Abstract
Psoriasis is an immune-mediated inflammatory skin disease that affects millions worldwide. Studying immune cells involved in psoriasis pathogenesis is essential to identify effective and safe therapeutics for the disease. Using human psoriasis skin, activated macrophages were observed in both lesional and non-lesional skin, but were elevated in lesional skin. Activation of the IL-23/IL-17 pathway is integral to the development of psoriasis. To further characterize the monocyte/macrophage (Mon/Mac) population when the IL-23 pathway is activated, a murine model of intradermal injection of IL-23 was used. Flow cytometry revealed that Mon/Mac cells were the dominant immune population, particularly late in the model, highlighted by strong presence of Ly6ChiMHC IIhi cells. The Mon/Mac cells were also shown to have high expression for TNFα but not IL-17A. Prophylactic dosing of a CSF-1R inhibitor to deplete Mon/Mac cells significantly reduced several inflammatory mediators from the skin tissue suggesting a pathogenic role for Mon/Mac. Treatment dosing of the inhibitor produced a less robust effect. Mon/Mac cells were also differentiated by levels of Ki67 and TNFα expression. These data point to an important contribution of Mon/Mac cells in IL-23 related skin inflammation and suggest that these cells are a significant player in the underlying pathophysiology of psoriasis.
Similar content being viewed by others
Introduction
Psoriasis (Ps) is a chronic auto inflammatory skin disease that affects 1 to 2 percent of the U.S. population and 0.2 to 4.8 percent of the population world-wide1,2,3. The most common clinical variant of Ps is Psoriasis vulgaris (a.k.a. Plaque Psoriasis) affecting approximately 85 to 90 percent of diagnosed Ps patients2. Histopathologically, Ps vulgaris manifests with four distinctive features: thickening of epidermis, elongated rete ridges, parakeratosis, and infiltration of diverse types of leukocytes in both the epidermis and dermis. The leukocyte infiltration includes cells from both the innate and adaptive immune systems that reflect a complex interplay of the two immune systems during disease progression. A critical role for T cell subsets in the pathogenesis of Ps has been well established and reviewed by others3,4. In contrast, a defined role for myeloid lineage cells, monocytes and macrophages in particular, has continued to evolve. Previous studies linked macrophage involvement in Ps to its production of TNFα5 and also identified a subpopulation of CD163+ macrophages that were classically activated in Ps lesional skin6. Different preclinical models of Ps also have supported a role for macrophages in the pathogenesis of the disease7,8,9,10.
IL-23 is an IL-12 cytokine family member composed of two subunits, p19 and p40. Human genetic association studies have revealed a strong linkage between IL-23R and Ps11,12,13. IL-12 and IL-23 have independent roles in Ps14,15,16. Confirmation of the importance of the IL-23 pathway in the pathogenesis of Ps follows with the recent development of successful therapeutics that modulate this pathway17. Thus, murine models that activate the IL-23/IL-17 axis in skin have been developed to further interrogate the mechanistic consequences associated with stimulation of this pathway18,19,20,21. Indeed, a model using intradermal injection of IL-23 in mice has demonstrated a relatively strong transcriptional match to human Ps when compared to other murine models20. Similar IL-23 murine models have shown marked infiltration of T cells followed by robust accumulation of macrophages18 and also a role for monocyte derived dendritic cells (moDCs) in the observed inflammation19.
In this study, the role of monocyte/macrophage (Mon/Mac) was further examined in relation to Ps and IL-23 related inflammation. In human lesional skin, activated macrophages increased with a unique distribution pattern by congregating in sites of active inflammation. Using the IL-23 intradermal injection model, a robust accumulation of Ly6ChiMHCIIlo/hi Mon/Mac cells contributed to disease progression. Pharmacological depletion of the Mon/Mac population both prior to and after established inflammation significantly reduced the disease phenotype. Additionally, it was found that Mon/Mac cells were the main source of TNFα and they regulated IL-17A production during disease progression. Thus, the current data adds to the understanding of how Mon/Mac cells contribute to the pathogenesis of Ps and in particular their role following activation of the IL-23/IL-17 pathway.
Results
Activated macrophages were increased in lesional human psoriasis skin compared to non-lesional skin
Macrophages were reported to be abundant in Ps skin compared to normal skin6. In order to confirm the observation and to further evaluate the levels and distribution of macrophages in Ps patient skin, immunohistochemistry was performed using IBA-122,23 to identify activated macrophages. The level of IBA-1+ signal was significantly elevated in Ps lesional skin compared to non-lesional skin taken from the same patient (Fig. 1). IBA-1+ macrophages were present in both dermal and epidermal layers of skin. The most robust signal was within the dermis beneath the parakeratotic area, suggesting that macrophages were recruited to the site of active inflammation within the lesional plaque.
