Abstract
In this study, a rational combination of 200 pre-selected Carbohydrate-Active enzymes (CAZymes) and sulfatases were tested, individually or combined, according to their ability to degrade Chlorella vulgaris cell wall to access its valuable nutritional compounds. The disruption of microalgae cell walls by a four-enzyme mixture (Mix) in comparison with the control, enabled to release up to 1.21 g/L of reducing sugars (p < 0.001), led to an eight-fold increase in oligosaccharides release (p < 0.001), and reduced the fluorescence intensity by 47% after staining with Calcofluor White (p < 0.001). The Mix treatment was successful in releasing proteins (p < 0.001), some MUFA (p < 0.05), and the beneficial 18:3n-3 fatty acid (p < 0.05). Even if no variation was detected for chlorophylls (p > 0.05), total carotenoids were increased in the supernatant (p < 0.05) from the Mix treatment, relative to the control. Taken together, these results indicate that this four-enzyme Mix displays an effective capacity to degrade C. vulgaris cell wall. Thus, these enzymes may constitute a good approach to improve the bioavailability of C. vulgaris nutrients for monogastric diets, in particular, and to facilitate the cost-effective use of microalgae by the feed industry, in general.
Similar content being viewed by others
Introduction
Autotrophic microalgae are currently considered an attractive source of high-value chemicals for biofuel, nutraceutical and pharmaceutical industries1, as well as sustainable animal production2. While the nutritional profile of microalgae varies considerably with the species, a large majority are characterised by having high protein, carbohydrate, lipid, vitamin, mineral and pigment contents3, which are comparable, if not superior, to conventional feedstuffs. These alternative feedstuffs are rich in beneficial n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFA)4. The enriched concentration of n-3 LCPUFA by microalgae represents a largely untapped natural resource with well-known beneficial health implications for both animals and humans5.
Chlorella vulgaris, a freshwater unicellular eukaryotic microalga, is one of the most cultivated microalgae worldwide. Although it is known for its relative ease of cultivation and high biomass productivity6, C. vulgaris, like the majority of microalgae, is endowed with a recalcitrant cell wall that confers resistance against invaders and harsh environmental conditions such as desiccation during growth, and is therefore refractory to breakage and drying, and hence to product extraction7. These cell walls have shown to contain an incredibly diverse and complex matrix of cross-linked insoluble carbohydrates, which trap valuable nutrients, thus limiting their direct use. The cell wall structure and composition were recently reviewed by Baudelet et al.1 for Chlorellae genus, and Safi et al.8 for Chlorella species.
Due to their recalcitrant nature, microalgal cell walls are largely indigestible by monogastric animals. For microalgae species, contrary to macroalgae, mechanical methods, as hammer mills, are not usually applied9. In turn, bead milling is used to incorporate Chlorella cells as food additives and this is a successfully, rising process in the food industry. However, this mechanical process is characterised as being hard working and expensive whereupon cells are massive destroyed. It is therefore imperative to find novel technologies to disrupt Chlorella vulgaris cells whereby cell wall disruption would be under a strictly controlled process to improve microalgal nutrient utilization, in particular to gain access to their protein and lipids2,10. Another essential prerequisite for the large-scale use of algal biomass as a feed supplement is to achieve a low production cost6. In addition, Chlorella is also considered as a potential source of microalgae oils for biofuel production and recognised as one of the alternatives to current biofuel crops, such as soybean, corn, rapeseed and lignocellulosic feedstock because it does not compete with food and does not require arable lands to grow11. The exploitation of biofuel production by Chlorella is thus attracting considerable attention. Chlorella has the ability to fix carbon dioxide efficiently and to remove nutrients rich in nitrogen and phosphorous, making it a good candidate for greenhouse gas biomitigation and wastewater bioremediation3.
Exogenous Carbohydrate-Active enzymes (CAZymes), mainly xylanases and beta-glucanases, are now widely used to supplement diets of monogastric livestock species to improve feed nutritive value and directly impact on animal performance and health12. The use of feed enzymes is currently a cost-effective strategy to improve the nutritional value of cereal-based diets for monogastric animals, although it remains to be established for microalgae biomass. In line with this, we hypothesised that the efficiency of C. vulgaris microalgae could be fine-tuned using individually or combined CAZymes and sulfatases, due to the degradation of recalcitrant cell wall and subsequent increase in nutrients bioavailability. Herein, cell disruption induced by enzymatic treatment was assessed by optical and fluorescence microscopies, and by measuring the reducing sugars and the oligosaccharides profile. The release of bioactive compounds with nutritional interest was assessed by quantifying proteins and pigments, as well as the fatty acid content and detailed composition, in both supernatant and residue fractions after incubation with the enzymatic treatment.
Results
Individual screening of enzymes in Chlorella vulgaris cell wall disruption
In order to evaluate which CAZymes and sulfatases of the library created in this work have the capacity to degrade C. vulgaris cell wall, each one of the enzymes was individually incubated with a microalgae suspension. Although a great majority of the enzymes were unable to deconstruct the marine biomass, 29 individual enzymes displayed a measurable capacity to degrade the cell wall of C. vulgaris, as described in Table 1. The ability to degrade the microalgae was assessed by the capacity to release reducing sugars as evaluated through the 3,5-dinitrosalicylic acid (DNSA) method. Table 1 data is presented in a qualitative scale of the amount of reducing sugars released (g/L): −, 0.00 < 0.005; +, 0.05 < 0.200; ++, 0.200 < 0.300; +++, >0.300. Although the release of reducing sugars was undetected for four enzymes, with identification numbers (ID) 69, 73, 77 and 82, they were included in this selection because their predicted substrates (1,3-α-glucans; agar and neoagarooligosaccharides; 1,3-β-glucans and insoluble 1,3-β-glucans, respectively) are major constituents of C. vulgaris cell walls8,13. Within this group of enzymes, CAZymes with ID 36, 47 and 60 exhibited the highest release of reducing sugars from the marine biomass, whereas the remaining enzymes displayed a low to moderate capacity to attack the complex polysaccharides.
