Abstract
In this study, the effect of post-deposition tetrafluoromethane (CF4) plasma treatment on the physical and electrical characteristics of an In2TiO5 based electrolyte-insulator-semiconductor (EIS) sensor was investigated. Post-deposition CF4 plasma treatment typically improved the crystalline structure and repaired dangling bonds at the grain boundaries. We used the newly fabricated device to detect several ions, such as sodium and potassium, which are essential for many biological processes. The as-deposited and CF4 plasma treated In2TiO5 sensing window with an EIS structure was also able to detect the pH of a solution, different alkali ions (Na+ and K+), glucose, and urea. The sensing membrane after a 60-sec CF4 plasma treatment displayed improved biosensing characteristics, such as higher sensitivity (59.64 mV/pH), better drift rate, and a smaller hysteresis voltage of approximately 0.424 mV/h. The In2TiO5 sensing membrane treated with CF4 plasma is a promising material for use in EIS biosensing applications.
Similar content being viewed by others
Introduction
Over the last few decades, major advances have been made in the field of biosensors for the monitoring and control of many biochemical activities. Biosensors are extensively used for monitoring food and soil quality, as well as human biochemistry and other medical applications1. Ion-sensitive field-effect transistors (ISFETs) are considered excellent transducers for sensing biochemical reactions due to their small size, fast response, and reliability. Electrolyte-insulator-semiconductor (EIS) materials operate with the same working principle as ISFETs, but their fabrication process is relatively simple and inexpensive as compared to that of ISFETs2. The surface potential of the exposed insulator sensing area changes in response to the H+ concentration of the solution3. Therefore, selecting a proper insulating material with suitable characteristics is important to achieve high sensitivity and long-term reliability of the device.
Several metal oxides like indium-gallium-zinc oxide (InGaZnO, IGZO), zinc oxide (ZnO), and indium tin oxide (InSnO, ITO) have been developed as sensor membranes owing to their excellent sensing characteristics4,5,6. Indium oxide (In2O3) thin films have attracted significant attention as a sensing membrane because of its higher mobility (160 cm2/(V·s))7, higher melting point (1910 °C), larger band gap (3.5–3.7 eV)8, and insolubility in water. In2O3 films can easily be reduced to create an oxygen deficiency, where insufficient oxygen atoms are contained in the crystal structure9. This results in the formation of a nonstoichiometric In2O3−x film with an increased number of defects. Thus, the main free carriers in In2O3−x are the internal oxygen vacancies of the film, which also affect the chemical stability of the film. As a potential solution, CF4 plasma treatment of the In2O3 and In2TiO5 film can enhance the In-O bonding. In2O3 is widely used for gas sensing10, transparent conducting oxides11, and thin film transistors12 because of its excellent material characteristics.
The performance of a biosensor is conferred by doping with a transition metal, such as titanium (Ti). Ti affects the sensing membrane through the passivation of the defects in the bulk to the sensing performance and production of dangling bonds at the oxide interface13,14. Ti internalization also enhances device capacitance and reduces the reactivity with the surrounding moisture15,16.
Recent evidence demonstrated CF4 plasma treatment that improves the sensing performance of oxide materials for biosensing applications17. Fluorine incorporation improves the dielectric properties of the material by fixing the dangling bonds and replacing the weak bonds at the grain boundaries18. Fluorine introduces a net negative surface charge owing to its high electronegativity and improves the ability to capture positively charged H+ ions19. Moreover, pre- and post-deposition CF4 plasma treatment reduces metal-silicate formation at the oxide/silicon (Si) interface20.
Here, we used a post-deposition CF4 plasma treatment on In2O3 and In2TiO5 thin films to improve their electrical and physical characteristics. We describe the consequence of the post-deposition CF4 plasma treatment on the sensing and physical characteristics of a deposited In2TiO5 thin film as a sensing membrane. The detailed process flow of the EIS structure fabrication is shown in Fig. 1. After fabricating the device, sensing characteristics including sensitivity, hysteresis, and drift of the device were measured. To determine the physical characteristics, X-ray diffraction (XRD) atomic force microscopy (AFM), and secondary ion mass spectroscopy (SIMS) analyses were done.