IBA-1+ macrophages were increased in lesional human Ps skin. IBA-1 IHC staining of non-lesional and lesional skin of the Ps patients (n = 4) (a) representative images of IHC staining of IBA-1 (red) in epidermis and dermis (black filled triangle) area. Inset image represents active parakeratosis area within the lesional skin biopsy. IBA-1+ macrophages resided in both epidermis and dermis area and congregated under the active parakeratosis region. Section of whole skin biopsies of both lesional and non-lesional were scanned with digital scanner at 20X objective. The representative images within the skin section were from 8X digital magnification. Inset area of the lesional skin was from 32X digital magnification. (b) quantification of IBA-1+ signal of non-lesional and lesional skin of Ps patients. IBA1+ staining was normalized to the length of biopsy epidermis analyzed for each donor and presented as area (μm2) divided by length (μm). Data are shown as mean ± SEM.; *P < 0.05.
CD64+ Mon/Mac dominated the increase of immune cells following IL-23 induced skin inflammation
In a previous study, we observed an increase of Mon/Mac cells in a murine model of IL-23 induced skin inflammation18. To delineate the role of Mon/Mac population in the pathogenesis of this inflammation, we further characterized these cells in this model (Fig. 2). Daily intradermal injections of IL-23 induced a robust ear inflammation represented by significantly increased ear thickness up to the end of the study (i.e. day 4, Fig. 2a), and by elevated leukocyte infiltration compared to sham injected animals (Fig. 2b). CD64, a high affinity IgG receptor FcγRI24, was used to discriminate between dendritic cells (DC) and Mon/Mac populations. On day 2 of the IL-23 model, 43% (P < 0.05) of the infiltrated leukocytes were CD64+ Mon/Mac and this continued to rise to 56% (P < 0.001) by day 4 (Fig. 2b). Thus, as the disease progressed, the accumulation of Mon/Mac cells predominated the immune cell influx. This suggests that Mon/Mac may be involved in the maintenance and/or exacerbation of the observed ear inflammation.
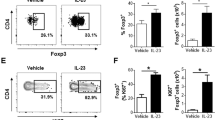
CD64+ Mon/Mac population dominated the increase of immune cells in the murine model of IL-23 induced skin inflammation. (a) Daily ear thickness measurement in IL-23 and PBS + 0.1% BSA (i.e. sham) injected mice (n = 6–8). (b) Enumeration of ear leukocytes (CD45+ live cells, left) and ear Mon/Mac (CD45+CD64+ live cells, center) and frequency of ear Mon/Mac (CD45+CD64+ live cells, right) on day 2 and day 4 of IL-23 and sham injected mice (n = 4). All values were determined by flow cytometry. (c) Representative 2-dimentional couture plot (left) of cell surface expression of Ly6C and MHC II on Mon/Mac cells from IL-23 injected ears at day 2 and 4 determined by flow cytometry and respective enumeration (right) of ear Mon/Mac subsets (CD45+CD64+) based on Ly6C/MHC II expression levels on days 2 and 4 of IL-23 and sham injected mice (n = 4). Flow cytometry gating strategy is detailed in Supplemental Fig. S2. Numerical data are shown as mean ± SEM.; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To further discriminate the CD64+ Mon/Mac population in the IL-23 injected skin, the expression levels of Ly6C/MHC II was analyzed (Fig. 2c, Supplementary Fig. S2). Blood monocytes that are Ly6Chi (classical monocytes) have been reported to be recruited into inflamed tissue and are precursors for mononuclear phagocytes25. Differential expression levels of Ly6C and MHC II can facilitate further discrimination of Mon/Mac subsets in skin26. Four sub-populations were identified in the skin of IL-23 injected mice: Ly6ChiMHC IIlo, Ly6ChiMHC IIhi, Ly6CloMHC IIhi, and Ly6CloMHC IIlo. Both Ly6ChiMHC IIlo and Ly6ChiMHC IIhi cells were significantly increased (P < 0.001) over the course of the IL-23 model. The increase of these two populations was equally dominant on day 2, 44% and 45% respectively (Fig. 2c, Supplementary Fig. S1). However on day 4, the Mon/Mac compartment was dominated by Ly6ChiMHC IIhi cells (53% of Ly6ChiMHC IIhi vs. 30% of Ly6ChiMHC IIlo, P < 0.001). Although both of these two Ly6Chi Mon/Mac subsets were elevated on day 2, the higher proportion of Ly6ChiMHC IIhi cells within the CD64+ Mon/Mac compartment on day 4 suggests that these cells may be derived from the Ly6ChiMHC IIlo monocytes and accumulated progressively in the tissue. A similar observation has been reported in the inflamed gut27. This is consistent with the view that a dynamic Ly6Chi monocyte population exists in inflamed tissue28.