Composition of a four-enzyme constituted Mix based on reducing sugars released
With the purpose of finding synergistic actions between the individual enzymes identified, the 29 enzymes presented in Table 1 were combined and tested in a mixture for the capacity to release reducing sugars from the microalgae. A mixture (Mix) consisting of four-enzymes was found to be the most restricted combination in terms of enzyme numbers and displaying the highest level of released sugars. This Mix was composed of an exo-β-glucosaminidase, an alginate lyase, a peptidoglycan N-acetylmuramic acid deacetylase and a lysozyme (CPE1314) and is presented in detail in Table 1. When this Mix was incubated with C. vulgaris suspension, a value of 1.21 g/L (p < 0.001) of reducing sugars released was observed, which represents an increase of 1.6-fold in relation to the highest value found in the individual enzyme screening. The rates for released sugars were found to be: for Mix vs Control = 333.7%; for Mix vs exo-β-glucosaminidase = 38.1%; for Mix vs alginate lyase = 198.4%; for Mix vs peptidoglycan N-acetylmuramic acid deacetylase = 248.1%; for Mix vs lysozyme (CPE1314) = 248.1%.
Thermostability and proteolysis assays
The four enzymes that constitute the Mix treatment were subjected individually to different temperatures to test their thermostability. Figure 1 illustrates the variation of protein concentration across the range of temperatures tested. At 37 and 40 °C, representing the internal temperature of mammals and poultry, respectively, all enzymes maintained their stability. However, the stability of ID 93 and ID 66 decayed abruptly from 37 and 40 °C, respectively. ID 66 even reached complete degradation at 55 °C, while ID 60 and ID 101 remained stable up to 80 °C. To investigate the capacity of the four enzymes to resist to the proteolytic attack, to which feed enzymes are subjected in the animal gastrointestinal tract, the same enzymes were treated with pancreatin at 37 °C. Table 2 shows the proteolytic resistance of these enzymes. ID 60 and ID 101 had partial resistance over the entire assay time; in turn, ID 66 and ID 93 showed complete degradation after 15 minutes.
Effect of Mix treatment on Chlorella vulgaris cell number and cell wall integrity
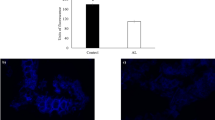
No significant differences were found on the number of cells observed between the control and the Mix (p > 0.05) (Fig. 2A). The number of cells counted was around 20000 cells for both treatments (Fig. 2B,C). The fluorescence intensity was reduced by 47% (Fig. 2D; p < 0.001) when C. vulgaris was incubated with the Mix (Fig. 2F), compared to the control (Fig. 2E).
(A) Cell counting using a Neubauer chamber for control and Mix treatments. (B,C) Light microscopy images (×400) of Chlorella vulgaris suspension for control and Mix treatments, respectively (scale bar: 20 µm). (D) Fluorescence intensity derived from Calcofluor White staining for control and Mix treatments. Asterisk denotes statistical difference at p < 0.001. (E,F) Fluorescence images (×400) of Chlorella vulgaris suspension stained with Calcofluor White for control and Mix treatments, respectively.
Effect of Mix treatment on the release of oligosaccharides from Chlorella vulgaris cell wall
Figure 3 shows the chromatogram on the release of oligosaccharides from C. vulgaris cell wall after treatment with control enzyme mixtures and the Mix enzymes identified in this work. A large peak in the oligosaccharides region was observed in the Mix treatment chromatogram (Fig. 3B) in relation to the control (Fig. 3A), corresponding to an 8-fold increase of oligosaccharides amount (p < 0.001; Fig. 3C).
Effect of Mix treatment on the release of proteins
In order to understand if the treatment with the Mix triggered the release of proteins from C. vulgaris cells to the external environment, the protein content in supernatant and residue fractions was determined (Table 3). In the supernatant, the Mix treatment caused a 23.4-fold increase in protein content relative to the control (p < 0.001), whereas in the residue, the Mix treatment led to a 1.7-fold decrease relative to the control (p < 0.001).
Effect of Mix treatment on the release of chlorophylls and carotenoids
Following the rationale of the previous point, the release of pigments from C. vulgaris cells to the external environment was determined in supernatant and residue fractions (Table 3). No significant variations were observed for chlorophylls (p > 0.05). Total carotenoids displayed significant differences with a 1.1-fold increase in the supernatant fraction of the Mix treatment relative to the control (p = 0.032).