Results and Discussion
Physical characteristics
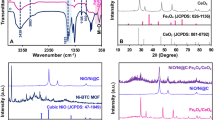
XRD was used to probe the crystalline structure of the materials. Figure 2(a,b) depicts XRD patterns of as-deposited and post-deposition CF4 plasma treated In2O3 and In2TiO5 membranes, respectively. The diffraction angles (2θ) ranged from 20 to 60°. All the In2O3 membrane samples displayed a cubic crystalline phase with two diffraction peaks, (222) and (321), at a diffraction angle of 30.5° and 33.10°, respectively21. A strong In2O3 (321) peak was observed for the sample annealed at 60 sec. In2TiO5 displayed a strong peak at (222) at a 2θ of 30.5. The major phase changed from (321) to (222) in XRD pattern of In2TiO5. The intensities of these three peaks were enhanced by the CF4 plasma treatment and further increased by increasing the plasma treatment time from 15 to 60 sec due to the improvement in the crystalline structure. The crystallinity improvement arises from the formation of a stronger fluorinated bond between indium and oxygen18. Therefore, after CF4 plasma treatment for 60 sec the In2O3 and In2TiO5 membranes would display higher intensity peaks than those prepared under other conditions. The XRD data indicated that internalization of Ti upgrades the thin film stoichiometry because it minimizes the number of dangling bonds on the dielectric surface and improves the temperature stability compared with In2O3 thin film22,23.
AFM was used to study the surface morphology of the devices treated with post-deposition plasma. Figure 3(a–d) show two-dimensional (2D) AFM images of the as-deposited and post-deposition CF4 plasma treated In2O3 and In2TiO5 samples. The AFM images of as-deposited In2O3 and In2TiO5 membranes revealed surface roughness of 0.557 nm and 0.628 nm, respectively. The roughness increased with In2O3 and In2TiO5 samples with increased CF4 plasma treatment time from 15 to 60 sec, as shown in Fig. 3(e). Samples doped with Ti achieve better surface roughness compared to bare In2O3. Ti has a higher affinity toward oxygen, and thus provides a larger grain size. This produces a rougher surface23,24. CF4 plasma treatment increases the interior grain size and causes plasma-induced morphological changes, which increase surface roughness and the number of surface sites, which in turn increase the sensitivity and linearity25.
AFM of the surface of the (a) as-deposited In2O3 sample (Rrms = 0.557 nm), (b) In2O3 sample CF4 plasma treated for 60 sec (Rrms = 1.77 nm), (c) as-deposited In2TiO5 sample (Rrms = 0.628 nm), (d) In2TiO5 sample CF4 plasma treated for 60 sec (Rrms = 2.92 nm), and (e) AFM of the sensing membrane with CF4 plasma treatment for various times.
Secondary ion mass spectrometry analysis data of the In2O3 and In2TiO5 thin films with and without CF4 plasma treatment are shown in Fig. 4. The as-deposited sample displayed no trace of fluorine atoms. For all the samples with CF4 plasma treatment, fluorine atoms were evenly distributed throughout the entire oxide bulk areas. Fluorine content increased as the CF4 plasma treatment time increased from 15 to 60 sec. Ti-doped In2O3 membranes have less excess etching of the sensing membrane. Fluorine incorporation reduces interfacial defect states at the oxide/silicon interface and increases the overall quality of the deposited oxide layer26.
Sensing characterization
The most important parameter of EIS structure is its flat-band voltage, which is defined as the voltage applied to produce a silicon surface potential of zero. The flat-band voltage of EIS is calculated as27:
where Eref is the reference electrode potential with respect to vacuum;\(\,\frac{1}{q}\)фSi is the work function of Si, which is equal to 4.7 V; Ψ0 is the potential drop in the electrolyte at the insulator-electrolyte interface; Ci and Qi are the insulator capacitance and effective charge per unit area, respectively, χSol is the surface dipole potential of the solvent; and δχ is the number of variations of χ potentials. The surface potential Ψ0 changes by changing the pH of the solutions, which causes a change in flat-band voltage at the different pHs. The surface potential Ψ0 is calculated as25,28:
where pHpzc is the value of pH for which the oxide surface is electrically neutral and β is the parameter that depends on the sensitivity of the sensing surface. The site-binding model can also be explained using the equation for capacitance, Q = CV, where Q is the surface charge, C is the double layer capacitance at the insulator interface, and V is the surface potential (Ψ0). The surface potential can be expressed as:
where α is the dimension-less sensitivity parameter consisting of the differential double layer capacitance. The ability of the oxide surface to protonate and deprotonate can be represented by the symbol β.