Mon/Mac is a major contributor to IL-23 model pathogenesis
Generation, proliferation, and maintenance of the Mon/Mac population depend on signals mediated through the CSF-1R protein29. To assess the pathophysiological role of Mon/Mac in the IL-23 model, JNJ-40346527, a selective oral CSF-1R tyrosine kinase inhibitor30,31 was administered to deplete the Mon/Mac population. Animals dosed with the inhibitor prior to the first injection of IL-23 displayed a significant decrease in ear swelling (78%, P < 0.0001) as well as epidermal (59%, P < 0.001) and dermal areas (68%, P < 0.0001) compared to vehicle dosed animals on day 4 (Fig. 3a). IBA-1 immunohistochemistry confirmed complete depletion of macrophages (P < 0.001) following this prophylactic administration of JNJ-40346527. Additionally, the mRNA expression of pro-inflammatory genes, TNFα, IL-17A, IL-1β and β-defensin 4 in the ear skin was decreased in a range of 63–84% (P < 0.01) after JNJ-40346527 administration (Fig. 4a). Interestingly, gene transcripts of IL-22, a T cell cytokine that has been reported to drive epidermal hyperplasia and keratinocyte proliferation32, was significantly elevated (P < 0.0001) after IL-23 exposure but was not affected by the JNJ-40346527 induced Mon/Mac depletion.
Both prophylactic and treatment dosing of small molecule CSF-1R inhibitor (JNJ-40346527) reduced IL-23 induced skin pathophysiology. (a) Prophylactic and (b) treatment dosing of JNJ-40346527 (CSF-1Ri + IL-23) or vehicle (Veh + IL-23) in IL-23 injected mice. Sham injection controls dosed with vehicle (Veh + Sham) were also included in both studies. Daily ear thickness measurement (left), epidermal and dermal area measurements (center), and IBA-1+ staining (right) are shown for both studies (n = 6–8). For each type of staining, one 5 mm length of ear sample section was analyzed per animal. Staining was reported as tissue area (mm2) for H&E and tissue staining area (µm2) for IBA-1. Data are shown as mean ± SEM. **P < 0.01; ***P < 0.001; ****P < 0.0001.
JNJ-40346527 modulated IL-23 induced proinflammatory mediators in ear skin. (a) Prophylactic dosing of JNJ-40346527 reduced the mRNA expression levels of TNFα, IL-1β, IL-17A, and β-defensin 4 but not IL-22 that were all elevated by IL-23 injections. (b) Treatment dosing of JNJ-40346527 did not significantly alter the levels of these genes, but there was a trend for reduction in TNFα, and IL-1β levels. Sham injection controls dosed with vehicle were also included in both studies. Data calculated as fold change compared to sham injected vehicle dosing group. Data are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
In the next experiment, JNJ-40346527 was delivered after inflammation was established in the IL-23 model to determine effects of attenuating Mon/Mac levels at this stage of the disease. Thus, JNJ-40346527 was administered on day 2 (Fig. 3b) when there was already a significant rise (Fig. 2a, P < 0.0001) in ear thickness. Except for mRNA expression, treatment dosing of JNJ-40346527 showed similar trends to the prophylactic dosing but the degree of effect was more modest (Fig. 3b). IBA-1 staining of skin samples from day 4 confirmed a partial depletion of macrophages in dosed animals (P < 0.001), and correspondingly JNJ-40346527 reduced ear swelling by only 42% (P < 0.01), as well as epidermal and dermal area by 34% (non-significant) and 55% (P < 0.001), respectively. This partial reduction of inflammation was also accompanied by weak non-significant decreases of TNFα (28%) and IL-1β (20%), but no changes in mRNA expression for IL-17A, or β-defensin 4 (Fig. 4b), which were decreased with prophylactic dosing. Taken together, these data strongly suggest that Mon/Mac cells play a significant role during the pathogenesis and progression of IL-23 mediated skin inflammation.