Effect of Mix treatment on the release of fatty acids
The fatty acid content in residue and supernatant fractions, after incubation with the Mix treatment, was analysed to understand if the activity of Mix in the cell wall favoured the release of fatty acids from C. vulgaris cells to the external environment (Table 3). For the supernatant fraction, the predominant fatty acids were saturated fatty acids (SFA) > monounsaturated fatty acids (MUFA) > PUFA > n-6 PUFA > n-3 PUFA, while for the residue higher MUFA percentages were found, in the following order: MUFA > SFA > PUFA > n-6 PUFA > n-3 PUFA. In the supernatant, the percentage of 18:0 was increased in the control relative to the Mix treatment (p = 0.009). Conversely, the percentages of 16:1c7, 16:1c9, 17:1c9, 18:3n-3 and n-3 PUFA were found increased in the Mix treatment in comparison to the control (p = 0.014, p = 0.028; p = 0.003, p = 0.015 and p = 0.015, respectively). For the residue fraction, the Mix treatment presented higher percentages of 16:1c7, 16:1c9, 18:2n-6, 18:3n-3 and n-6 PUFA (p = 0.043, p = 0.003, p = 0.041, p = 0.044 and p = 0.033, respectively), and lower percentages of 16:0 (p = 0.002) and 22:2n-3 (p = 0.033) in relation to the control.
Discussion
To test the hypothesis that nutrients bioavailability of C. vulgaris could be largely improved after disruption of its recalcitrant cell wall, a large library of 178 CAZymes and 22 sulfatases, with well-defined and carefully thought-out enzymatic characteristics, was established by recombinant expression in E. coli cells. These 200 enzymes were selected taking into account the composition of the known matrix polysaccharides of microalgae cell walls, which comprises pectin, chitin agar, alginates or the aliphatic polymer algenan14. The selected enzymes were produced in a high-throughput (HTP) platform that involves gene synthesis, gene cloning, protein expression and protein purification. These enzymes were screened individually to degrade C. vulgaris cell wall, which was firstly assessed by measuring the release of reducing sugars. In the next stage, the 29 recombinant enzymes able to degrade C. vulgaris cell wall (see Table 1) were tested in combination to obtain the maximum disruption of C. vulgaris cell wall. As a result of these combinations, a four-enzyme mixture (Mix) was identified as the most active in the degradation of C. vulgaris cell wall and applied throughout.
The selected Mix was composed of four recombinant enzymes, an exo-β-glucosaminidase, an alginate lyase, a peptidoglycan N-acetylmuramic acid deacetylase and a lysozyme. The exo-β-glucosaminidase, included in the category of glucosaminidases15, has cellooligosaccharides and chitooligosaccharides as main substrates. The alginate lyase belongs to the family 5 of PL and has polyguluronate and polymannuronate as main substrates16,17,18. The peptidoglycan N-acetylmuramic acid deacetylase, which is included in the category of acetylglucosamine, has peptidoglycan as main substrate19. Finally, lysozyme, also known as muramidase belongs to GH family 2516 and peptidoglycan containing muramic acid δ-lactam is the main substrate of this enzyme20. The four enzymes constituting the Mix were biochemically characterised in terms of their thermostability and resistance to proteolysis. Both ID 60 and ID 101 were stable throughout the range of temperatures tested and resistant to the proteolytic action of pancreatin. The tertiary structure of protein, which confers thermotolerance to enzymes, could also confer inherent proteinase resistance, as demonstrated by Fontes et al.21. In contrast, enzymes ID 66 and ID 93 were shown to be sensitive to temperature rise and to proteolysis.
With the aim of evaluating the capacity of enzymes to digest the C. vulgaris cell wall for lipid extraction, Gerken et al.22 focused on the inhibition of C. vulgaris growth by a variety of enzymes. C. vulgaris is typically sensitive to chitinases and lysozymes, both enzymes degrading polymers containing N-acetylglucosamine. This observation corroborates our results with the introduction of a lysozyme, a glucosaminidase and an acetylglucosamine deacetylases in the Mix. Even if the composition of C. vulgaris cell wall is not entirely known, it is formed by a complex matrix constituted by glucosamine or galactose and mannose, and a broad range of pentose and hexose sugars23. As discussed by Baudelet et al.1, certain viruses can infect C. vulgaris, digest the host cell wall, penetrate and let the newly synthesised virus to be released24,25. Baudelet et al.1 referenced the identification of cell wall degrading alginate lyase coding genes in the genome of C. vulgaris infecting virus. These insights remit to the importance of alginate lyase for C. vulgaris cell wall digestion.
The Mix was proven effective by the increase in reducing sugars released, indicating a likely synergistic effect of these enzymes, as described by Phong et al.26, and known when enzyme mixes degrade carbohydrate mixtures. Fu et al.27 with the aim to evaluate the capacity of an immobilised cellulase to hydrolyse the cell wall of Chlorella sp. under different conditions also used the measurement of reducing sugars. As Chlorella vulgaris has been described as having a residual content of polysaccharides inside the cell27, the oligosaccharides found came from the disruption of cell wall instead of cell interior. Moreover, when applying the enzymatic mixture treatment to Chlorella vulgaris cells, it is expected that the first structure to be affected and partially or entirely disrupted would be the cell wall with the concomitant release of reducing sugars, easily measurable by the 3,5-dinitrosalicylic acid method.
No significant differences were observed in C. vulgaris cell number after treatment with the four-enzyme Mix. However, the fluorescence intensity was reduced by 47% with the Mix, indicating that these exogenous enzymes do not lead to the complete degradation of the cell wall, although cell wall integrity was affected to a major degree. Safi et al.28,29 used the same fluorochrome on different species of microalgae, including C. vulgaris, before and after different cell wall disintegration methods (e.g. high-pressure and bead milling) were applied. This clear change in cell structure was reinforced in our study by an increase of oligosaccharides amount after the treatment with the Mix, as reported by Heo et al.30. Those authors observed a dramatic increase of glucose content in C. vulgaris after an osmotic shock treatment, which was related to an efficient cell wall disruption. Conversely, in our study, there was no complete degradation of carbohydrates from the cell wall, since a complex mixture of oligosaccharides rather than single sugars was obtained.
