Abstract
Aberrant activation of the Wnt/β-catenin signaling pathway promotes the progression of osteoarthritis (OA). We previously reported that R-spondin 2 (Rspo2), an activator of the Wnt/β-catenin signaling, facilitates differentiation of proliferating chondrocytes into hypertrophic chondrocytes by enhancing Wnt/β-catenin signaling in endochondral ossification. However, the role of Rspo2 in OA remains elusive. Here, we showed that the amounts of Rspo2 protein in synovial fluid were increased in OA patients. We searched for a preapproved drug that suppresses Rspo2-induced Wnt/β-catenin signaling in chondrogenic cells and reduces joint pathology in a rat model of OA. In Rspo2-treated ATDC5 cells, mianserin, a tetracyclic antidepressant, inhibited Wnt/β-catenin signaling, increased proteoglycan production, and upregulated chondrogenic marker genes. Mianserin suppressed Rspo2-induced accumulation of β-catenin and phosphorylation of Lrp6. We identified that mianserin blocked binding of Rspo2 to its receptor Lgr5. We also observed that intraarticular administration of mianserin suppressed β-catenin accumulation and prevented OA progression in a rat model of OA. We conclude that mianserin suppresses abnormally activated Wnt/β-catenin signaling in OA by inhibiting binding of Rspo2 to Lgr5.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is characterized by progressive loss of articular cartilage and concomitant loss of extracellular matrix (ECM), and causes pain and functional disorders in elderly people1,2. ECM is comprised of a highly hydrated fibrillar network of collagens embedded in a gel of negatively charged proteoglycans like aggrecan (Acan)3.
Wnt/β-catenin signaling pathway plays crucial roles in determination of cell fate, and controls tissue homeostasis4. Binding of secreted Wnts to their cell-surface receptor comprised of Frizzled and low-density lipoprotein receptor-related proteins 5/6 (Lrp5/6) phosphorylates Lrp5/6 and accumulates β-catenin. Accumulated β-catenin is translocated into the nucleus, and interacts with transcriptional factors, T-cell factor/lymphoid enhancer factor (TCF/LEF), to regulate gene expressions. Excessive activity of Wnt/β-catenin signaling has been implicated in many human diseases including OA5,6. Activation of Wnt/β-catenin signaling promotes hypertrophic differentiation of articular chondrocytes which, in turn, induces cartilage degradation and subsequent OA aggravation7. Indeed, OA progression is mitigated by inhibiting the Wnt/β-catenin signaling8,9,10.
R-spondins (Rspos) were originally identified as secreted positive-feedback activators of Wnt/β-catenin signaling in Xenopus11 and often co-expressed with Wnts12. Rspos binds to two cell-surface receptors: Lgr4-6 (leucine-rich repeat containing G protein-coupled receptors) and RNF43 (ring finger protein 43)/ZNRF3 (zinc and ring finger 3)13,14,15,16. RNF43/ZNRF3 are E3 ubiquitin ligases that degrade the Wnt receptors, Frizzled and Lrp5/6. Rspos bridge Lgr4-6 and ZNRF3/RNF43, and induce endocytosis of Lgr4-6 and ZNRF3/RNF43, which suppresses the E3 ubiquitin ligase activity of ZNRF3/RNF4317,18. The effects of monoclonal antibodies against Rspo1, Rspo2, and Rspo3 to suppress Rspo-induced signaling are reported in human tumor xenografts19.
Rspo2 is an important factor for regulating cell proliferation and differentiation, as well as tissue development. Single nucleotide variations in RSPO2 are associated with proliferative bone and soft tissue diseases in human20,21. We recently reported that Rspo2 activates Wnt/β-catenin signaling and reduces expressions of chondrogenic marker genes of Sox9 (sex-determining region Y-Box 9; a master gene for chondrocyte differentiation), Col2a1 (collagen type II α1), and Acan, which subsequently facilitates differentiation of proliferating chondrocytes into hypertrophic chondrocytes in the growth cartilage22. Hypertrophic differentiation of articular chondrocytes is similarly observed in human OA and animal OA models23,24. Excessive Wnt/β-catenin signaling activity facilitates hypertrophic differentiation of articular chondrocytes, which, in turn, induces cartilage-degrading metalloproteinase expression and subsequent OA aggravation7,25. However, involvement of Rspo2 in OA remains to be elucidated. Here, we observed that Rspo2 is increased in synovial fluid in OA patients. We next searched for a drug to antagonize the effect of Rspo2 using the drug repositioning strategy, in which a drug currently used to treat a specific disease is applied to another disease26,27. We found that mianserin, a tetracyclic antidepressant, suppressed Rspo2-induced activation of Wnt/β-catenin signaling in chondrocytes, which subsequently ameliorated loss of proteoglycans and increased expressions of Sox9, Col2a1, and Acan. Mianserin exerted its anti-Rspo2 effect by directly blocking binding of Rspo2 to Lgr5. In a rat model of OA, intraarticular administration of mianserin reduced accumulation of β-catenin in articular chondrocytes and prevented OA progression.