Mon/Mac is the major immune contributor for TNFα production in IL-23 mediated skin inflammation
TNFα and IL-17A are two inflammatory cytokines that have been clinically proven to be pathogenic for Ps33,34. Our group has shown that neutralizing either TNFα or IL-17A with antibodies at clinically relevant doses was efficacious in the IL-23 model18 and the current study demonstrated that both of these cytokines were elevated in the model as well. However, these cytokines were differentially modulated by administration of JNJ-40346527 (Fig. 4). In order to place these results in context, intracellular protein staining was performed using flow cytometry to identify immune cells that were TNFα or IL-17A positive from skin samples taken on day 4 of the IL-23 model (Fig. 5 and Supplementary Fig. S4). The TNFα+ leukocytes were predominantly from the Mon/Mac population and to a lesser extent from conventional T cells. The IL-17A+ leukocytes were primarily localized to the T cell population, in contrast to Mon/Mac, as well as neutrophils which were IL-17A negative and very few of them were TNFα positive (Fig. 5 and Supplementary Fig. S4). These data suggests that Mon/Mac may contribute to disease pathogenesis in the IL-23 model by directly producing TNFα and by indirectly regulating IL-17A levels from T cells.
Mon/Mac and T cells were the predominant immune cells that produced TNFα and IL-17A, respectively. Number of (a) TNFα+ cells and (b) IL-17A+ cells in the ears of sham and IL-23 injected mice at day 4 (n = 6–8). The cellular lineage, TNFα and IL-17A expression were all determined by flow cytometry (detailed gating hierarchy in supplemental materials). Data are shown as mean ± SEM. ****P < 0.0001. B, B cells; ILC, innate lymphocytes; Conv.T, conventional T cells (i.e. αβ T cells and dermal γδ T cells); DETC, Dendritic epidermal T cells; Gran/DC, granulocytes and dendritic cells; Mon/Mac, monocytes/macrophages; other Mon, CD11bloCD64− monocytes.
It was noteworthy that in addition to a TNFα+ Mon/Mac population in the IL-23 model, a separate population that was Ki67+ was also recognized (Fig. 6a). Ki67 is a well-known cellular proliferative marker35. Like the TNFα+ Mon/Mac population, Ki67+ Mon/Mac population was also significantly elevated (P < 0.01) by day 4 in the IL-23 model (Fig. 6b). The ratio of Ki67+ Mon/Mac to TNFα+ Mon/Mac in the Day 4 diseased ear was 1.35 indicating the presence of more of Ki67+ cells than TNFα+ cells. Thus, there are two functionally distinct Mon/Mac populations that increase following activation of the IL-23 pathway. One population is proliferative and the other is differentiated towards producing TNFα.
Two distinct Mon/Mac (Ki67+ and TNFα+) subsets increased in IL-23 inflamed skin. (a) Representative 2-dimentional couture plot of TNFα and Ki67 expression in Mon/Mac cells from IL-23 injected ears at day 4 determined by flow cytometry. The quad gate that separates TNFα and Ki67 positive/negative was determined based on comparing fluorescent staining from IL-23 ears to those from naive ears and IL-23 injected ears without the specific fluorescent antibody. Numbers indicate the frequencies of the cell population within the quad gates. (b) Enumeration of Ki67+ and TNFα+ Mon/Mac cells in ears of sham and IL-23 injected animals (n = 6–8). Frequencies of Ki67+ and TNFα+ Mon/Mac cells were determined as described in (a) and were used to calculate the number of cells per ear. Data are shown as mean ± SEM. **P < 0.01.
Discussion
Ps is an auto-inflammatory skin disease with a complex pathophysiology that includes diverse cell types and cytokine pathways4. Within this complex biology, T cells are the most studied immune cell population with multiple subsets linked to the development of skin lesions36,37,38,39. Meanwhile, understanding the role of innate immune cells in Ps pathogenesis, in particular macrophages, is still evolving. Consistent with a previous report6, macrophage levels in the current study were increased in the lesional skin of human Ps patients relative to adjacent non-lesional skin from the same patient. These macrophages were localized to both dermal and epidermal areas of the lesional skin and tended to congregate beneath the parakeratotic areas in the diseased skin. This implies that macrophages were recruited to a site of active inflammation within the lesional skin, and supports the idea that macrophages are important for the pathogenesis of the disease, as reported by others using preclinical models7,8,9,10.
