Abstract
Exposure to fine particulate matter (PM) with diameter <2.5 µm (PM2.5) causes epithelium injury and endothelial dysfunction. Primary cilia are sensory organelles that transmit extracellular signals into intracellular biochemical responses and have roles in physiology. To date, there have been no studies investigating whether PM2.5 affects primary cilia in skin. We addressed this in the present study using normal human epidermal keratinocytes (NHEKs) and retinal pigment epithelium (RPE) cells. We found that formation of primary cilium is increased in differentiated NHEKs. However, treatment with PM2.5 blocked increased ciliogenesis in NHEKs and RPE cells. Furthermore, PM2.5 transcriptionally upregulated small proline rich protein 3 (SPRR3) expression by activating c-Jun, and ectopic expression of SPRR3 inhibits suppressed the ciliogenesis. Accordingly, treatment with c-Jun activator (anisomycin) induced SPRR3 expression, whereas the inhibitor (SP600125) recovered the ciliated cells and cilium length in PM2.5-treated cells. Moreover, c-Jun inhibitor suppressed upregulation of SPRR3 in PM2.5-treated cells. Taken together, our finding suggested that PM2.5 inhibits ciliogenesis by increasing SPRR3 expression via c-Jun activation in RPE cells and keratinocytes.
Similar content being viewed by others
Introduction
Atmospheric pollutants cause serious health problems. The premature death of 3.7 million people annually worldwide is linked to air pollution1. Particulate air pollutants include asian dust storm particles (ADSPs) and fine particulate matters (PMs), with the latter comprising coarse and fine fractions with aerodynamic diameters <10 and 2.5 μm (PM10 and PM2.5, respectively)2. PMs are heterogeneous pollutants composed of several molecules, including toxic heavy metals, ionic elements, and polycyclic aromatic hydrocarbons, which constitute the primary hazardous components of air pollutants. Recently, numerous deaths and other health problems have been reported to be associated with particulate pollution3.
PMs are known to cause epithelium injury and endothelial dysfunction4,5. Since PMs penetrate the nasal cavity and bronchial cilia, PMs cause inflammation, asthma, and chronic bronchitis4,5. In addition, the airborne particles could cause the development and aggravation of symptoms of skin diseases such as atopic dermatitis and psoriasis by increasing oxidative stress and inflammatory response6,7. Moreover, PMs also induce eye injury and increase the risk of cardiovascular damage and neurotoxicity8,9,10,11,12. The skin consists of two layers, the epidermis and dermis, which are involved in protection, regulation, and sensation. The main function of skin is to act as a physical barrier to protect the interior from harmful the effects of ultraviolet (UV) radiation, microorganism, and toxic molecules. Skin is intensively connected with nerve system and senses environmental changes13.
Because the primary cilium is a major cellular sensory organelle that functions as an antenna for sensing extracellular information, they mediate the interactions between cells and external stimuli including chemical and mechanical signals14,15. Primary cilia are highly conserved, dynamic, microtubule-based organelles, which emanate from the surface of many human cell types. The major role of primary cilia is to recognize extracellular signals such as growth factors, nutrients, and hormones16,17. The process of formation of primary cilia, called ciliogenesis, is regulated by the intraflagellar transport (IFT) protein complexes, IFT-A and IFT-B18. The cilium membrane harbors a number of receptors, ion channels, and signaling components such as sonic hedgehog (Shh) and Wnt receptors14; thus, primary cilia play an important role in signal transduction during development, cell migration, the cell cycle, and apoptosis19,20. As ADP-ribosylation factor-like protein 13B (ARL13B), a small GTPase of the Arf/Arl family, and Smoothened (Smo) are specifically localized to primary cilia and regulate Shh21,22, these proteins are wildly used as a marker for primary cilia. Ciliogenesis is induced by serum starvation or highly confluent cell culture conditions23. Recent studies showed that ciliogenesis is promoted by various cellular stresses, including UV radiation, heat shock, actin destabilization, and loss of mechanical stresses as well as serum starvation24,25. Therefore, ciliogenesis is highly linked with the arrest of cell growth and proliferation. Furthermore, differentiation of stem cells requires the presence of primary cilia and associated IFT26. It was recently shown that the absence of primary cilia inhibits differentiation of mesenchymal stem cells26,27.
Since primary cilia play important roles in tissue development, cell differentiation, and homeostasis, defects in their formation are associated with a wide range of human disorders, including various ciliopathies19. In this study, we hypothesized that PM as a toxic molecule may induce dysfunction via primary cilia in the skin, and found that ciliogenesis was increased in differentiated normal human epidermal keratinocytes (NHEKs) and PM2.5 negatively regulated ciliogenesis. In addition, PM2.5 increased the expression of small proline rich protein 3 (SPRR3) via activation of c-Jun in retinal pigment epithelium (RPE) cells and keratinocytes. Our results provide insight into the molecular and cellular bases for tissue damage caused by exposure to atmospheric pollutants.