Results and Discussion
Rspo2 in synovial fluid in patients with knee OA increases with increasing OA severities
We first confirmed that glycosaminoglycans (GAG), which are degradation products of GAG chains in cartilage proteoglycans28, were increased in synovial fluid with increasing OA severities that was evaluated by the Kellgren-Lawrence (KL) radiographic grading system29 (Fig. 1A–C). We next observed that intraarticular Rspo2 gradually increased with worsening of OA up to KL grade 3, but was decreased at KL grade 4. This may represent that the number of intraarticular Rspo2-producing and other cells is reduced at KL grade 4. Alternatively, the increase of Rspo2 is associated with only initiation and progression of OA (Fig. 1D–F, Supplementary Table S1). The amounts of Rspo2 were much higher in female patients than male patients (Fig. 1D,E). In contrast to Rspo2, the amounts of Wnt antagonists including dickkopf (DKK)-1, DKK-2, and sclerostin in synovial fluid are inversely correlated with OA severity30,31,32.
Rspo2 in synovial fluid of knee joints is increased in OA patients with Kellgren-Lawrence (KL) grades 2 and 3. (A–C) Concentrations of glycosaminoglycans (GAG) in synovial fluid were measured by a spectrophotometric dye binding assay using 1,2, dimethylmethylene blue (DMMB), and were plotted against the KL grading scale representing OA severity for females (n = 36) (A), males (n = 16) (B), and all patients (n = 52) (C). (D–F) The amount of Rspo2 was normalized to the amount of total protein in synovial fluid. Rspo2 in females (n = 36) (D), males (n = 16) (E), and all patients (n = 52) (F) are plotted against the KL grading scale. Bars indicate average values for each KL grade. *P < 0.05 and **P < 0.01 by one-way ANOVA followed by Tukey’s post-hoc test. Values of each patient are shown in Supplementary Table S1.
Mianserin inhibits Rspo2-induced activation of Wnt/β-catenin signaling and increases the amounts of Rspo2-reduced ECM in human chondrosarcoma (HCS)-2/8 cells
We next attempted to identify a clinically applicable drug that inhibits Rspo2-induced activation of Wnt/β-catenin signaling and OA progression. We quantified Wnt/β-catenin signaling activity using the TOPFlash luciferase reporter assay in the presence of 1,271 FDA-approved drugs in HCS-2/8 cells, and searched for a drug that suppresses Rspo2-activated Wnt/β-catenin signaling. Recombinant human Rspo2 (rhRspo2) alone does not activate Wnt/β-catenin signaling in HCS-2/8 cells, but enhances the signaling in the presence of a low dose of recombinant human Wnt3a (rhWnt3a) (Supplementary Fig. S1A)17. We thus performed drug screening with 120 ng/ml rhRspo2 and 20 ng/ml rhWnt3a, and found that a tetracyclic antidepressants (TeCA), mianserin, that is an antagonist or inverse agonist of the histaminergic H1 receptor, serotoninergic 5-HT1–7 receptors, and α2-adrenergic receptor, suppressed the TOPFlash reporter activity in a dose-dependent manner (Fig. 2A). Interestingly, mianserin did not reduce Wnt/β-catenin signaling activated by rhWnt3a alone (Fig. 2B). We observed that 120 ng/ml rhRspo2 and 20 ng/ml rhWnt3a upregulated mRNA expression of Wnt/β-catenin-responsive AXIN233 in HCS-2/8 cells, and that mianserin attenuated the upregulation of AXIN2 (Supplementary Fig. S1B). We also observed similar tendencies in two other Wnt/β-catenin-responsive genes of CCND134 and MYC35 in HCS-2/8 cells (Supplementary Fig. S1B–D). We next tested two other TeCAs: maprotiline, the first TeCA ever to be developed36, and mirtazapine, one of analogues for mianserin37. In contrast to mianserin, neither maprotiline nor mirtazapine suppressed the TOPFlash reporter activity at 10 μM (1.000 ± 0.272 and 1.018 ± 0.289, mean and SD, n = 3, respectively). Taken together, mianserin inhibited the TOPFlash reporter activity via Rspo2, and the effect is unlikely to be associated with its tetracyclic structure.
Mianserin suppresses Rspo2-induced activation of Wnt/β-catenin signaling in chondrogenic cell lines. (A,B) HCS-2/8 human chondrosarcoma cells transfected with the TOPFlash reporter plasmid to quantify Wnt/β-catenin signaling activity were treated with either 120 ng/ml recombinant human Rspo2 (rhRspo2) and 20 ng/ml recombinant human Wnt3a (rhWnt3a) (A) or 90 ng/ml rhWnt3a alone (B) along with increasing concentrations of mianserin for 24 h. Firefly luciferase activity of the TOPFlash reporter was normalized to the TK promoter-driven Renilla luciferase activity (n = 5). (C,D) ATDC5 cells were cultured with 1% ITS for 2 weeks to induce chondrogenic differentiation and subsequently treated with either 200 ng/ml rhRspo2 (C) or 90 ng/ml rhWnt3a (D) for 48 h in the presence of the indicated concentrations of mianserin. After staining with Alcian blue, proteoglycans in the cell lysates were quantified by measuring optical densities at 620 nm (n = 4). (E–H) Chondrogenically differentiated ATDC5 cells were treated with 200 ng/ml rhRspo2 for 48 h in the presence of the indicated concentrations of mianserin. Expression levels of each mRNA were quantified by quantitative RT-PCR, and were normalized for those of Gapdh and also for untreated cells (n = 4). Mean and SD are indicated. *P < 0.05 and **P < 0.01 by one-way ANOVA followed by Tukey’s post-hoc test.