Recent genetic and clinical data have demonstrated that the IL-23/IL-17 pathway is a key driver in human Ps17. Thus, animal models that activate this axis have utility to study its mechanistic undertones and understand possible contributions to Ps. Intradermal injection of IL-23 in rodents, which has been used by several groups including ours18,19,20,21, has been reported to be a mechanistically useful model, and when compared to other murine models, better resembles human Ps at a transcriptomic level20. Using a four-day IL-23 injection model, the current study showed that the Mon/Mac population was a significant component of the infiltrating leukocytes in the inflamed skin and was the predominant cell type by the final day of the model. Four subsets of the Mon/Mac population were identified based on the detection of high levels of surface CD64 expression and then sub-divided into groups with high or low levels of surface Ly6C and MHC II molecules. Two of the subsets with high expression of Ly6C (Ly6Chi) were similarly elevated by day 2 and continued to increase up to at least day 4, but the Ly6Chi MHC IIhi cells increased to a greater degree on day 4 than the Ly6Chi MHC IIlo cells.
The tissue Mon/Mac cells in the current study were also found to be the prevalent immune cell population expressing TNFα indicating a proinflammatory contribution. The proinflammatory role was further substantiated by significant attenuation of several related disease endpoints following depletion/reduction of the Mon/Mac population in the IL-23 model. The apparent proinflammatory/pathogenic role of Mon/Mac in the current study is similar to the proposed role of moDCs in a previous report using an IL-23 injection model19. However, the majority of the Mon/Mac cells in the current study did not fall under the moDC lineage since they were high in CD64 expression, a marker that Singh’s group employed to differentiate moDCs from macrophages. Thus, monocyte derived cells other than moDCs, in particular Ly6C/MHC II expressing macrophages, can also be recruited following activation of the IL-23 pathway and contributed to the psoriasis-like skin inflammation.
The dynamic change towards a predominant Ly6C/MHC II Mon/Mac subset over time suggests that like moDCs, these Mon/Mac cells can mature during the progression of inflammation. The Ly6ChiMHC IIlo cells may be monocyte precursors in blood that enter tissue27 and are still highly proliferative which may have been reflected by the high levels of Ki67+ cells. These cells may then give rise to the MHC II “high” cells, which would be fully differentiated macrophages that no longer proliferate but produce inflammatory cytokines such as TNFα. Thus, it appears that IL-23 induced skin inflammation is partially a consequence of monocytes that were recruited to the tissue and then further differentiated into subsets that contributed to the disease pathophysiology. Since this observation was induced by IL-23 injection, this finding may be generalizable and possibly translatable to diseases like Ps that are linked to IL-23 pathway. The diversity of monocyte derived subsets may reflect a heightened cellular plasticity of monocytes during inflammation28. A deeper characterization of the Mon/Mac/DC subsets in the inflamed skin tissue is needed to further elucidate the individual and possibly redundant roles.
A selective CSF-1R tyrosine kinase inhibitor, JNJ-40346527, was used to deplete/reduce Mon/Mac levels in the IL-23 model31. CSF-1R is a hematopoietic growth factor receptor for CSF-1 and IL-34 that specifically regulates the homeostasis and development of mononuclear phagocytes, particularly monocytes and macrophages25,29. Both prophylactic and treatment dosing of JNJ-40346527 significantly reduced the IBA1+ macrophages and subsequently ear swelling in IL-23 injected mice. These effects on ear thickness, particularly with prophylactic dosing, were accompanied by reduction of TNFα mRNA expression. The effect on TNFα appears likely to be a direct consequence of reducing the Mon/Mac population as these cells were shown to be TNFα positive. Prophylactic dosing of JNJ-40346527 also significantly reduced IL-17A mRNA expression. The effect on IL-17A appears to be a downstream consequence of decreasing the Mon/Mac cells as it was demonstrated that T cells, but not the Mon/Mac cells, were the major class expressing IL-17A. Meanwhile, administration of JNJ-40346527 after establishing inflammation significantly reduced ear swelling but did not affect IL-17A expression. This suggests that there is a need for substantial reduction in Mon/Mac levels (and probably TNFα expression) to modulate IL-17A. Thus, treatment with JNJ-40346527 at a more advanced stage of the disease is likely not sufficient to totally overcome the “inflammatory momentum” resulting in partial efficacy. In contrast to the regulation of IL-17A, IL-22 expression was unaffected, suggesting a separate feedback loop regulating IL-22 production and possibly a different cellular subset (i.e., Th22) not affected by Mon/Mac reduction. An alternative approach to reduce Mon/Mac levels would have been to use clodronate liposomes which act intracellularly to induce apoptosis in phagocytic cells40. Administration of clodronate liposomes was efficacious in different mouse models of Ps-like inflammation7,8,10. Both approaches can functionally reach the same goal of reducing Mon/Mac levels, but ease of use and reduction of animal stress favors oral delivery of JNJ-40346527 (i.e., vs. added intra-dermal injections). Taken together, reducing Mon/Mac levels can significantly decrease skin inflammation suggesting that targeting Mon/Mac may be an effective means to prevent new inflammation and to, at least partially, treat existing disease. Confirmatory studies using other Ps like animal models are needed to further test the idea.