The Mix treatment effect on C. vulgaris cell wall was a massive release of (hydro-) soluble proteins found in the supernatant, which was counterbalanced by a considerable decrease of proteins in the residue. These results agree with Safi et al.28,29, who observed an increase in soluble protein concentration after the application of different mechanical and chemical cell wall disruption methods in C. vulgaris. C. vulgaris has a high protein content, up to 68%3, with great nutritional quality since its amino acid composition meets the human dietary requirements proposed by World Health Organization (WHO) and Food and Agricultural Organization (FAO)8.
The Mix treatment also promoted the beneficial release of total carotenoids to the supernatant. Carotenoids, in particular β-carotene, astaxanthin, cantaxanthin and lutein, have various therapeutic properties, such as prevention of retina degeneration31 and regulation of blood cholesterol32, which are associated with their antioxidant activity33 and account for 1% in C. vulgaris29,34. Chlorophyll is the most abundant pigment in C. vulgaris, reaching 1–2% of the microalga dry weight. Even though the Mix treatment promoted the release of total carotenoids to the supernatant, no variation was observed for chlorophylls a and b. Safi et al.29 showed that the disruption of cell wall through the application of several mechanical and chemical methods allowed to release chlorophylls and carotenoids to the aqueous phase29, and at least for carotenoids, their results concur with ours. We speculate that the Mix treatment was unable of penetrating the phospholipid bilayer of the chloroplast in which pigments, such as chlorophylls and primary carotenoids, are embedded inside the thylakoids, therefore justifying the absence of differences for chlorophylls29. Alternatively, the presence of chlorophylls and carotenoids in the supernatant indicates the formation of micellar structures29, which are in line with their amphiphilic characteristics sharing different degrees of polarity35.
The fatty acid content and composition described herein for C. vulgaris cells agree with previous reports36,37, regardless the enzymatic treatment. Several studies, including those of Heo et al.30, Zheng et al.36, Cho et al.38 and Liang et al.39, were performed to improve the yield of lipid extraction from microalgae. A substantial cell wall disruption was observed by Cho et al.38 using a mixture of cellulases and β-glucosidases, and by Zheng et al.36 using a mixture of snailase, lysozyme and cellulase. In both cases, the enzymatic treatment led to an increase in lipid extraction efficiency, highlighting in the case of Zheng et al.36, the good performance exhibited by lysozyme. In our study, the focus was not on whether the Mix led to an increase in lipid extraction yield but, instead, on the release of fatty acids from C. vulgaris, through the disruption of microalgae cell wall. The major differences were found at the level of some MUFA, with a higher release of 16:1c7, 16:1c9 and 17:1c9, when C. vulgaris was submitted to the Mix treatment, justifying a higher percentage in the corresponding supernatant. The same applies to α-linolenic acid (18:3n-3), an essential n-3 LCPUFA, with important health properties, in particular for the prevention of cardiovascular diseases, cancer, autoimmune diseases and type 2 diabetes5,40. Due to its benefits, the increase release of α-linolenic acid when using this Mix deserves to be further exploited.
Conclusion
The results reported in this work indicate that this four-enzyme Mix has capacity to partially degrade C. vulgaris cell wall. These findings open new opportunities to develop a novel generation of biocatalysts to supplement diets for monogastric animals, in particular those incorporating C. vulgaris microalga. Data indicate that exogenous enzymes may disrupt microalgae cell walls to a significant extent, allowing the release of trapped nutrients with important nutritional value. Consequently, exogenous enzymes may promote the use of microalgae in animal diets at higher incorporation levels (>1%), leading to the release of highly beneficial bioactive compounds in an economically viable way. Further work is ongoing at our research laboratories to assess how effective these combined enzyme activities are for the supplementation of monogastric diets with C. vulgaris microalga as a feed ingredient. In addition to the animal feed industry, these results may increase the yield in obtaining valuable constituents of C. vulgaris for other biotechnological industries, in particular those related with biofuel, food and nutraceutical applications.
Methods
Microalgae production
Chlorella vulgaris is an unicellular freshwater microalgae of the genus Chlorella characterised by a relative ease of cultivation, high productivity and high content of proteins, lipids and other valuable components6. It has emerged as a promising alternative feedstock that represents an enormous biodiversity with multiple benefits exceeding the potential of conventional agricultural feedstock8.
C. vulgaris was cultivated through inoculation of axenic microalgal cultures (from the Institutes algal banks) in a medium that stimulates the growth of C. vulgaris: NaNO3 (250 mg/L), KH2PO4 (105 mg/L), MgSO4 (75 mg/L), CaCl2 (25 mg/L), NaCl (25 mg/L), K2HPO4 (75 mg/L), and 3 mL of trace metal solution: FeCl3 (0.194 g/L), CoCl2 (0.16 g/L), MnCl2 (0.082 g/L), Na2MoO4·2H2O (0.008 g/L) and ZnCl2 (0.005 g/L), using the adapted Krauss medium41.