Figure 5(a–d) represent the capacitance versus substrate bias (C-V) curves of the In2O3 and In2TiO5 sensing membranes with and without CF4 plasma treatment. The inset of the figures shows the pH sensing properties of the films extracted from the C-V curves. The as-deposited In2O3 sample displayed a very low sensitivity of 21.67 mV/pH and linearity of 91.66%. The In2O3 sample treated with CF4 plasma for 15 sec displayed higher sensitivity and linearity of 47.03 mV/pH and 98.76%, respectively. The sensitivity and linearity increased to 52.43 mV/pH and 98.83% for samples treated for 30 sec. When samples were treated for 60 sec, the sensitivity and linearity further increased to 56.15 mV/pH and 98.18%, respectively. The as-deposited In2TiO5 sample displayed a decent sensitivity of 36.34 mV/pH and linearity of 97.65%. For CF4 plasma treatments of 15 and 30 sec, the sensitivity and linearity increased to 49.98 mV/pH and 94.23% at 15 sec, respectively, and 55.06 mV/pH and 99.67% at 30 sec, respectively. With a 60-sec CF4 plasma treatment, In2TiO5 samples displayed highest sensitivity and linearity values of 59.64 mV/pH and 99.68%, respectively. The CF4 plasma treatment increased the overall sensing surface area by increasing the surface roughness. Thus, the larger sensing surface area will lead to higher sensitivity with more surface sites being present and capable of binding ions present in the electrolyte solution18. Therefore, pH sensing membrane treated with CF4 plasma for 60 sec can possess high sensitivity and excellent linearity. These properties can be attributed to the formation of a greater number of fluorinated bonds (F-In bonds) on the In2O3 and In2TiO5 film surface due to CF4 plasma treatment. The deviation in surface potential of the sensing surface after the surface is dipped into solutions with different pH values was determined by the C-V shift. H+ and OH−attachment at the corresponding binding sites at the sensing surface can alter the surface potential29. The present results demonstrate that Ti doping increases the numbers of binding sites on the sensing surface. The grain size becomes larger with higher surface roughness due to incorporation of Ti. Correspondingly the number of binding sites increases with the larger surface area23. Therefore, the sensitivity and linearity of In2TiO5 membranes that are plasma-treated for 60 sec is much higher than those aspects of In2O3 membranes.
To investigate the hysteresis effects of the membrane, the samples were immersed in buffer solutions with different pH values in an alternating cycle (pH 7 → 4 → 7 → 10 → 7). Moreover, to test the long-term reliability of the devices, the drift effect of the In2O3 with CF4 plasma treated sensing membrane was measured by a C-V curve in a pH 7 buffer solution for 12 h. The hysteresis voltage and drift rate under the different CF4 plasma treatment conditions reveal that the 60-sec In2O3 film plasma treatment produced the lowest hysteresis voltage and lowest drift rate (4.33 mV and 0.95 mV/h, respectively (Fig. 6(a–d)). In2TiO5 CF4 plasma-treated sensing membrane displayed a hysteresis voltage 25.75, 9.32, 6.85 and 2.72 mV in the as-deposited condition and following 15, 30, and 60 sec of annealing, respectively. Figure 6(d) shows the drift rate for In2TiO5 devices with CF4 plasma treatment. The drift rate of the as-deposited sample was 9.23 mV/h, whereas drift rates of 4.92, 1.09, and 0.42 mV/h were obtained following annealing for 15, 30, and 60 sec, respectively. This was likely because fluorine compensated for the dangling bonds and replaced weaker bonds in the grain boundaries. Thus, the CF4 plasma treatment can reduce the trap states in the oxide/silicon interface and improve the hysteresis and drift rate characteristics of the films18. The dangling bonds are negated by Ti ions and the defects located underneath of the dielectric surface are compensated16,23.
(a,c) Hysteresis of In2O3 and In2TiO5 sensing membranes for the as-deposited and samples with CF4 plasma treatment of various times during the pH loop of 7 → 4 → 7 → 10 → 7 over a period of 30 min. (b,d) Drift rate of In2O3 and In2TiO5 sensing membranes for the as-deposited and samples with CF4 plasma treatment for various times in pH 7 buffer solution.