In conclusion, the current study confirmed that macrophages were upregulated in dermal and epidermal layers of human lesional Ps skin. In mice, activation of an important Ps pathogenic pathway, the IL-23 pathway, induced significant tissue levels of TNFα+ Mon/Mac. Mon/Mac cells were the predominant immune population in the inflamed skin highlighted by high levels of the Ly6ChiMHC IIhi subset. Pharmacological reduction of the Mon/Mac population ameliorated several inflammatory endpoints demonstrating a pathophysiological role for these cells and suggesting potential value in the treatment of Ps.
Materials and Methods
More detailed materials and methods are available in the Supplementary Materials.
Human skin
Punch biopsies (4 mm diameter) of psoriatic lesional and adjacent non-lesional skin were obtained from four Ps patients under an AbbVie Institutional Review Board-approved protocol. Informed consent was obtained and the study was performed in adherence with the Declaration of Helsinki Principles. Biopsies were placed in HypoThermosol FRS tissue transport solution (BioLife Solutions, Bothell, WA) for site-to-site transfer.
Animal models
Female C57BL/6 mice were purchased from Charles River Labs (Portage, MA). All animal studies were conducted under a protocol approved by AbbVie’s Institutional Animal Care and Use Committee and in accordance with the relevant guidelines and regulations. All studies were conducted in a blinded manner.
IL-23 induced skin inflammation was achieved as described previously18. Briefly, 1 μg of recombinant IL-23 (AbbVie), or PBS + 0.1% BSA (Sham) was intradermally injected into the dorsal side of one ear once a day (morning) for four days starting on day 0. Ear thickness was measured daily and mice were euthanized on either day 2 or day 4 for tissue harvesting.
To deplete/reduce macrophages, JNJ-40346527, or vehicle (ASD (i.e. amorphous solid dispersion) formulation) was dosed orally at 100 mg•kg−1, once a day in either a prophylactic or treatment regime (Fig. 3 and Supplementary materials).
Histology and Immunohistochemistry
Standard procedures were used for histology and immunohistochemistry as previously described18 and detailed in the supplemental materials. All routine H&E staining and IHC were conducted using ST5010 autostainer and BondRX immunostainer, respectively (both from Leica, Wetzlar, Germany). Standardized IHC protocol was used for anti-IBA-1 (Wako Chemicals, Richmond, VA) staining. Stained tissue slides were digitized with a P250 pathology slide scanner (Perkin Elmer, Waltham, MA) and analyzed by HALO software (Indica Labs, Corrales, NM).
For human and mouse skin samples, 4 µm microtome sections were collected on slides for H&E and IHC staining. An analysis technique was chosen to minimize the impact of variations in samples since total skin area as well as the proportion of epidermis to dermis can change as the disease progresses. Also, human biopsy collection techniques can result in “edge effects” in the dermis. Thus, for human biopsies, IBA1+ staining was normalized to the length of epidermis evaluated, and data are presented as area (µm2) divided by length (µm). For mouse ear samples, a standard 5 mm length was analyzed for each type of staining, and data are reported as area (mm2 or µm2) for both the H&E and IHC assessment.
Tissue processing and Flow Cytometry
Ear skin preparation for flow cytometry analysis was conducted as previously described18 and in supplementary materials.
To determine cellular lineage, skin cell extracts were treated with standard flow cytometry surface staining procedures. For intracellular staining, skin cell extracts were incubated at 37 °C plus 5% CO2 for 3 hours in presence of 1X protein transporter inhibitor (eBioscience, Carlsbad, CA) prior to the standard surface and intracellular staining procedures (Supplementary Materials). Subsequently, cells were acquired using BD FACSAria III flow cytometer (BD Biosciences, San Jose, CA). The FCS files were analyzed using FlowJo 10.2 software (FlowJo LLC, Ashland, OR).
