
Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res 30, e47 (2002).
Acknowledgements
This study was supported in part by National Key Research & Development Program (2016YFC1000200, 2016YFC1000204), the State Key Program of National Natural Science of China (31530047), National Natural Science Foundation of China (81602927, 81701475), Cheung Kong Scholars Programme of China, the QingLan Project of the Jiangsu Province, Jiangsu Specially-Appointed Professor project, Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Innovation fund of state key laboratory of reproductive medicine (SKLRM-GC201802), Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015A067), Natural Science Foundation of Jiangsu Province (BK20161031), Natural Science Fund Project of Colleges in Jiangsu Province (17KJB330001). The authors thank all of medics of Drum Tower Clinical Medical College of Nanjing Medical University for their participating in subject recruitment and data collection in current study through the years. We also appreciate all the subjects’ contribution that donated blood samples and completed questionnaires for this study. Additionally, we are grateful to Dr. Hailong Li from the University of South Carolina for revising English language.
Author information
Authors and Affiliations
Contributions
Obtained funding: J.D. and Q.W. study concept and design: J.D., Q.W., K.D. and Y.H. critical revision of the manuscript for important intellectual content: all coauthors; statistically analysis: K.D., Y.D. and F.W. Molecular analysis and technical support: J.D. and J.C. DNA samples preparing: Q.W., Y.J. and J.C. subjects recruit and diagnostic evaluation: Q.W., J.L., Y.H. and Y.D. Study supervision: Q.W. and G.J. This manuscript has been read and approved by all the authors.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41598_2019_44968_MOESM1_ESM.pdf
Leukocyte telomere length, lipid parameters and gestational diabetes risk: a case-control study in a Chinese population.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weng, Q., Deng, K., Wu, F. et al. Leukocyte telomere length, lipid parameters and gestational diabetes risk: a case-control study in a Chinese population. Sci Rep 9, 8483 (2019). https://doi.org/10.1038/s41598-019-44968-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44968-9
This article is cited by
-
Telomere Length in Patients with Gestational Diabetes Mellitus and Normoglycemic Pregnant Women: a Systematic Review and Meta-analysis
Reproductive Sciences (2024)
-
Which one of LDL-C /HDL-C ratio and non-HDL-C can better predict the severity of coronary artery disease in STEMI patients
BMC Cardiovascular Disorders (2022)
-
Cardiometabolic profile and leukocyte telomere length in a Black South African population
Scientific Reports (2022)
-
Mendelian randomization implies no direct causal association between leukocyte telomere length and amyotrophic lateral sclerosis
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.