Results
PM2.5 negatively regulates ciliogenesis in NHEKs and RPE cells
Primary cilia have various functions and play a role in cell differentiation, which can be initiated by cell-cell contact. To investigate the presence of primary cilia on differentiated keratinocytes, NHEKs were cultured under confluent condition for 2, 4, and 6 days to induce cell differentiation. Primary cilia formation by NHEKs was observed by immunostaining with ARL13B, which is a small G protein localized in the primary cilia. Both the number of cells with primary cilia and length of the cilium were significantly increased in differentiation-induced keratinocytes (Fig. 1a,b). Then, we further investigate the effect of primary cilia on differentiation in NHEKs by treatment with ciliobrevin A1/Hedgehog pathway inhibitor 4 (HPI4). Ciliobrevin A1 is the first specific small-molecule antagonist of the cytoplasmic dyneins, and was reported to chemically inhibit primary cilium formation28. During cell differentiation, the expression of various differentiation markers, such as involucrin, loricrin, keratin1, and keratin10 increase with time29. We found that differentiation markers including keratin1 and keratin10 was notably decreased in ciliobrevin A1-treated keratinocytes (Fig. 1c,d).
Differentiation induces ciliogenesis in normal human epidermal keratinocytes (NHEKs). (a,b) NHEKs grown to confluence were cultured for 6 days. Cells were stained with E-cadherin (red), ARL13B (green), and DAPI (blue) to obtain cell images (lower: enlarged image, scale bar = 10 μm) (a). Then, both ciliated cells and cilium length were determined as described in Material and Method part (b). (c,d) NHEKs grown to confluence were cultured for 4 days with or without ciliobrevin A1 (Cilio A, 5 uM). The cells were imaged with a bright microscopy (scale bar = 50 μm) (c), and harvested to analyze the level of Keratin1 and Keratin10 by using RT-PCR assay (d). Data were obtained from at least three independent experiments and values are means ± SE (*p < 0.05 and **p < 0.01).
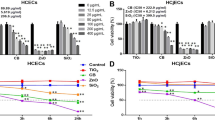
Keratinocytes form the outermost layer of the skin and provide a barrier against environmental damage. External environmental stimuli, including chemical, mechanical, and paracrine signals can regulate ciliogenesis and, therefore, we examined the effect of PM2.5 on cilium formation in keratinocytes. NHEKs grown to confluence were cultured for 4 days in the presence or absence of PM2.5. Interestingly, the number of cells with primary cilia and their length were notably decreased in PM2.5-treated NHEKs when compared with the untreated control cells (Fig. 2a,b). Treatment with ciliobrevin A1 suppressed cell differentiation as well as ciliogenesis in keratinocytes (Fig. 2a,b). Consistently, we found that the mRNA expression levels of keratinocyte differentiation markers and primary cilia marker, IFT88 were also reduced by PM2.5-treated NHEKs (Fig. 2c). Taken together, these results suggested that PM2.5 regulates ciliogenesis in differentiated keratinocytes.
PM2.5 suppresses ciliogenesis and in NHEKs and RPE cells. (a–c) NHEKs grown to confluence were cultured for 4 days with either PM2.5 (50 µg/mL) or ciliobrevin A1 (5 μM). Cells were stained with E-cadherin antibody (red), ARL13B antibody (green), and DAPI (blue) to acquire cell images (a). Both ciliated cells and cilium length were determined using fluorescence microscopy (b). Differentiation of NHEKs was examined using RT-PCR asssay with indicated markers (c). (d,e) RPE cells pretreated with PM2.5 (50 µg/mL) were further incubated in serum-free medium (SF) condition for 12 hours [PM (24 h) → SF (12 h)]. Or RPE cells cultured in SF media for 12 h were further exposed to PM2.5 [(25 and 50 µg/mL, SF (12 h) → PM (24 h)]. Then the cells were stained with acetylated tubulin antibody (AT, red) and ARL13B antibody (green). (yellow: red-green merged image) (d). (e) Both ciliated cells and cilium length were counted using fluorescence microscopy. Data are means ± standard error (SE) of three independent experiments (**p < 0.01, scale bar = 5 μm).
Then, we further confirmed the effect of PM2.5 on ciliogenesis in other cell types. Because RPE cells show clear primary cilium, the cells are widely used to study the role of primary cilia23. To confirm the effect of PM2.5 on ciliogenesis, RPE cells were cultured in serum-free conditional medium either before or after PM2.5 treatment. To image the primary cilia, the cells were stained with both anti-acetylated tubulin antibody and ARL13B antibody. Consistent with HNEKs, incubation of RPE cells in serum-free condition highly increased the cilium length and cell numbers (Fig. 2d,e). However, increased ciliogenesis by serum deprivation was significantly blocked by PM2.5 in RPE cells, suggesting that PM2.5 accelerated disassembly of primary cilia (Fig. 2d,e). Moreover, we found that pre-treatment with PM2.5 efficiently reduced ciliogenesis in serum-deprived RPE cells (Fig. 2d,e), further indicating that PM2.5 decreased ciliogenesis and increased the degradation of primary cilia. Taken together, these results suggest that PM2.5 negatively regulates primary cilia in RPE cells and NHEKs.