C. vulgaris was first grown in 1 L capacity airlift bioreactors and then scaled-up until 25 L capacity polyethylene bag bioreactors (40 cm diameter) with bubbling filtered air (without CO2 addition), at low incident light conditions (150 µE.m−2.s−1), and at the optimal temperature of 25 °C for C. vulgaris. The harvesting step was done after reaching the stationary growth phase. Microalgal biomass was harvested without flocculation by simply removing agitation, followed by centrifugation in a continuous centrifuge LPX 40 (Alfa Laval, Sweden) (25 L). The concentrated biomass slurry was then frozen at −20 °C and freeze dried (Powerdry LL 3000, Thermo, Denmark) for further analysis.
High-throughput gene synthesis, cloning and protein expression/purification of recombinant enzymes
One-hundred and seventy-eight CAZymes with high potential for degradation of microalgae cell wall were selected from a diverse repertoire, including glycoside hydrolases (GH), pectate lyases (PL) and carbohydrate esterases (CE). In addition, twenty-two sulfatases were also selected for screening, as they are also likely involved in microalgae cell wall degradation22. The coding genes for the selected enzymes were synthesised in vitro using NZYGene Synthesis kit (Nzytech, Portugal). The protein sequence of each enzyme is presented as Supplementary Material (Table S1). Synthetic genes were codon optimised for expression in Escherichia coli, using NZYTech′s codon optimization software ATGenium42. All genes included the required 16 bp overhangs on both 5′ and 3′-ends for direct cloning into the bacterial expression vector pHTP1 (Nzytech, Portugal), following the procedure described in the NZYEasy Cloning & Expression kit I (Nzytech, Portugal). The generated recombinant plasmids were subjected to inducible T7 promoter control, while encoding the 200 enzymes fused to an N-terminal His6-tag that facilitates purification through Immobilised Affinity Chromatography (IMAC). The two-hundred plasmids were sequenced to ensure that no mutations accumulated during gene synthesis and were used to transform E. coli BL21 (DE3) cells. Transformed cells were grown on solid media and resulting colonies were used to inoculate 5 mL of NZY Auto-Induction LB medium (Nzytech, Portugal) supplemented with kanamycin (50 mg/L) at 37 °C to early-exponential phase (A600 nm = 1.5–2.0). Recombinant protein production occurred following a further incubation at 25 °C for 16 hours. All steps were carried out in 24 deep-well plates42. Cells were harvested by centrifugation at 75,000 g at 4 °C for 15 min and lysed in NZY Bacterial Cell Lysis Buffer (NZYTech, Portugal). The His6-tagged recombinant enzymes were purified from cell-free extracts by IMAC, based on an automated protocol that allows the purification of 96 proteins by day, as described previously43. Briefly, the crude cell lysates were incubated with Sepharose chelating beads (200 μL with bound Ni2+) and then transferred into 96-well filter plates (Macherey-Nagel). The wells were washed twice with buffer A (50 mM Na-HEPES, pH 7.5, 500 mM NaCl, 10 mM imidazole). The recombinant fusion proteins were eluted from the resin beads with 200 μL of elution buffer (50 mM Na-HEPES, pH 7.5, 500 mM NaCl, 300 mM imidazole) into 96-deep-well plates. All protein purification steps were automated on a Tecan robot (Tecan, Switzerland) containing a vacuum manifold. Homogeneity of purified proteins and molecular mass of recombinant enzymes were determined by SDS-PAGE in 14% (w/v) acrylamide gels. Protein concentration of enzymes stock solutions varied between 0.5–20 g/L, as determined spectrophotometrically by the Bradford method44.
Preparation of microalga cells suspension
Chlorella vulgaris suspension was prepared at 20 g/L, as follows: dry microalgae were weighed, re-suspended in phosphate buffered saline (PBS) and incubated for 30 min at 37 °C in an orbital incubator shaker at 200 rpm. After incubation, the suspension was centrifuged at 2500 g for 30 min, the supernatant was discarded and the pellet re-suspended in the same amount of PBS.
Enzymatic cell wall disruption
The cell wall disruption assay was performed in a 24 well microplate (VWR Chemicals, West Chester, PA, USA). Two mL of microalgae suspension was added to each well along with the respective enzyme added at a final concentration of 20 mg/L. Control wells took the same amount of PBS. The microplate was then sealed and incubated overnight in an orbital incubator shaker at 37 °C and 140 rpm. Microplate was centrifuged for 15 min at 3210 g and the supernatants and pellets were recovered. To precipitate and remove the enzymes, the supernatant for DNSA and HPLC analyses was boiled for 5 min and centrifuged for 5 min at 10,000 g and the supernatant recovered.
Reducing sugars measurement
3,5-Dinitrosalicylic acid (DNSA) method45, was employed as a standard protocol to quantify the released amount of reducing sugars. In this method, the aldehyde and ketone groups of the reducing sugars reduce the yellow 3,5-dinitrosalicylic acid to orange 3-amino-5-nitrosalicylic acid. Glucose was used as standard. After mixing 0.6 mL of glucose solutions or supernatants with 0.6 mL of DNSA reagent, samples were heated at 100 °C for 15 min. Then, samples were cooled on ice for 5 min and detected by ultraviolet-visible spectrophotometry at 570 nm.
Thermostability and proteolysis experiments
Each enzyme from the four-enzyme constituted mixture (Mix; Provisional Patent number 20181000067928, INPI, Portugal) was subjected individually to 12 different temperature conditions (without incubation and with incubation at 30 °C, 37 °C and 40 °C to 80 °C at 5 °C intervals) for 30 min. Then, the incubation was cooled on ice for 10 min and centrifuged at 16,100 g for 8 min at 4 °C. The supernatant was recovered and the protein amount was quantified in triplicate using a NanoDrop 2000/2000c (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA). To validate results, the supernatants were also analysed by 14% SDS-PAGE gels and the images were acquired with BioRad ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA, USA).