We evaluated the effects of mianserin on ECM production in mouse chondrogenic ATDC5 cells, which produce high levels of ECM when Wnt/β-catenin signaling is not activated22. Quantitative analysis of Alcian blue staining revealed that mianserin ameliorated rhRspo2-induced, but not rhWnt3a-induced, reduction of proteoglycans (Fig. 2C,D). We also confirmed that mianserin mitigated Rpos2-induced upregulation of Axin2 (Fig. 2E), as well as Rspo2-induced downregulation of Sox9, Col2a1, and Acan (Fig. 2F,G,H). These results indicate that mianserin mitigates Rspo2-induced suppression of ECM production. As far as we know, the effect of mianserin on Rspo2 has not been reported previously. We previously reported that another antidepressant, fluoxetine, ameliorates cartilage degradation in OA by inhibiting Wnt/β-catenin signaling. The putative target of fluoxetine, however, is likely to be a degradation complex including β-catenin or its downstream signaling, and not Rspo210.
Mianserin reduces Rspo2-induced β-catenin accumulation and Lrp6 phosphorylation, and blocks binding of Rspo2 to Lgr5
We first confirmed that Rnf43/Znrf3 mRNAs, Lgr4-6 mRNAs, and Lgr5 protein were expressed in differentiated ATDC5 cells (Supplementary Fig. S2A,B). Rspo2 did not alter mRNA expressions of Rnf43/Znrf3 (Fig. 3A,B) and Lgr4/5/6 in 48 h in differentiated ATDC5 cells (Fig. 3C–E). In contrast, as in HEK293 cells38, Rspo2 increased the expressions of Lrp6, Lrp5, Frizzled6 (Fzd6), and β-catenin proteins in 48 h in differentiated ATDC5 cells (Fig. 3F and Supplementary Fig. S2C), which was likely to be initiated by increased phosphorylation at Ser1490 of Lrp639 in 1.5 h (Fig. 3G). Mianserin suppressed rhRspo2-mediated increases of Lrp6, Lrp5, Fzd6, and β-catenin proteins, as well as Lrp6 phosphorylation, in differentiated ATDC5 cells and in HEK293 cells (Fig. 3F,G and Supplementary Fig. S2C,D). These observations prompted us to hypothesize that the target of mianserin is either upstream or on the cell membrane. Rspos activate Wnt/β-catenin signaling by forming a complex with the extracellular domains of both Lgr4/5/6 and RNF43/ZNRF317,18. As Lgr5 was highly expressed in both OA cartilage (OAC) cells stated below and ATDC5 cells (Supplementary Fig. S2A,B), we evaluated the effect of mianserin on the binding of human Rspo2 to Lgr5 on the surface of HEK293 cells. We found that mianserin suppressed binding of Rspo2 to Lgr5 in a dose-dependent manner (Fig. 4H). We similarly evaluated the effect of mianserin on the binding of human Rspo2 to RNF43 on the surface of HEK293 cells, but observed no effect (Fig. 4H). Thus, mianserin directly suppresses binding of Rspo2 to Lgr5, and subsequently attenuates Lrp6 expression and β-catenin accumulation in chondrocytes.
Mianserin reduces Rspo2-induced accumulation of β-catenin and phosphorylation of Lrp6 by suppressing binding of Rspo2 to Lgr5. (A–E) ATDC5 cells were cultured with 1% ITS for 2 weeks to induce chondrogenic differentiation and subsequently treated with 200 ng/ml rhRspo2 for 48 h in the presence of the indicated concentrations of mianserin. Expression levels of each mRNA by quantitative RT-PCR were normalized for those of Gapdh, and also for untreated cells. Mean and SD are indicated (n = 4). No statistical differences were observed by one-way ANOVA followed by Tukey’s post-hoc test. (F) Differentiated ATDC5 cells were cultured with 10 μM mianserin and 200 ng/ml rhRspo2 for 48 h. Representative Western blots and intensities of Lrp6 and β-catenin normalized for those of β-actin and also for untreated cells are shown. Mean and SD are indicated (n = 3). *P < 0.05 by one-way ANOVA followed by Tukey’s post-hoc test. (G) Differentiated ATDC5 cells were cultured with 10 μM mianserin and 200 ng/ml rhRspo2 for 1.5 h. Representative Western blots and intensities of phosphorylated Lrp6 normalized for those of total Lrp6 and also for untreated cells are shown. Mean and SD are indicated (n = 3). *P < 0.05 by one-way ANOVA followed by Tukey’s post-hoc test. (H) Cell surface binding assay of Rspo2-AP in the presence of increasing concentrations of mianserin on HEK293 cells expressing human Lgr5 or RNF43. Bound Rspo2-AP was normalized for the added Rspo2-AP. Mean and SD are indicated (n = 3). *P < 0.05 and **P < 0.01 compared to control cells expressing neither Lgr5 nor RNF43 by one-way ANOVA followed by Tukey’s post-hoc test.