PM2.5 upregulates SPRR3 expression and ectopic expression of SPRR3 blocks ciliogenesis
Our previous transcriptome analysis in keratinocytes showed that treatment with PM2.5 upregulated several SPRR family proteins, such as SPRR2, SPRR3, and SPRR430. In addition, SPRR3 was screened as a potential ciliogenesis regulator from a small-scale short interfering RNA (siRNA) library screening, thus, we further investigated the effect of SPRR3 on ciliogenesis in PM2.5-treated cells. To confirm the mRNA-sequencing (RNA-seq) analysis, we examined the expression of SPRR3 in PM2.5-treated keratinocytes. Consistent with our previous data, both mRNA and protein levels of SPRR3 were remarkably increased in PM2.5-treated NHEKs (Fig. 3a,b). ADSP also upregulated SPRR3 expression in NHEKs (Fig. 3a). Furthermore, treatment with a protein translation inhibitor, cycloheximide, blocked SPRR3 expression in PM2.5-treated RPE cells (Fig. 3c), suggesting that PM2.5 upregulated SPRR3 expression in keratinocytes and RPE cells. Moreover, we observed that ectopic expression of SPRR3 in RPE cells decreases the serum deprivation-induced ciliogenesis (Fig. 3d,e). Taken together, these results suggest that the upregulation of SPRR3 induced by PM2.5 can contribute to suppression of ciliogenesis.
PM2.5 upregulates SPRR3 to suppress ciliogenesis. (a) NHEKs were exposed to either ADSP (25 ug/mL) or PM2.5 (50 µg/mL) for 48 h. Then, mRNA level of SPRR3 was determined using a RT-PCR analysis. (b) NHEKs were exposed to PM2.5 for either 2 days or 4 days, then cells were analyzed by Western blotting with SPRR3 antibody. GAPDH was used as an internal loading control. (c) RPE cells were exposed to PM2.5 for 24 h in the presence or absence of cycloheximide (CHX, 10 µg/mL) for 14 h and were analyzed using Western blotting with SPRR3 and Actin antibodies. (d,e) Retinal pigment epithelium (RPE) cells transiently transfected with pHA-SPRR3 were incubated with normal culture medium (Cont) or serum-free condition (SF). (d) After 48 h, cells were imaged by staining with anti-HA antibody (red), ADP-ribosylation factor-like protein 13B (ARL13B) antibody (green), and Hoechst dye (blue). (e) Then, both ciliated cells and cilium length were counted under a fluorescence microscopy. Data are means ± standard error (SE) of three experiments (*p < 0.05).
Depletion of SPRR3 enhances ciliogenesis in PM2.5-treated cells
To further investigate the function of SPRR3 in ciliogenesis, we performed a loss-of-function experiment in which SPRR3 expression was silenced using siRNA. Depletion of SPRR3 enhanced both ciliated cells and their length in RPE cells (Fig. 4a,b). Additionally, knockdown of SPRR3 efficiently reversed decreased primary cilia induced by PM2.5 in RPE cells (Fig. 4c,d). In addition to RPE cells, depletion of SPRR3 expression also restored loss of primary cilia in NHEKs (Fig. 4e,f), implying that SPRR3 was closely involved in the regulation of ciliogenesis in PM2.5-treated cells.
Depletion of SPRR3 restores reduced primary cilia in PM2.5-treated cells. (a,b) Retinal pigment epithelium (RPE) cells were transiently transfected with small interfering RNA (siRNA) targeting SPRR3 (siSPRR3) or scrambled siRNA (Sc). After 3 days, cells were stained with ARL13B antibody (green), acetylated tubulin (red) and Hoechst dye (blue) (yellow: red-green merged image) (scale bar = 5 μm) (a). Then both ciliated cells and cilium length were counted using fluorescence microscopy (b). (c,d) RPE cells transiently transfected with siSPRR3 or Sc were treated with PM2.5 (50 µg/mL) for 24 h, were stained with acetylated tubulin antibody (red), ARL13B antibody (green), and Hoechst dye (blue) (yellow: red-green merged image) (c, scale bar = 10 μm). (d) Then both ciliated cells and cilium length were counted using fluorescence microscopy. (e,f) NHEKs transiently transfected with siRPRR3 or Sc were treated with PM2.5 (50 µg/mL) for 24 h, and stained with acetylated tubulin (red), ARL13B (green), and DAPI (blue, scale bar = 10 μm). (f) Then, both ciliated cells and cilium length were counted using fluorescence microscopy. Data are from at least three independent experiments and are means ± standard error (SE, *p < 0.05 and **p < 0.01).