Two-hundred microliters of each enzyme that compose the Mix, at a concentration of 1 g/L, was placed in individual tubes. For each enzyme, there was a control and a treatment: 200 µL of PBS was added for the control; 200 µL of porcine pancreatin (VWR Chemicals, West Chester, PA, USA) at 5 g/L was added for the treated sample. The reactions were incubated at 37 °C, at regular intervals of 15 min until 2 h. The samples were then removed and analysed by 14% SDS-PAGE gels to validate results. The images from gels were acquired with BioRad ChemiDoc XRS imaging system (Bio-Rad). A qualitative scale was created to assess the proteolytic resistance based on the visualisation of protein fragments in SDS-PAGE gels. The qualitative scale was defined as follows: −, no resistant (only fragmentation bands) which means the complete disappearance of the protein band along with visualisation of protein fragments from enzymatic digestion; + , partially resistant (protein and fragmentation bands) meaning that the protein band is visualised associated with protein fragments bands from enzymatic digestion.
Determination of total oligosaccharides
The mono and oligosaccharide profiles from the supernatants of C. vulgaris after control and Mix treatments were analysed by High Performance Liquid Chromatography (HPLC) on an Agilent system (Agilent 1200 Series, Agilent Technologies Inc., Palo Alto, CA), equipped with an electrochemical detector (Coulochem III, ESA Dionex Thermo Fisher Scientific Inc, USA). The HPLC analysis was performed using a Dionex CarboPac PA10 column (4 × 250 mm, Thermo Fisher Scientific Inc, USA) fitted to a CarboPac PA10 guard column (4 × 50 mm), following the procedure described by Thermo Fisher Scientific46 with slight modifications. The separation of mono and oligosaccharides was achieved using a mobile phase with a flow rate of 1 mL/min for 60 min at 25 °C, as follows: isocratic elution with 18 mM NaOH (eluent A) during 18 min, gradient with 100–0 mM NaOH (eluent B) and 0–75 mM sodium acetate in 100 mM NaOH (eluent C) from 18–40 min, and re-equilibration to 18 mM NaOH during 20 min. The quantification of total oligosaccharides was based on a standard curve, using a range of concentrations from 0.025 mM to 0.2 mM of glucose. The results were expressed as equivalent moles of glucose released per gram of microalgae.
Optical and fluorescence microscopic observations
The pellets from the enzymatic cell wall disruption assay were re-suspended in 2 mL of PBS. The number of cells in the microalgae suspension was determined using a Neubauer counting chamber by direct observation on a bright-field Olympus CH30 microscope (Olympus, Center Valley, PA, USA) and images were acquired with an Olympus DP21 (Olympus) digital camera. The fluorochrome Calcofluor White (Sigma-Aldrich, St. Louis, Mo, USA) that binds to the cell wall28 was added to the same suspensions used for optical microscopy. Cells were observed with an epifluorescence microscope and images were captured with a Leica DFC-340FX (Leica, Wetzlar, Germany) camera system. When excited at λ = 488 nm, cells were identified as blue coloured. The fluorescence intensity, expressed as arbitrary units, was measured using the Image J software47.
Determination of protein content
The N content in lyophilised supernatants and residues from C. vulgaris suspension after control and Mix treatments was determined by the Kjeldahl method (984.13)48, assuming that no nitrogen from the media interfere with the assay. The crude protein was calculated as N × 6.25.
Pigment analysis
The content of chlorophyll a, chlorophyll b and total carotenoids in supernatants and residues from C. vulgaris suspension after control and Mix treatments was measured according to Hynstova et al.49, with slight modifications. For the pigment determination in the residue fraction, 4 mL of acetone was added to 40 mg of residue and incubated in the dark during 1 h at 45 °C and 200 rpm. After incubation, the samples were analysed using UV–Vis spectrophotometer (Ultrospec 3100 pro, Amersham Biosciences, Little Chalfont, UK) and the pigment content was calculated according to equations described by Hynstova et al.49. The supernatant fraction was red directly after treatment using UV–Vis spectrophotometer and the pigment content was calculated as defined above.
Determination of fatty acid composition
Fatty acids from the lyophilised supernatants and pellets of C. vulgaris after control and Mix treatments were extracted based on the method of Folch et al.50, replacing chloroform:methanol (2:1, v/v) by dichloromethane:methanol (2:1, v/v), according to Carlson51. Fatty acids were esterified to methyl esters (FAME) by acid catalysis with acetylchloride-methanol solution (1.25 M Sigma-Aldrich, St. Louis, Mo, USA) at 80 °C for 60 min as described by Batista et al.52. The analysis of FAME was done using a gas chromatograph HP7890A (Hewlett-Packard) coupled with a flame ionization detector (GC-FID). The separation was performed in a SupelcowaxTM10 capillary column (30 m × 0.20 mm i.d., 0.20 μm film thickness; Supelco Inc., Bellefonte, PA) with helium as a carrier gas at a flow rate of 1.3 mL/min. The injector and detector temperatures were 250 and 280 °C, respectively. The oven temperature program was started at 150 °C and held for 11 min, then increased to 210 °C at a rate of 3 °C/min and maintained for 30 min. The identification of FAME was achieved by comparison with retention times of fatty acids standards (37 Component FAME mixture from Supelco Inc.). The quantification of total FAME was carried out using heneicosanoic acid (21:0) as internal standard. Each fatty acid was expressed as a percentage of the sum of identified fatty acids (% total fatty acids).