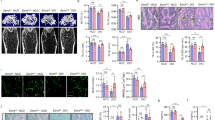
Mianserin ameliorates OA progression and reduces β-catenin expression in a rat model of OA. (A) Destabilization of medial meniscus (DMM) surgery was performed on the right knees of 6 Wistar/ST rats. Sham surgery was performed on the contralateral left knees by incising the skin and joint capsules. Fifty microliters PBS (n = 3) or 50 μM mianserin dissolved in 50 μl PBS (n = 3) was injected intraarticularly into both knees once weekly for 8 weeks after surgery. Representative histological sections of the medial compartments in the knee joints stained with Safranin-O and Fast-green are shown. (B) OA severity in the medial compartment of the knee joint was quantified by modified Mankin score at 8 weeks after the surgery. The sum of femoral and tibial scores is shown by mean and SD. *P < 0.05 by one-way ANOVA followed by Tukey’s post-hoc test. There was no statistical significance between PBS and mianserin in the DMM group (P = 0.079). (C) Left panels show magnifications of the boxed areas in A. Right panels show immunostaining with anti-β-catenin antibody in serial sections of the left panels. (D) Magnifications of the boxed areas in (C). Articular chondrocytes are stained with anti-β-catenin antibody and DAPI. (E,F) Blinded morphometry of β-catenin signals of the chondrocytes at the medial compartments with MetaMorph. (E) Signal intensities for β-catenin in the total cellular area and the DAPI-positive nuclear area are normalized to the number of cells and also to that in total cellular area in PBS-treated cartilage. **P < 0.01 by one-way ANOVA followed by Tukey’s post-hoc test. Mean and SD are indicated (n = 3 knees in each group). (F) The number of β-catenin-positive cells is divided by the number of DAPI signals to calculate the ratio of nuclear β-catenin-positive cells. The number of β-catenin-positive cells is divided by the number of DAPI signals to calculate the ratio of nuclear β-catenin-positive cells. *P < 0.05 by one-way ANOVA followed by Tukey’s post-hoc test. Scale bar = 200 μm (A), 100 µm (C), and 25 µm (D). (G) Model of Rspo2-mediated OA development, and the effect of mianserin.
Mianserin inhibits β-catenin accumulation in chondrogenic cells in OA cartilage and mitigates articular cartilage degradation in a rat OA model
To examine the effects of mianserin on OA cartilage (OAC), we isolated OA cells from OA patients undergoing total knee replacement surgery. We first confirmed that Rnf43/Znrf3 mRNAs, Lgr4-6 mRNAs, and Lgr5 protein were expressed in OAC cells, as we observed in ATDC5 cells (Supplementary Fig. S2A,B). Cell viability assay showed that less than 100 µM of mianserin had no toxicity on OAC cells (Supplementary Fig. S3A). Immunoblotting showed that mianserin decreased rhRspo2-induced accumulation of β-catenin (Supplementary Fig. S3B,C), indicating that mianserin suppresses Wnt/β-catenin signaling in human OAC cells without overt toxicity.
We also examined the effects of mianserin on articular chondrocytes in a rat OA model. Rats were intraarticularly injected with 50 µl phosphate-buffered saline (PBS, control) or 50 µM mianserin once weekly for 8 weeks after OA induction by destabilization of the medial meniscus (DMM) surgery. At 8 weeks after surgery, mianserin improved Safranin-O staining on the articular surfaces and preserved articular cartilage structures in DMM-operated knees (Fig. 4A,B). We also observed that mianserin reduced the cellular and nuclear intensities of β-catenin (Fig. 4C–E), and the ratio of β-catenin-positive cells (Fig. 4F).
Thus, intraarticular injection of mianserin suppresses Wnt/β-catenin signaling in articular chondrocytes and ameliorates OA progression in a rat OA model (Fig. 4G). As the loss-of-function of β-catenin in cartilage also causes osteoarthritic changes by increasing apoptotic cells40, a certain level of Wnt/β-catenin signaling is required for articular cartilage41. Optimization of administration protocols, scrutinized analysis of adverse effects, and examination of an additive effect of concomitant treatment for a rat OA model need to be performed before mianserin is applied to human OA patients. Evaluation of the suppressive effect of long-term mianserin in depressed patients on OA development in epidemiological studies will also help elucidate the effect of mianserin on OA patients. In addition to OA, dysregulation of Rspo2 is also reported in several cancers4,18,42 and in proliferative diseases of bone and soft tissue20,43. We expect that mianserin is a potential repositioned drug that ameliorates human diseases associated with Rspo2-mediated abnormal activation of Wnt/β-catenin signaling.
Materials and Methods
Quantification of Rspo2 amounts in patient synovial fluid with knee OA
All studies involving human synovial fluid were performed in accordance with the protocol in Institutional Review Board of Nagoya University Hospital (The protocol No. 2015-0239-2) and approved by the Ethical Review Committee of the Nagoya University Graduate School of Medicine. After an appropriate written informed consent was obtained at Nagoya University Hospital, synovial fluid samples were acquired from 52 patients with knee OA (16 males and 36 females; ages, 36–93 years; and mean age, 74.6 years) with needle puncture at the outpatient clinic when the patients had hydrarthrosis. The diagnosis of knee OA was based on the criteria of the American Rheumatism Association44. Two authors independently evaluated plain knee radiographs and severity of knee OA was decided using the Kellgren-Lawrence (KL) radiographic grading system (grade 1, 14 patients; grade 2, 17 patients; grade 3, 13 patients; and grade 4, 8 patients)29. Each KL grade is characterized as follows: grade 1, doubtful OA with minimal osteophytes of dubious importance; grade 2, mild OA with definite osteophytes but unimpaired joint space; grade 3, moderate OA with moderate osteophytes and definite joint space narrowing; and grade 4, severe OA with considerably impaired joint space and sclerosis of subchondral bone45.