Activation of c-Jun by PM2.5 increases SPRR3 expression, but inhibition of c-Jun recovers ciliogenesis in PM2.5-treated cells
We further explored the potential molecular mechanism of PM2.5-regulated ciliogenesis. Interestingly, it was recently reported that SPRR3 expression is upregulated by the transcriptional factor, c-Jun, which is activated by phosphorylation31. In addition, we found that SPRR3 is increased in PM2.5-treated cells. Therefore, we further examined c-Jun activation in PM2.5-treated cells and found that phosphorylation of c-Jun was notably increased by PM2.5 treatment (Fig. 5a). In addition, treatment with anisomycin, a potent activator of c-Jun, highly induced SPRR3 expression as well as c-Jun phosphorylation (Fig. 5b). In contrast, treatment with a c-Jun inhibitor (SP600125) strongly blocked the increased expression of SPRR3 in PM2.5-treated cells (Fig. 5c). More importantly, immunostaining assay with primary cilia revealed that down-regulated ciliogenesis in PM2.5-treated cells was further recovered by inhibition of c-Jun (Fig. 5d,e). We additionally confirmed the effect of c-Jun activation by PM2.5 in NHEKs. Consistent with RPE cells, inhibition of c-Jun by treatment with SP600125 suppressed the loss of primary cilia by PM2.5 exposure in NHEKs (Fig. 6a,b). Accordingly, SP600125 treatment significantly recovered down-regulated differentiation markers such as Keratin1 and Keratin10 in PM2.5-treated NHEKs (Fig. 6c). Taken together, these results suggest that c-Jun-mediated SPRR3 expression negatively regulated ciliogenesis and differentiation in PM2.5-treated keratinocytes.
Inhibition of C-Jun induces ciliogenesis by decreasing SPRR3 expression in PM2.5-treated RPE cells. (a,b) RPE cells were incubated PM2.5 (25 and 50 µg/mL) for 24 h or anisomycin (0.1 and 1 µg/mL) for 1 h and expression of indicated proteins was detected using western blot analysis. (c,d) RPE cells were treated with PM2.5 (50 µg/mL) in the presence or absence of SP600125 (10 µM) for 24 h. (c) Cells were stained with acetylated tubulin antibody (red) and ARL13B antibody (green) Hoechst dye (blue, yellow: merged image, scale bar = 5 μm). (d) Then, both ciliated cells and cilium length were determined using fluorescence microscopy. (e) RPE cells were exposed to PM2.5 (50 µg/mL) in the presence or absence of SP600125 (10 µM) for 24 h. Then, cells were analyzed using western blotting with indicated antibodies. Data are means ± standard error (SE) of at least three independent experiments (*p < 0.05).
PM2.5-mediated down-regulation of ciliogenesis and differentiation was restored by inhibition of c-Jun in keratinocytes. (a–c) NHEKs were treated with PM2.5 (25 µg/mL) with or without SP600125 (10 µM) for 24 h (a). Then, cells were stained with acetylated tubulin antibody (red), ARL13B (green), and DAPI (blue, scale bar = 10 μm). Then, both ciliated cells and cilium length were counted under a fluorescence microscopy (b), and harvested to analyze the level of Keratin1 and −10 by using RT-PCR (c). Data are means ± standard error (SE) of at least three independent experiments (*p < 0.05 and **p < 0.01).
Discussion
Many toxicological and epidemiological studies have suggested that PMs negatively exert various human physiological systems, including respiratory system, circulatory system, nervous system, and integumentary system32,33,34. Among them, skin is directly exposed to PMs. Therefore, PM can easily penetrate the skin and induce abnormal skin states such as allergic reactions, aging, and delayed wound healing35,36. PMs can exert skin barrier dysfunction by decreasing filaggrin, which is a major structural protein in maintain the skin barrier function and influences allergic skin disease via Cox2 expression37. In addition, PMs also aggravate skin damage by increasing Ca++ influx which stimulates production of proinflammatory such as cytokines interleukin-1β and TNF-α in keratinocytes38.