Statistical analysis
All experiments were conducted in triplicate, and the mean values are presented. The error bars on figures indicate the standard error. Data were checked for normality and analysed using the Generalised Linear Mixed (GLM) model test. p value lower than 0.05 was considered to be statistically significant. All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Data Availability
All data generated during this study are included in this published article. The datasets generated during the current study are available from the corresponding author on demand.
References
Baudelet, P. H., Ricochonb, G., Lindera, M. & Munigliaa, L. A new insight into cell walls of Chlorophyta. Algal Research 25, 333–371 (2017).
Lum, K., Kim, J. & Lei, X. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. Journal of Animal Science and Biotechnology 4, 53 (2013).
Liu, J. & Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. Advances in Biochemical Engineering/Biotechnology 153, 1–35 (2016).
Madeira, M. S. et al. Microalgae as feed ingredients for livestock production and meat quality: a review. Livestock Science 205, 111–121 (2017).
Calder, P. Mechanisms of action of (n-3) fatty acids. The Journal of Nutrition 142, 592–599 (2012).
Kotrbáček, V., Doubek, J. & Doucha, J. The chlorococcalean alga Chlorella in animal nutrition: a review. Journal of Applied Phycology 27, 2173–2180 (2015).
Acton, Q. Cellular structures - advances in research and application, 946 p (Scholarly Editions, 2013).
Safi, C., Zebib, B., Merah, O., Pontalier, P.-Y. & Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renewable and Sustainable Energy Reviews 35, 265–278 (2014).
Makkar, H. P. S. et al. Seaweeds for livestock diets: A review. Animal Feed Science and Technology 212, 1–17 (2016).
Austic, R., Mustafa, A., Jung, B., Gatrell, S. & Lei, X. Potential and limitation of a new defatted diatom microalgal biomass in replacing soybean meal and corn in diets for broiler chickens. Journal of Agricultural and Food Chemistry 61, 7341–7348 (2013).
Singh, A., Nigam, P. S. & Murphy, J. D. Renewable fuels from algae: an answer to debatable land based fuels. Bioresource Technology 102, 10–16 (2011).
Ravindran, V. & Son, J. Feed enzyme technology: present status and future developments. Recent Patents on Food, Nutrition & Agriculture 3, 102–109 (2011).
Chen, C. Y. et al. Microalgae-based carbohydrates for biofuel production. Biochemical Engineering Journal 78, 1–10 (2013).
Scholz, M. J. et al. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryotic Cell 13, 1450–1464 (2014).
Honda, Y., Shimaya, N., Ishisaki, K., Ebihara, M. & Taniguchi, H. Elucidation of exo-β-d-glucosaminidase activity of a family 9 glycoside hydrolase (PBPRA0520) from Photobacterium profundum SS9. Glycobiology 21, 503–511 (2011).
Cantarel, B. L. et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Research 37, 233–238 (2009).
Yoon, H. J. et al. Overexpression in Escherichia coli, purification, and characterization of Sphingomonas sp. A1 alginate lyases. Protein Expression and Purification 19, 84–90 (2000).
Yamasaki, M., Ogura, K., Hashimoto, W., Mikami, B. & Murata, K. A structural basis for depolymerization of alginate by polysaccharide lyase family-7. Journal of Molecular Biology 352, 11–21 (2005).
Blair, D. E. & van Aalten, D. M. Structures of Bacillus subtilis PdaA, a family 4 carbohydrate esterase, and a complex with N-acetyl-glucosamine. FEBS Letters 570, 13–19 (2004).
Chen, Y., Miyata, S., Makino, S. & Moriyama, R. Molecular characterization of a germination-specific muramidase from Clostridium perfringens S40 spores and nucleotide sequence of the corresponding gene. Journal of Bacteriology 179, 3181–3187 (1997).
Fontes, C. M. G. A., Hall, J., Hirst, B. H., Hazlewood, G. P. & Gilbert, H. J. The resistance of cellulases and xylanases to proteolytic inactivation. Applied Microbiology and Biotechnology 3, 52–57 (1995).
Gerken, H. G., Donohoe, B. & Knoshaug, E. P. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 237, 239–253 (2013).
Lee, S. Y., Cho, J. M., Chang, Y. K. & Oh, Y. K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresource Technology 244, 1317–1328 (2017).
Meints, R. H., Lee, K., Burbank, D. E. & Van Etten, J. L. Infection of a chlorella-like alga with the virus, PBCV-1: ultrastructural studies. Virology 138, 341–346 (1984).
Reisser, W. & Kapaun, E. Entry of a chlorella-virus into its host cell 1. Journal of Phycology 27, 609–613 (1991).
Phong, W. N. et al. Mild cell disruption methods for bio-functional proteins recovery from microalgae - Recent developments and future perspectives. Algal Research 31, 506–516 (2018).
Fu, C. C., Hung, T. C., Chen, J. Y., Su, C. H. & Wu, W. T. Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresource Technology 101, 8750–8754 (2010).
Safi, C. et al. Aqueous extraction of proteins from microalgae: effect of different cell disruption methods. Algal Research 3, 61–65 (2014).
Safi, C. et al. Understanding the effect of cell disruption methods on the diffusion of Chlorella vulgaris proteins and pigments in the aqueous phase. Algal Research 8, 61–68 (2015).