All patients had knee effusion caused by synovitis and underwent an arthrocentesis of the knee under aseptic condition. After centrifugation at 18,000 × g for 20 min at 4 °C to remove cells and other debris, concentrations of Rspo2 and total protein in the collected synovial fluid samples were quantified. Concentrations of Rspo2 were determined by the Human R-Spondin 2 (RSPO2) ELISA Kit (MyBioSource) according to the manufacturer’s instructions and using PowerScan4 (DS Pharma Biomedical) to measure absorbance at 450 nm. Concentrations of total protein were quantified by the Pierce 660 nm Protein Assay Kit (Pierce Biotechnology). We also evaluated concentrations of glycosaminoglycans (GAG) in synovial fluid by a spectrophotometric dye binding assay using 1,2, dimethylmethylene blue (DMMB; Santa Cruz Biotechnology)46. A positive correlation between GAG concentration in synovial fluid and KL grade was demonstrated in a previous report28.
Screening of 1,271 FDA-approved drugs with TOPFlash luciferase reporter assay
We screened 1,271 FDA-approved chemical compounds (Prestwick Chemical) to identify a drug that specifically suppresses Rspo2-mediated activation of Wnt/β-catenin signaling. We quantified Wnt/β-catenin signaling activity with TOPFlash luciferase reporter assay. Human chondrosarcoma (HCS-2/8) cells were kindly provided by Dr. Masaharu Takigawa at Okayama University47. HCS-2/8 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific). After 60–70% cellular confluency in a 100-mm dish, HCS-2/8 cells were transfected with 10 µg TOPFlash firefly luciferase reporter vector (M50 Super 8x TOPFlash plasmid, Addgene) and 0.25 µg Renilla luciferase plasmid (phRL-TK, Promega) using FuGENE 6 (Promega). At 24 h after transfection, the cells were seeded in a 96-well culture plate at 4 × 104 cells/well and incubated for 24 h in the presence of 10 μM of each drug. Recombinant human Rspo2 protein (rhRspo2, R&D Systems) and/or recombinant human Wnt3a protein (rhWnt3a, R&D Systems) were added to the medium. Luciferase activity was measured with the Dual Luciferase Reporter Assay System (Promega) in PowerScan MX (DS Pharma Biomedical). Firefly luciferase activity was normalized by Renilla luciferase activity.
Immunoblotting for β-catenin, total Lrp6, and phosphorylated Lrp6 in differentiated ATDC5 cells and human OAC cells
Differentiated ATDC5 cells and OAC cells were treated with 0–20 μM mianserin in the presence of 200 ng/ml rhRspo2 for the indicated times. Cells were harvested with ice-cold RIPA Lysis Buffer (Santa Cruz Biotechnology) with 0.1 mM dithiothreitol, 1 mg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin and phosphatase inhibitors (PhosSTOP; Sigma-Aldrich). Whole-cell lysates were separated on SDS-PAGE and transferred to a nitrocellulose membrane. Expression levels of β-catenin, β-actin, Lrp6, phosphorylated Lrp6, Lrp5, Frizzled 6 and Gapdh were determined using antibodies against β-catenin (dilution 1:1,000, 610154, Becton Dickinson), β-actin (1:250, sc47778, Santa Cruz Biotechnology), Lrp6 (1:500, sc15399, Santa Cruz Biotechnology), phospho-Lrp6 at Ser1490 (1:1,000, #2568, Cell Signaling Technology), Lrp5 (1:1,000, #5731, Cell Signaling Technology), Frizzled 6 (1:1,000, #5158, Cell Signaling Technology), and Gapdh (1:1,000, sc-47724, Santa Cruz Biotechnology), respectively. Signal intensities were normalized either to the β-actin or the total Lrp6 level. Three independent experiments were performed, and quantified using the NIH ImageJ software.
Preparation of Rspo2-MycAP and cell surface binding of Rspo2 to Lgr5 and RNF43
The full-length human RNF43 cDNA in pcDNA3-HA was kindly provided by Drs. Shigetsugu Hatakeyama and Tadasuke Tsukiyama at Hokkaido University, Japan48. We previously reported the full-length human LGR5 (BC099650) cDNA in pcDNA3.149. The C-terminal-deleted human RSPO2 cDNA (amino acids 1–218) and the C-terminal of rat Agrn cDNA (amino acids 1141–1937 of M64780.1) were cloned into APtag-5 (GenHunter Corp.) at the HindIII and SnaBI sites to make the myc-and-alkaline phosphatase-tagged Rspo2 (Rspo2-mycAP) and the myc-and-alkaline phosphatase-tagged Agrn (Agrn-mycAP) as described previously49.