Primary cilia play various roles in the skin such as mediating the initiation of hair follicle morphogenesis39. In addition, increased ciliogenesis, which activates the Smo-signalling pathway suppresses skin pigmentation in melanocytes40. Epidermal differentiation is suppressed by ciliary defective mutants and the elimination of cilia induces hyperproliferation of keratinocytes in mice during skin development41. In this study, we found that the formation of primary cilium is involved in differentiation in NHEKs (Fig. 1). Our findings suggest that primary cilia possibly are linked with cell differentiation in skin. The SPRR family members are known as cross-bridging proteins between other structural molecules just beneath the plasma membrane, which reinforce the cornified envelope of the skin epidermis. In this study, we found that the mRNA expression of SPRR family proteins was increased in PM2.5-treated cells, which decreased primary cilia formation. Among the SPRR family proteins, SPRR3 contributed to the alteration of primary cilia in keratinocytes in response to PM2.5 (Figs 2 and 3). Nonetheless, the upregulation of SPRR3 by PM has not been elucidated. Hu et al.42 recently reported that cigarette smoke transcriptionally increases SPRR3 expression in human bronchial epithelial (HBE) cells by favouring c-Jun/Fra1 heterodimerization42. In response to injury induced by chronic cigarette smoke exposure, HBE cells express various proteins associated with keratinized squamous cells43. Cigarette smoke is the main source of PM constituting indoor air pollution, which is a risk factor for chronic obstructive pulmonary diseases. Meaningfully, we also found that PM2.5 upregulated SPRR3 expression in an exposure-dependent manner in keratinocytes and RPE cells. In addition, inhibition of c-Jun activation blocked SPRR3 upregulation in PM2.5-treated cells (Fig. 5). Together with our findings, these results strongly indicated that the expression of SPRR3 is mainly regulated by c-Jun activation in PM-exposed cells.
The results of our study showed that loss of SPRR3 induces ciliogenesis, whereas SPRR3 overexpression had the opposite effect. Although the precise regulatory mechanism was not elucidated, we recently showed that ectopic expression of SPRR3 increased AKT phosphorylation in SW480 colorectal cancer cells44. The AKT pathway regulates various intracellular signaling events related to cell proliferation and differentiation. AKT is a component of the primary cilium maintenance signaling network. Hamamoto et al.45 reported that AKT phosphorylation is an important stage in melanin-concentrating hormone (MCH)-induced cilia length shortening in RPE cells45,46. Treatment with LY294002 and wortmannin, inhibitors of phosphoinositide 3-kinase (PI3K), which is an upstream AKT activator, significantly blocked MCH-induced cilia shortening. In addition, glycogen synthase kinase 3 β (GSK3β) and mammalian target of rapamycin (mTOR) are downstream of AKT, and were reported to be regulators of ciliogenesis47,48. However, the role of the AKT pathway in ciliogenesis is not simple and seems to be dependent on the ciliated cell types. For instance, inactive phosphorylation of GSK3 β by ATK was reported to occur in leptin-induced ciliogenesis in N1 hypothalamic neurons49. Depletion of phosphatase and tensin homolog (PTEN) and GSK3β increased ciliogenesis in leptin-treated cells. Therefore, further studies are needed to clarify the contribution of AKT signaling to the regulation of primary cilia by SPRR3.
In conclusion, we found that PM2.5 disrupts the formation of primary cilia via c-Jun-mediated regulation of SPRR3 expression in ciliated cell types of the skin and eye. These findings provide molecular-level insight into the damaging effects of PM on tissues exposed to the atmosphere as well as a justification for stricter regulations to limit human exposure to harmful air pollutants.
Materials and Methods
Cell culture
Normal human epidermal keratinocytes (NHEKs) from the neonatal foreskin donors were purchased from Lonza (Basel, Switzerland) and were cultured in KBM-GOLD medium supplemented with insulin, human epidermal growth factor, bovine pituitary extract, hydrocortisone, epinephrine, transferrin, and gentamicin/amphotericin B (all supplements from Lonza). The cells were serially passaged at 70–80% confluence, and experiments were performed using subconfluent cells within two or three passages. NHEKs grown to confluence were cultured with PM 2.5 (50 μg/mL) in KBM-Gold medium without supplements for 2 or 4 days. Human telomerase-immortalized retinal pigmented epithelial (RPE) cells were provided by Dr. Joon Kim (KAIST, Korea)25. RPE cells were cultured in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA).
Reagents and siRNA transfection
PM2.5 was collected on a Teflon filter (Zefluor, Pall Life Science, Ann Arbor, MI, USA) using a low-volume air sampler consisting of a cyclone (2.5-μm size cut, URG-2000-30EH), two upstream denuders (annular, URG-2000-30x242-3CSS), Teflon filter (ZefluorTM 2.0 μm Pall Life Sciences, Mexico), backup filter, and backup denuder in the series50. The collection started at approximately 10:00 a.m., the filters were replaced every 24 h, and the flow rate was 16.7 L/min. The sampling region was located 35 km southeast of downtown Seoul, Korea (37.34°N, 127.27°E, 167 m above sea level). Detailed information of the location and collection methods is provided in our previous report43. The filter was sonicated in ethanol (EtOH) for 30 min, the EtOH was evaporated, and then the PM2.5 was resuspended in deionized water. ADSPs were also sampled from the roof of the Korea University Science Building, located in the north-eastern region of Seoul, Korea (37.35°N, 127.01°E). The PMs were collected using a high-capacity air collector with a quartz filter, as described previously51.