Heo, Y. M. et al. An integrative process for obtaining lipids and glucose from Chlorella vulgaris biomass with a single treatment of cell disruption. Algal Research 27, 286–294 (2017).
Fernández-Sevilla, J. M., Fernández, F. G. A. & Grima, E. M. Obtaining lutein-rich extract from microalgal biomass at preparative scale. Methods in Molecular Biology 892, 307–314 (2012).
Granado, F., Olmedilla, B. & Blanco, I. Nutritional and clinical relevance of lutein in human health. British Journal of Nutrition 90, 487–502 (2003).
Gouveia, L., Raymundo, A., Batista, A. P., Sousa, I. & Empis, J. Chlorella vulgaris and Haematococcus pluvialis biomass as colouring and antioxidant in food emulsions. European Food Research and Technology 222, 362 (2006).
Mendes, R. L. et al. Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chemistry 53, 99–103 (1995).
Háda, M., Nagy, V., Deli, J. & Agócs, A. Hydrophilic carotenoids: recent progress. Molecules 17, 5003–5012 (2012).
Zheng, H. et al. Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Applied Biochemistry and Biotechnology 164, 1215–1224 (2011).
Yeh, K. L. & Chang, J. S. Effects of cultivation conditions and media composition non cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresource Technology 105, 120–127 (2012).
Cho, H. S., Oh, Y. K., Park, S. C., Lee, J. W. & Park, J. Y. Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris. Renewable Energy 54, 156–160 (2013).
Liang, K., Zhang, Q. & Cong, W. Enzyme-assisted aqueous extraction of lipid from microalgae. Journal of Agricultural and Food Chemistry 60, 11771–11776 (2012).
Abedi, E. & Sahari, M. A. Long‐chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Science & Nutrition 2, 443–463 (2014).
Vonshak, A. Laboratory techniques for the cultivation of microalgae in CRC Handbook of Microalgal Mass Culture (ed. Richmond, A.) 117–143 (CRC Press, 1986).
Sequeira, A. F. et al. Gene design, fusion technology and TEV cleavage conditions influence the purification of oxidized disulphide-rich venom peptides in Escherichia coli. Microbial Cell Factories 16, 4 (2017).
Saez, N. J. & Vincentelli, R. High-throughput expression screening and purification of recombinant proteins in E. coli. Methods in Molecular Biology 1091, 33–53 (2014).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254 (1976).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31, 426–428 (1959).
Thermo Scientific. Dionex CarboPac PA10 in Column Product Manual (2009).
NIH. Image J software. available at, https://imagej.nih.gov/nih-image/.
AOAC. Official methods of analysis, 17th ed (Association of Official Analytical Chemists, 2000).
Hynstova, V. et al. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. Journal of Pharmaceutical and Biomedical Analysis 148, 108–118 (2018).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 226, 497–509 (1957).
Carlson, L. A. Extraction of lipids from human whole serum and lipoproteins and from rat liver tissue with methylene chloride-methanol: a comparison with extraction with chloroform-methanol. Clinica Chimica Acta 149, 89–93 (1985).
Batista, A. P., Gouveia, L., Bandarra, N. M., Franco, J. M. & Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Research 2, 164–173 (2013).
Acknowledgements
This study was supported by Fundação para a Ciência e a Tecnologia (FCT, Lisbon, Portugal; grant PTDC/CVT-NUT/5931/2014), Portugal2020 (grant 08/SI/3399/2015), CIISA (project UID/CVT/00276/2019), a PhD fellowship to D.C. (SFRH/BD/126198/2016) and a Post-Doc fellowship to P.A.L. (08/SI/3399/2015).
Author information
Authors and Affiliations
Contributions
D.C. carried out the microalgae cultivation and prepared biomass for the subsequent experiments. D.C. and P.A.L. assisted the experiments and contributed to the discussion of the experimental results. D.C., V.C., J.B. and P.P. were responsible for high-throughput production of recombinant enzymes. D.C., P.A.L., M.S.M. and C.M.A. analysed reducing sugars, oligosaccharides amount and fat composition of microalgae samples. D.C. and M.S.M. performed statistics. D.C., P.A.L. and J.A.M.P. performed the manuscript preparation and literature review. N.M.B., H.G.G., C.M.G.A.F. and J.A.M.P. contributed to the overall research design and interpretation of results. All authors have revised, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coelho, D., Lopes, P.A., Cardoso, V. et al. Novel combination of feed enzymes to improve the degradation of Chlorella vulgaris recalcitrant cell wall. Sci Rep 9, 5382 (2019). https://doi.org/10.1038/s41598-019-41775-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41775-0
This article is cited by
-
Optimization and Characterization of Spirulina and Chlorella Hydrolysates for Industrial Application
Applied Biochemistry and Biotechnology (2023)
-
Integrated Omics analysis of pig muscle metabolism under the effects of dietary Chlorella vulgaris and exogenous enzymes
Scientific Reports (2022)
-
Impact of dietary Chlorella vulgaris and feed enzymes on health status, immune response and liver metabolites in weaned piglets
Scientific Reports (2022)
-
Influence of Chlorella vulgaris on growth, digestibility and gut morphology and microbiota of weaned piglet
Scientific Reports (2022)
-
A novel Penicillium sumatraense isolate reveals an arsenal of degrading enzymes exploitable in algal bio-refinery processes
Biotechnology for Biofuels (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.