HEK293 cells were transfected with Rspo2-mycAP to make Rspo2-CM. Rspo2-mycAP and Agrn-mycAP in the CM were concentrated ~100-fold using Amicon Ultra-4 filters (Merck Millipore) for the cell surface binding assay. HEK293 cells were transfected with 3 μg RNF43/pcDNA3-HA, 5 μg Lgr5/pcDNA3.1, or 5 µg empty pcDNA3.1 using Lipofectamine 2000 (Thermo Fisher Scientific). Cells were incubated for 24 h with concentrated CM containing either Rspo2-mycAP or Agrn-mycAP then placed on ice for 1.5 h. Cells were washed with HABH buffer (0.5 mg/ml BSA, 0.1% NaN3, and 20 mM HEPES (pH 7.0) in Hank’s balanced salt solution), and then fixed in 60% acetone for 10 min on ice followed by 4% paraformaldehyde in 20 mM HEPES (pH 7.0) in Hank’s balanced salt solution for 10 min on ice. Fixed cells were washed once with 20 mM HEPES (pH 7.0) and 150 mM NaCl, incubated at 65 °C for 30 min, washed once with 0.1 M Tris-HCl (pH 8.0), and lysed with 0.1% Triton-X in Tris-HCl (pH 8.0). AP activity in the lysed solutions was measured using LabAssay ALP (Wako Pure Chemical Industries Ltd.) to quantify bound Rspo2-mycAP or Agrn-mycAP. Agrn-mycAP was used to confirm that it did not bind to the cell surface of RNF43-transfected or Lgr5-transfected HEK293 cells (data not shown).
In vivo surgical induction of OA and intraarticular administration of mianserin
All animal experiments were performed in accordance with the recommendations in the Regulations on Animal Experiments in Nagoya University and approved by the Animal Care and Use Committee of the Nagoya University Graduate School of Medicine (The protocol No. 2017–29469). Ten-week-old male Wistar/ST rats were anesthetized with isoflurane and their right knee joints were induced to OA by surgical destabilization of the medial meniscus (DMM)50. Briefly, the knee joint was aseptically exposed with a medial capsular incision and the medial menisco-tibial ligament was resected to destabilize the medial meniscus. Sham surgery was performed on the contralateral left knee with incisions of the skin and joint capsule. Rats were randomly divided into PBS and mianserin groups (n = 3 each), and 50 µl PBS (control group) and 50 μM mianserin in 50 µl PBS (mianserin group) was intraarticularly injected into both knees once weekly, respectively. At 8 weeks after surgery, the rats were sacrificed and their knee joints were fixed overnight in 4% paraformaldehyde at 4 °C, decalcified, and embedded in paraffin. We made multiple sections around the middle portion of the medial femoral condyle (i.e. the weight-baring area) of each sample. A single section crossing the narrowest interarticular portion was stained with Safranin O and Fast-green. OA progressions were graded according to the modified Mankin histologic score51 on both tibial and femoral sides of articular cartilage.
The modified Mankin histological score is the sum of seven parameters. These include the articular cartilage structure (grades 0–11); tidemark duplication (grades 0–3); Safranin O staining (grades 0–8); fibrocartilage (grades 0–2); chondrocyte clones in uncalcified cartilage (grades 0–2); hypertrophic chondrocytes in calcified cartilage (grades 0–2); and subchondral bone (grades 0–2). Using a single well-cut and well-stained sagittal section crossing the narrowest interarticular portion in the medial compartment of the knee joint, the grades of OA were scored by a single blinded observer and averaged in each group of mice.
For immunofluorescence staining, the paraffin-embedded sections were deparaffinized and rehydrated. They were unmasked with 10 mM sodium citrate buffer (pH 6.0) at 90 °C for 15 min and incubated in 3% H2O2 for 15 min to inactivate endogenous peroxidases. They were then treated with a blocking buffer containing 5% goat serum in TBS-T for 1 h at room temperature and incubated with anti-β-catenin antibody (dilution 1:100; Cell Signaling Technology) at 4 °C overnight. The sections were then washed with TBS-T and incubated with Alexa 488-conjugated goat anti-rabbit IgG (dilution 1:500; Invitrogen) at room temperature for 1 h. The specimens were then mounted in VectaShield containing 2 µg/ml diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc.) and visualized with an A1Rsi microscope (Nikon Corp.). The average signal intensities of β-catenin in cytoplasm and nucleus of articular chondrocytes were blindly quantified with MetaMorph software (Molecular Devices, LLC). When the intensity of β-catenin in the nucleus was similar to or more than that in the cytoplasm, the cell was recognized as nuclear β-catenin-positive.
Statistical analysis
All continuous variables are expressed as mean ± standard deviation (SD). Statistical significance was estimated either by Student’s t-test or one-way ANOVA followed by Tukey’s post-hoc test. P-values less than 0.05 was considered statistically significant. All statistical analyses were performed with SPSS ver. 23 (IBM Corp.).
Other Materials and Methods
Details of other materials and methods are available in the Supplementary Information. This section includes detailed descriptions of total RNA extraction, real-time quantitative polymerase chain reaction (qPCR), Alcian blue staining, MTS assay, and isolation of human osteoarthritic chondrocyte (OAC) cells.
Change history
14 February 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Bijlsma, J. W., Berenbaum, F. & Lafeber, F. P. Osteoarthritis: an update with relevance for clinical practice. Lancet 377, 2115–2126, https://doi.org/10.1016/S0140-6736(11)60243-2 (2011).
Heinegård, D. & Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 7, 50–56, https://doi.org/10.1038/nrrheum.2010.198 (2011).