All chemical reagents, including ciliobrevin A1 (2,4-Dichloro-a-(3,4-dihydro-4-oxo-2(1H)-quinazolinylidene)-β-oxo-benzenepropanenitrile/Hedgehog pathway inhibitor 4), Hoechst 33342, 4′,6-diamidino-2-phenylindole (DAPI), cycloheximide, anisomycin, and SP600125 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Previously validated siRNAs targeting SPRR3 (5ʹ-CAGACAAGCCCUUGAGAA-3ʹ)52 was synthesized by Genolution (Seoul, Korea). Cultured cells were transfected with siRNA using Lipofectamine® RNAi MAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After incubation with siRNA for 48 h, the cells were treated with each chemical for 24 h.
RNA extraction and quantitative real-time reverse transcription polymerase chain reaction (q-RT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The RNA concentration was spectrophotometrically determined, and RNA integrity was analyzed using a BioAnalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA); 4 μg were reverse transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen). The expression levels of target genes were evaluated by qRT-PCR using TaqMan technology (7500 Fast; Applied Biosystems, Foster City, CA, USA). The cycling protocol was as follows: 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 s and 60 °C for 1 min. The target genes were as follows: keratin 1, Hs00196158_m1; keratin 10, Hs00166289_m1; IFT88, Hs00544051_m1; and SPRR3, Hs00271304_m1. Ribosomal protein L13A (Hs04194366_g1; Applied Biosystems) was used to normalize the expression levels of target genes.
Western blot analysis
All lysates were prepared in 2 × Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8, 25% [v/v] glycerol, 2% [w/v] sodium dodecyl sulphate [SDS], 5% [v/v] β-mercaptoethanol, and 0.01% [w/v] bromophenol blue, Bio-Rad, Hercules, CA, USA). All cellular proteins were quantified using the Bradford solution (Bio-Rad) according to the manufacturer’s instructions. The samples were then separated using SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane (Bio-Rad). After blocking with 4% (w/v) skim milk in Tris-buffered saline plus Tween (TBST; 25 mM Tris, 140 mM sodium chloride [NaCl], and 0.05% [v/v] Tween® 20), the membranes were incubated overnight with the following specific primary antibodies: HA (1:3000, Santa Cruz), SPRR3 (1:3000, Proteintech), Actin (1:10000, EMD Millipore), GAPDH (1:10000, Cell Signaling Technology), phosphor-c-Jun (1:1000 Cell Signaling Technology) and c-Jun (1:3000 Cell Signaling Technology). For protein detection, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies and the signals were detected using SuperSignal® West Dura HRP detection kit (Pierce, Rockford, IL, USA).
Immunofluorescence analysis using confocal microscopy
Cells cultured on Lab-Tek™ glass chamber slides (Thermo Scientific, Waltham, MA, USA) were washed with cold phosphate-buffered saline (PBS), fixed for 20 min with 4% (w/v) paraformaldehyde, washed twice with PBS, and incubated in PBS containing 0.1% (v/v) Triton™ X-100 (PBS-T) for 3 min. The cells were further washed three times with PBS-T and then incubated with the following antibodies: anti-acetylated-tubulin (1:1000, Sigma-Aldrich), anti-cadherin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-HA (1:1000, Santa Cruz Biotechnology), and anti-ARL13B (1:1000, Proteintech) diluted in PBS-T containing 5% (v/v) normal goat serum for 1 h at room temperature. After washing with PBS, the cells were incubated with appropriate conjugated secondary antibodies and then mounted on glass slides to observe the fluorescence using a confocal laser scanning microscope (model LSM510; Carl Zeiss Microimaging Inc., Thornwood, NY, USA).
Measurement of increased cilium number and length
Changes in the cilium numbers in cells were measured by counting the immunofluorescent stained primary cilia. The cilium length was determined using the cell-Sense standard software (Olympus, Japan). The average cilium length was calculated using the Free-hand Line selection tool. The length of an individual cilium was examined from randomly selected cells, and the images were captured and digitized using the cell-Sense standard software and GraphPad Prism 8 (30–200 cells per experiment, n = 3).
Statistical analysis
Data were obtained from at least three independent experiments and are presented as means ± standard error (SE). The results were statistically evaluated using one-way or two way analysis of variance using the statistical package for the social sciences (SPSS, software, version 21).
References
Krutmann, J. et al. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 76, 163–168 (2014).
Choi, J. H. et al. Comparative study of PM2.5 - and PM10 - induced oxidative stress in rat lung epithelial cells. J. Vet. Sci. 5, 11–18 (2004).
Anderson, J. O., Thundiyil, J. G. & Stolbach, A. J. Clearing the air: a review of the effects of particulate matter air pollution on human health. Med. Toxicol. 8, 166–175 (2012).