Measurement of gene expression
Animal whole ears were homogenized and the expression of various genes was determined using QuantiGene multiplex kit (ThermoFisher Scientific, Waltham, MA). Probe sets used in the multiplex measurement are detailed in the supplementary materials. All procedures followed manufacturer’s instructions. Data was acquired using FlexMAP 3D cytometer (Luminex, Austin, TX). All data were normalized by GeoMean to two housekeeping genes, Hprt and Gapdh. Normalized data were than compared to the control group (i.e. vehicle treated Sham animals) and presented as fold changes.
Statistics
Prism 7 (GraphPad Software, San Diego, CA) was used for all the statistical analyses. All studies have been repeated at least once and confirmed. Data were analyzed using either an unpaired T-test, or a one-way/two-way ANOVA followed by a Bonferroni’s multiple comparison post-hoc analysis if the ANOVA revealed significance. A P-value less than 0.05 was considered significant. Data are shown as mean ± SEM.
References
Gudjonsson, J. E. & Elder, J. T. Psoriasis: epidemiology. Clinics in dermatology 25, 535–546, https://doi.org/10.1016/j.clindermatol.2007.08.007 (2007).
Nestle, F. O., Kaplan, D. H. & Barker, J. Psoriasis. The New England journal of medicine 361, 496–509, https://doi.org/10.1056/NEJMra0804595 (2009).
Greb, J. E. et al. Psoriasis. Nature reviews. Disease primers 2, 16082, https://doi.org/10.1038/nrdp.2016.82 (2016).
Kim, J. & Krueger, J. G. The immunopathogenesis of psoriasis. Dermatologic clinics 33, 13–23, https://doi.org/10.1016/j.det.2014.09.002 (2015).
Marble, D. J., Gordon, K. B. & Nickoloff, B. J. Targeting TNFalpha rapidly reduces density of dendritic cells and macrophages in psoriatic plaques with restoration of epidermal keratinocyte differentiation. Journal of dermatological science 48, 87–101, https://doi.org/10.1016/j.jdermsci.2007.06.006 (2007).
Fuentes-Duculan, J. et al. A subpopulation of CD163-positive macrophages is classically activated in psoriasis. The Journal of investigative dermatology 130, 2412–2422, https://doi.org/10.1038/jid.2010.165 (2010).
Wang, H. et al. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. The Journal of clinical investigation 116, 2105–2114, https://doi.org/10.1172/JCI27180 (2006).
Stratis, A. et al. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. The Journal of clinical investigation 116, 2094–2104, https://doi.org/10.1172/JCI27179 (2006).
Leite Dantas, R. et al. Macrophage-mediated psoriasis can be suppressed by regulatory T lymphocytes. The Journal of pathology 240, 366–377, https://doi.org/10.1002/path.4786 (2016).
Ward, N. L. et al. Depletion of antigen-presenting cells by clodronate liposomes reverses the psoriatic skin phenotype in KC-Tie2 mice. The British journal of dermatology 164, 750–758, https://doi.org/10.1111/j.1365-2133.2010.10129.x (2011).
Nair, R. P. et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nature genetics 41, 199–204, https://doi.org/10.1038/ng.311 (2009).
Cargill, M. et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. American journal of human genetics 80, 273–290, https://doi.org/10.1086/511051 (2007).
Capon, F. et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Human genetics 122, 201–206, https://doi.org/10.1007/s00439-007-0397-0 (2007).
Kulig, P. et al. IL-12 protects from psoriasiform skin inflammation. Nature communications 7, 13466, https://doi.org/10.1038/ncomms13466 (2016).
Guenova, E. et al. IL-4 abrogates T(H)17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proceedings of the National Academy of Sciences of the United States of America 112, 2163–2168, https://doi.org/10.1073/pnas.1416922112 (2015).
Tonel, G. et al. Cutting edge: A critical functional role for IL-23 in psoriasis. Journal of immunology 185, 5688–5691, https://doi.org/10.4049/jimmunol.1001538 (2010).
Puig, L. The role of IL 23 in the treatment of psoriasis. Expert review of clinical immunology 13, 525–534, https://doi.org/10.1080/1744666X.2017.1292137 (2017).
Gauld, S. B. et al. Mechanistic and pharmacological assessment of murine IL-23 mediated psoriasiform dermatitis; implications for drug discovery. Journal of Dermatological Science 92, 45–53, https://doi.org/10.1016/j.jdermsci.2018.08.001 (2018).
Singh, T. P. et al. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nature communications 7, 13581, https://doi.org/10.1038/ncomms13581 (2016).
Suarez-Farinas, M. et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PloS one 8, e84634, https://doi.org/10.1371/journal.pone.0084634 (2013).