Kannu, P., Bateman, J. F., Belluoccio, D., Fosang, A. J. & Savarirayan, R. Employing molecular genetics of chondrodysplasias to inform the study of osteoarthritis. Arthritis Rheum. 60, 325–334, https://doi.org/10.1002/art.24251 (2009).
Clevers, H. & Nusse, R. Wnt/β-catenin signaling and disease. Cell 149, 1192–1205, https://doi.org/10.1016/j.cell.2012.05.012 (2012).
Zhu, M. et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J. Bone Miner. Res. 24, 12–21, https://doi.org/10.1359/jbmr.080901 (2009).
van den Bosch, M. H. et al. Induction of Canonical Wnt Signaling by Synovial Overexpression of Selected Wnts Leads to Protease Activity and Early Osteoarthritis-Like Cartilage Damage. Am. J. Pathol. 185, 1970–1980, https://doi.org/10.1016/j.ajpath.2015.03.013 (2015).
Dong, Y. F., Soung, D. Y., Schwarz, E. M., O’Keefe, R. J. & Drissi, H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J. Cell. Physiol. 208, 77–86, https://doi.org/10.1002/jcp.20656 (2006).
Deshmukh, V. et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 26, 18–27, https://doi.org/10.1016/j.joca.2017.08.015 (2018).
Takamatsu, A. et al. Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin signaling. PLoS One 9, e92699, https://doi.org/10.1371/journal.pone.0092699 (2014).
Miyamoto, K. et al. Fluoxetine ameliorates cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin signaling. PLoS One 12, e0184388, https://doi.org/10.1371/journal.pone.0184388 (2017).
Kazanskaya, O. et al. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell 7, 525–534, https://doi.org/10.1016/j.devcel.2004.07.019 (2004).
Nam, J. S. et al. Mouse R-spondin2 is required for apical ectodermal ridge maintenance in the hindlimb. Dev. Biol. 311, 124–135, https://doi.org/10.1016/j.ydbio.2007.08.023 (2007).
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297, https://doi.org/10.1038/nature10337 (2011).
Hao, H. X. et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200, https://doi.org/10.1038/nature11019 (2012).
Carmon, K. S., Gong, X., Lin, Q., Thomas, A. & Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452–11457, https://doi.org/10.1073/pnas.1106083108 (2011).
Chen, P. H., Chen, X., Lin, Z., Fang, D. & He, X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 27, 1345–1350, https://doi.org/10.1101/gad.219915.113 (2013).
Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779, https://doi.org/10.1038/nrm3470 (2012).
de Lau, W., Peng, W. C., Gros, P. & Clevers, H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 28, 305–316, https://doi.org/10.1101/gad.235473.113 (2014).
Chartier, C. et al. Therapeutic Targeting of Tumor-Derived R-Spondin Attenuates beta-Catenin Signaling and Tumorigenesis in Multiple Cancer Types. Cancer Res. 76, 713–723, https://doi.org/10.1158/0008-5472.Can-15-0561 (2016).
Balaji, K. N., Kaveri, S. V. & Bayry, J. Wnt signaling and Dupuytren’s disease. N. Engl. J. Med. 365, 1740, author reply 1740, https://doi.org/10.1056/NEJMc1110094 (2011).
Masahiro N. et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nature Genetics 46(9):1012–1016 (2014).
Takegami, Y. et al. R-spondin 2 facilitates differentiation of proliferating chondrocytes into hypertrophic chondrocytes by enhancing Wnt/β-catenin signaling in endochondral ossification. Biochem. Biophys. Res. Commun. 473, 255–264, https://doi.org/10.1016/j.bbrc.2016.03.089 (2016).
Rita D. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Research & Therapy 12(5), 216 (2010).
van der Kraan, P. M. & van den Berg, W. B. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis and Cartilage 20(3), 223–232 (2012).
Marlies, B. et al. The Reason for Discontinuation of the First Tumor Necrosis Factor (TNF) Blocking Agent Does Not Influence the Effect of a Second TNF Blocking Agent in Patients with Rheumatoid Arthritis. The Journal of Rheumatology 36(10), 2171–2177 (2009).
Abbott, A. Neurologists strike gold in drug screen effort. Nature 417, 109, https://doi.org/10.1038/417109a (2002).
Chong, C. R. & Sullivan, D. J. New uses for old drugs. Nature 448, 645–646, https://doi.org/10.1038/448645a (2007).
Kulkarni, P. et al. Glycosaminoglycan measured from synovial fluid serves as a useful indicator for progression of Osteoarthritis and complements Kellgren-Lawrence Score. BBA Clin 6, 1–4, https://doi.org/10.1016/j.bbacli.2016.05.002 (2016).
Kellgren, J. H. & Lawrence, J. S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16, 494–502 (1957).
Honsawek, S. et al. Dickkopf-1 (Dkk-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskelet. Disord. 11, 257, https://doi.org/10.1186/1471-2474-11-257 (2010).
Mabey, T. et al. Plasma and synovial fluid sclerostin are inversely associated with radiographic severity of knee osteoarthritis. Clin. Biochem. 47, 547–551, https://doi.org/10.1016/j.clinbiochem.2014.03.011 (2014).
Liao, W. et al. Proteomic analysis of synovial fluid in osteoarthritis using SWATHmass spectrometry. Mol Med Rep 17, 2827–2836, https://doi.org/10.3892/mmr.2017.8250 (2018).