Guo, J. F. et al. Highly stable and efficient Ag/AgCl@TiO2 photocatalyst: preparation, characterization, and application in the treatment of aqueous hazardous pollutants. J. Hazard. Mater. 15(211–212), 77–82 (2012).
Wang, T. et al. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part. Fibre. Toxicol. 9, 35, https://doi.org/10.1186/1743-8977-9-35 (2012).
Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy. Clin. Immunol. 134, 993–999; discussion 1000 (2014).
Gehring, U. et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am. J. Respir. Crit. Care. Med. 181, 596–603 (2010).
Hwang, S. H. et al. Potential Importance of Ozone in the Association Between Outdoor Air Pollution and Dry Eye Disease in South Korea. JAMA Ophthalmol, https://doi.org/10.1001/jamaophthalmol.2016.0139 (2016).
Buka, I., Koranteng, S. & Osornio-Vargas, A. R. The effects of air pollution on the health of children. Paediatr. Child. Health. 11, 513–516 (2006).
Chuang, K. J., Chan, C. C., Su, T. C., Lee, C. T. & Tang, C. S. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am. J. Respir. Crit. Care. Med. 176, 370–376 (2007).
Gillespie, P., Tajuba, J., Lippmann, M., Chen, L. C. & Veronesi, B. Particulate matter neurotoxicity in culture is size-dependent. Neurotoxicology 36, 112–117 (2013).
Lei, Y. C., Chan, C. C., Wang, P. Y., Lee, C. T. & Cheng, T. J. Effects of Asian dust event particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Environ. Res. 95, 71–76 (2004).
Roggenkamp, D. et al. Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model. J. Invest. Dermatol. 133, 1620–1628 (2013).
Satir, P., Pedersen, L. B. & Christensen, S. T. The primary cilium at a glance. J. Cell. Sci. 123, 499–503 (2010).
Kleene, S. J. & Van Houten, J. L. Electrical Signaling in Motile and Primary Cilia. Bioscience 64, 1092–1102 (2014).
Singla, V. & Reiter, J. F. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313, 629–633 (2006).
Berbari, N. F., O’Connor, A. K., Haycraft, C. J. & Yoder, B. K. The primary cilium as a complex signaling center. Curr. Biol. 19, R526–535 (2009).
Lechtreck, K. F. IFT-Cargo Interactions and Protein Transport in Cilia. Trends Biochem. Sci. 40, 765–778 (2015).
Quinlan, R. J., Tobin, J. L. & Beales, P. L. Modeling ciliopathies: Primary cilia in development and disease. Curr. Top. Dev. Biol. 84, 249–310 (2008).
Ishikawa, H. & Marshall, W. F. Ciliogenesis: building the cell’s antenna. Nat. Rev. Mol. Cell. Biol. 12, 222–234 (2011).
Caspary, T., Larkins, C. E. & Anderson, K. V. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell. 12, 767–778 (2007).
Mariani, L. E. et al. Arl13b regulates Shh signaling from both inside and outside the cilium. Mol. Biol. Cell, https://doi.org/10.1091/mbc.E16-03-0189 (2016).
Kim, J. et al. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464, 1048–1051 (2010).
Villumsen, B. H. et al. A new cellular stress response that triggers centriolar satellite reorganization and ciliogenesis. EMBO J. 32, 3029–3040 (2013).
Kim, J. et al. Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat. Commun. 6, 6781, https://doi.org/10.1038/ncomms7781 (2015).
Arrighi, N. et al. The primary cilium is necessary for the differentiation and the maintenance of human adipose progenitors into myofibroblasts. Sci. Rep. 7, 15248, https://doi.org/10.1038/s41598-017-15649-2 (2017).
Dalbay, M. T., Thorpe, S. D., Connelly, J. T., Chapple, J. P. & Knight, M. M. Adipogenic Differentiation of hMSCs is Mediated by Recruitment of IGF-1r Onto the Primary Cilium Associated With Cilia Elongation. Stem Cells 33, 1952–1961 (2015).
Firestone, A. J. et al. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 484, 125–129 (2012).
Son, E. D. et al. S100A7 (psoriasin) inhibits human epidermal differentiation by enhanced IL-6 secretion through IκB/NF-κB signalling. Exp. Dermatol. 25, 636–641 (2016).
Kim, H. J. et al. Transcriptome analysis of airborne PM2.5-induced detrimental effects on human keratinocytes. Toxicol. Lett. 273, 26–35 (2017).
Luo, A. et al. Differentiation-associated genes regulated by c-Jun and decreased in the progression of esophageal squamous cell carcinoma. PLoS One 9, e96610, https://doi.org/10.1371/journal.pone.0096610 (2014).
Xing, Y. F., Xu, Y. H., Shi, M. H. & Lian, Y. X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 8, E69–74 (2016).