Chan, J. R. et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. The Journal of experimental medicine 203, 2577–2587, https://doi.org/10.1084/jem.20060244 (2006).
Kohler, C. Allograft inflammatory factor-1/Ionized calcium-binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell and tissue research 330, 291–302, https://doi.org/10.1007/s00441-007-0474-7 (2007).
Utans, U., Arceci, R. J., Yamashita, Y. & Russell, M. E. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. The Journal of clinical investigation 95, 2954–2962, https://doi.org/10.1172/JCI118003 (1995).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938, https://doi.org/10.1016/j.immuni.2013.10.004 (2013).
Malissen, B., Tamoutounour, S. & Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nature reviews. Immunology 14, 417–428, https://doi.org/10.1038/nri3683 (2014).
Rodero, M. P., Hodgson, S. S., Hollier, B., Combadiere, C. & Khosrotehrani, K. Reduced Il17a expression distinguishes a Ly6c(lo)MHCII(hi) macrophage population promoting wound healing. The Journal of investigative dermatology 133, 783–792, https://doi.org/10.1038/jid.2012.368 (2013).
Zigmond, E. et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37, 1076–1090, https://doi.org/10.1016/j.immuni.2012.08.026 (2012).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nature reviews. Immunology 14, 571–578, https://doi.org/10.1038/nri3712 (2014).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661, https://doi.org/10.1126/science.1178331 (2010).
Genovese, M. C. et al. Results from a Phase IIA Parallel Group Study of JNJ-40346527, an Oral CSF-1R Inhibitor, in Patients with Active Rheumatoid Arthritis despite Disease-modifying Antirheumatic Drug Therapy. The Journal of rheumatology 42, 1752–1760, https://doi.org/10.3899/jrheum.141580 (2015).
George, D. M., Hoemann, M. & Loud, J. In 2017 Medicinal Chemistry Reviews Vol. 52 (ed Joanne J. Bronson) Ch. 9, 165–178 (ACS Division of Medicinal Chemistry, 2017).
Ma, H. L. et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. The Journal of clinical investigation 118, 597–607, https://doi.org/10.1172/JCI33263 (2008).
Thaci, D. et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. Journal of the American Academy of Dermatology 73, 400–409, https://doi.org/10.1016/j.jaad.2015.05.013 (2015).
Saurat, J. H. et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). The British journal of dermatology 158, 558–566, https://doi.org/10.1111/j.1365-2133.2007.08315.x (2008).
Scholzen, T. & Gerdes, J. The Ki-67 protein: from the known and the unknown. Journal of cellular physiology 182, 311–322, https://doi.org/10.1002/(SICI)1097-4652 (2000).
Cai, Y. et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 35, 596–610, https://doi.org/10.1016/j.immuni.2011.08.001 (2011).
Lowes, M. A. et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. The Journal of investigative dermatology 128, 1207–1211, https://doi.org/10.1038/sj.jid.5701213 (2008).
Paukkonen, K., Naukkarinen, A. & Horsmanheimo, M. The development of manifest psoriatic lesions is linked with the invasion of CD8+ T cells and CD11c+ macrophages into the epidermis. Archives of dermatological research 284, 375–379 (1992).
Bos, J. D. et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Archives of dermatological research 281, 24–30 (1989).
van Rooijen, N. & Hendrikx, E. In Liposomes: Methods and Protocols, Volume 1: Pharmaceutical Nanocarriers (ed Volkmar Weissig) 189-203 (Humana Press, 2010).
Acknowledgements
We extend our thanks to Drs Dawn George, Melissa Matzelle and Michael Kort for scientific input of the manuscript, as well as to Dr. Susan Huang, Loan Miller, and Dr. Philip Zocharski and the Comparative Medicine Group at AbbVie for excellent technical assistance.
Author information
Authors and Affiliations
Contributions
Y.W. and S.M. designed the study; Y.W., R.E., J.W., K.S., D.G., L.L., S.P., Z.S., I.W. and M.N. performed the experiments and analyzed data; S.M., S.B.G., P.H. and V.E.S. supervised work; Y.W. wrote the manuscript; S.M. and V.E.S. revised manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
All authors are employees, or former employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Edelmayer, R., Wetter, J. et al. Monocytes/Macrophages play a pathogenic role in IL-23 mediated psoriasis-like skin inflammation. Sci Rep 9, 5310 (2019). https://doi.org/10.1038/s41598-019-41655-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41655-7
This article is cited by
-
The Immunology of Psoriasis—Current Concepts in Pathogenesis
Clinical Reviews in Allergy & Immunology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.