Yan, D. et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc. Natl. Acad. Sci. USA 98, 14973–14978, https://doi.org/10.1073/pnas.261574498 (2001).
Sansom, O. J. et al. Cyclin D1 is not an immediate target of beta-catenin following Apc loss in the intestine. J. Biol. Chem. 280, 28463–28467, https://doi.org/10.1074/jbc.M500191200 (2005).
He, T. C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Andersen, J., Kristensen, A. S., Bang-Andersen, B. & Stromgaard, K. Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters. Chem. Commun. (Camb.), 3677–3692, https://doi.org/10.1039/b903035m (2009).
Gillman, P. K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 151, 737–748, https://doi.org/10.1038/sj.bjp.0707253 (2007).
Kim, K. A. et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588–2596, https://doi.org/10.1091/mbc.E08-02-0187 (2008).
Wei, Q. et al. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J. Biol. Chem. 282, 15903–15911, https://doi.org/10.1074/jbc.M701927200 (2007).
Zhu, M. et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 58, 2053–2064, https://doi.org/10.1002/art.23614 (2008).
Usami, Y., Gunawardena, A. T., Iwamoto, M. & Enomoto-Iwamoto, M. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab. Invest. 96, 186–196, https://doi.org/10.1038/labinvest.2015.142 (2016).
Hao, H. X., Jiang, X. & Cong, F. Control of Wnt Receptor Turnover by R-spondin-ZNRF3/RNF43 Signaling Module and Its Dysregulation in Cancer. Cancers (Basel) 8, https://doi.org/10.3390/cancers8060054 (2016).
Nakajima, M., Kou, I., Ohashi, H., Ikegawa, S. & Li, I. C. O. S. Identification and Functional Characterization of RSPO2 as a Susceptibility Gene for Ossification of the Posterior Longitudinal Ligament of the Spine. Am. J. Hum. Genet. 99, 202–207, https://doi.org/10.1016/j.ajhg.2016.05.018 (2016).
Altman, R. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 29, 1039–1049 (1986).
Link, T. M. et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology 226, 373–381, https://doi.org/10.1148/radiol.2262012190 (2003).
Sumantran, V. N. et al. Antiarthritic activity of a standardized, multiherbal, Ayurvedic formulation containing Boswellia serrata: in vitro studies on knee cartilage from osteoarthritis patients. Phytother. Res. 25, 1375–1380, https://doi.org/10.1002/ptr.3365 (2011).
Takigawa, M. et al. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 49, 3996–4002 (1989).
Shinada, K. et al. RNF43 interacts with NEDL1 and regulates p53-mediated transcription. Biochem. Biophys. Res. Commun. 404, 143–147, https://doi.org/10.1016/j.bbrc.2010.11.082 (2011).
Nakashima, H. et al. R-spondin 2 promotes acetylcholine receptor clustering at the neuromuscular junction via Lgr5. Sci. Rep. 6, https://doi.org/10.1038/srep28512 (2016).
Glasson, S. S., Blanchet, T. J. & Morris, E. A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15, 1061–1069, https://doi.org/10.1016/j.joca.2007.03.006 (2007).
Mankin, H. J., Dorfman, H., Lippiello, L. & Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 53, 523–537 (1971).
Acknowledgements
We thank Drs Shigetsugu Hatakeyama and Tadasuke Tsukiyama at Hokkaido University for providing RNF43 cDNA plasmid, and Dr. Masaharu Takigawa at Okayama University for providing HCS-2/8 cells. We thank Dr. Hiroaki Nakashima at Konan Kosei Hospital for critical discussion on this project. This study was supported by Grants-in-Aids from the Ministry of Education, Culture, Sports, Science and Technology (MEXT, 18K09062), the Ministry of Health, Labour, and Welfare of Japan (MHLW, H29-Nanchi-Ippan-030), the Japan Agency for Medical Research and Development (AMED, JP18gm1010002, JP18ek0109230, JP17ek0109281, JP18bm0804005), and the 24th General Assembly of the Japanese Association of Medical Sciences.
Author information
Authors and Affiliations
Contributions
T.O., B.O. and K.O. designed the study. T.O. and B.O. performed the experiments and collected the data. T.O., Y.T. and T.S. collected synovial fluid from the knee joints of patients with knee OA. Y.T. and T.S. evaluated OA severity. Y.T. blindly scored OA severity in a rat model of OA. T.O., B.O., M.I. and A.M. analyzed and interpreted the data. T.O., B.O., Y.T. and K.O. wrote the manuscript. All authors reviewed the manuscript. K.O. and N.I. supervised the project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okura, T., Ohkawara, B., Takegami, Y. et al. Mianserin suppresses R-spondin 2-induced activation of Wnt/β-catenin signaling in chondrocytes and prevents cartilage degradation in a rat model of osteoarthritis. Sci Rep 9, 2808 (2019). https://doi.org/10.1038/s41598-019-39393-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39393-x
This article is cited by
-
Ubiquitin-specific protease 49 attenuates IL-1β-induced rat primary chondrocyte apoptosis by facilitating Axin deubiquitination and subsequent Wnt/β-catenin signaling cascade inhibition
Molecular and Cellular Biochemistry (2020)
-
Wnt signaling: a promising target for osteoarthritis therapy
Cell Communication and Signaling (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.