Wei, T. & Tang, M. Biological effects of airborne fine particulate matter (PM2.5) exposure on pulmonary immune system. Environ. Toxicol. Pharmacol. 60, 195–201 (2018).
Wang, Y., Xiong, L. & Tang, M. Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J. Appl. Toxicol. 37, 644–667 (2017).
Ejaz, S., Ashraf, M., Nawaz, M. & Lim, C. W. Total particulate matter and wound healing: an in vivo study with histological insights. Biomed. Environ. Sci. 22, 278–287 (2009).
Vierkötter, A. et al. Airborne particle exposure and extrinsic skin aging. J. Invest. Dermatol. 130, 2719–2726 (2010).
Lee, C. W. et al. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 6, 27995 (2016).
Kwon, K. et al. Negative Cellular Effects of Urban Particulate Matter on Human Keratinocytes Are Mediated by P38 MAPK and NF-κB-dependent Expression of TRPV 1. Int. J. Mol. Sci. 19, E2660 (2018).
Lehman, J. M., Laag, E., Michaud, E. J. & Yoder, B. K. An essential role for dermal primary cilia in hair follicle morphogenesis. J. Invest. Dermatol. 129, 438–448 (2009).
Choi, H. et al. Primary Cilia Negatively Regulate Melanogenesis in Melanocytes and Pigmentation in a Human Skin Model. PLoS One 11, e0168025, https://doi.org/10.1371/journal.pone.0168025 (2016).
Ezratty, E. J. et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145, 1129–1141 (2011).
Hu, X. et al. Cigarette smoke upregulates SPRR3 by favoring c-Jun/Fra1 heterodimerization in human bronchial epithelial cells. Future Oncol, https://doi.org/10.2217/fon-2018-0043 (2018).
Rigden, H. M. et al. Squamous Metaplasia Is Increased in the Bronchial Epithelium of Smokers with Chronic Obstructive Pulmonary Disease. PLoS One 11, e0156009, https://doi.org/10.1371/journal.pone.0156009 (2016).
Cho, D. H. et al. Upregulation of SPRR3 promotes colorectal tumorigenesis. Mol. Med. 16, 271–277 (2010).
Hamamoto, A. et al. Modulation of primary cilia length by melanin-concentrating hormone receptor 1. Cell Signal. 28, 572–584 (2016).
Tomoshige, S. Cytoskeleton-related regulation of primary cilia shortening mediated by melanin-concentrating hormone receptor 1. Gen. Comp. Endocrinol. 253, 44–52 (2017).
Thoma, C. R. et al. pVHL and GSK3beta are components of a primary cilium-maintenance signaling network. Nat. Cell. Biol. 9, 588–595 (2007).
Boehlke, C. et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat. Cell. Biol. 12, 1115–1122 (2010).
Han, Y. M. et al. Leptin-promoted cilia assembly is critical for normal energy balance. J. Clin. Invest. 124, 2193–2197 (2014).
Kim, C. H. Characterization of Volatilization of Filter-Sampled PM2.5 Semi-Volatile Inorganic Ions Using a Backup Filter and Denuders. Aerosol Air Qual. Res. 15, 814–820 (2015).
Choi, H. et al. Asian dust storm particles induce a broad toxicological transcriptional program in human epidermal keratinocytes. Toxicol. Lett. 200, 92–99 (2011).
Kim, J. C. et al. Expression of SPRR3 is associated with tumor cell proliferation in less advanced stages of breast cancer. Breast Cancer Res. Treat. 133, 909–916 (2012).
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (2017R1A2B4005501). This research was also supported by Kyungpook National University Research Fund (2018) and the Amorepacific R&D Center.
Author information
Authors and Affiliations
Contributions
D.C., H.K., J.B. and H.C. designed the project and jointly supervised the study; J.B., H.C., D.S., H.N., N.P., J.K., D.J., M.C., J.L. and J.C. performed the experiments; J.B., H.C., H.K. and D.C. wrote the manuscript; and T.L. and E.L. discussed the data and edited the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bae, JE., Choi, H., Shin, D.W. et al. Fine particulate matter (PM2.5) inhibits ciliogenesis by increasing SPRR3 expression via c-Jun activation in RPE cells and skin keratinocytes. Sci Rep 9, 3994 (2019). https://doi.org/10.1038/s41598-019-40670-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40670-y
This article is cited by
-
Acute and continuous exposure of airborne fine particulate matter (PM2.5): diverse outer blood–retinal barrier damages and disease susceptibilities
Particle and Fibre Toxicology (2023)
-
ARP-T1-associated Bazex–Dupré–Christol syndrome is an inherited basal cell cancer with ciliary defects characteristic of ciliopathies
Communications Biology (2021)
-
Impact of airborne particulate matter on skin: a systematic review from epidemiology to in vitro studies
Particle and Fibre Toxicology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.