To analyse the sensing performance of our sensing membrane in the presence of potassium and sodium, we prepared a solution using a micropipette to control the concentrations of sodium and potassium ions ranging between 10−5 and 10−1 M by injecting 1 M NaCl/Tris-HCl and 1 M KCl/Tris-HCl into buffer electrolyte. Comparisons of H+, Na+, and K+ sensing performances of the as-deposited In2O3 and In2TiO5 membrane, and the membrane treated with CF4 plasma for 60 sec are presented in Fig. 7(a–d). In2TiO5 sample treated with CF4 plasma for 60 sec had best sensitivities (18.23 mV/pNa and 14.13 mV/pK) compared to the In2TiO5 as-deposited sample (9.93 mV/pNa and 7.98 mV/pK) and 60-sec CF4 plasma treated In2O3 sample (13.77 mV/pNa and 12.78 mV/pK). For sodium and potassium sensing, considerably lower sensitivity was obtained compared to H+ sensing. This can be explained by the heavier and larger Na+ and K+ ions compared to H+ ions.
We also measured the essential human biochemistry data of glucose and urea. They were detected by incorporating appropriate enzymes on the sensing oxide surface. The chemical equations for the reactions that can be used for glucose detection are30,31:
The chemical equation for the reactions that can be used for urea detection is30,31:
The glucose sensing properties of the In2TiO5 sensing membrane on the EIS structure with and without the 60-sec CF4 plasma treatment are presented in Fig. 8(a,b). The glucose concentration was controlled over the range of 2 to 7 mM. The as-deposited In2TiO5 sensing membrane exhibited a low glucose sensitivity of 2.85 mV/mM and linearity of 85.23%. After Ti doping and 60-sec CF4 plasma treatment, the sensitivity increased to 6.63 mV/mM and the linearity increased to 92.35%.
The urea concentration was controlled in a range between 5 and 40 mM (Fig. 9(a,b)). The sensitivity values of the In2TiO5 sensing membrane above as-deposited and CF4 plasma treatment in samples treated for 60 sec were 1.55 and 2.69 mV/mM, respectively. Therefore, the 60-sec CF4 plasma treated In2TiO5 sensing membrane has better sensitivity and linearity for the detection of urea. These results indicate that the CF4 plasma treatment can increase the interior grain size and increase the number of surface sites, resulting in better sensing performance for the detection of H+, Na+, K+, glucose, and urea.
The test sample of commercially available glucose devices, such as the ACCU-CHECK glucometer, uses a precious metal (Ag/AgCl)32. In this study, we used In and Ti as the sensing film. Their use can reduce the purchase price and enable reuse of material. These advantages also include a sensing membrane with high glucose sensitivity and the capability to accurately and sensitively measure other molecules.
Table 1 provides comparative data of the sensing parameters of drift rate, pH sensitivity, hysteresis voltage, glucose, and urea sensing for different EIS devices with TbYxOy33, CeO234, ZnO35, Sm2O336, Ti-doped ZnO37, and CeO with CF4 plasma treatment38. The pH sensitivity, hysteresis, drift rate, glucose and urea sensing of the EIS device prepared with In2TiO5 and incorporating a CF4 plasma sensing membrane was superior. CF4 incorporated into In2TiO5 film can enhance the In-O bonding at the In2TiO5 sensing membrane, which improves the sensing characteristics.
Conclusion
A CF4 plasma treated In2TiO5-based EIS sensor was fabricated for H+, Na+, K+, and glucose sensing applications. The effect of the post-deposition CF4 plasma treatment on the physical and electrical characteristics of the sensors was studied. The In2TiO5 EIS sensor with a 60-sec post-deposition plasma treatment exhibited good sensitivity of 59.64 mV/pH and linearity of 99.68%. Post-deposition CF4 plasma treatment improves the crystalline structure and repairs the dangling bonds at the grain boundaries. Furthermore, the 60-sec plasma treatment produced the best material and electrical properties, and achieved ideal sensing capabilities, likely due to defect passivation by fluorine. Furthermore, the Ti-doped In2O3-based EIS sensor treated with CF4 plasma was more responsive to H+ compared to Na+ and K+. The In2TiO5 sensing membrane with 60-sec CF4 plasma treatment also had better sensitivity and linearity than the as-deposited sample for glucose and urea detection.
Methods
The EIS incorporated In2O3 and In2TiO5 sensing membranes were fabricated on 4-inch n-type (100) silicon wafers with a resistivity of 5–10 Ω-cm. After a standard RCA cleaning process, the samples were dipped into a 1% hydrofluoric acid solution to remove native oxide from the surface. A 50 nm-thick SiO2 substrate was thermally oxidized on the silicon wafer. Then, (a) a 50 nm-thick In2O3 film was deposited on the SiO2/Si substrate by reactive radio frequency sputtering. (b) Both In2O3 and Ti were used to co-sputter In2TiO5 film on SiO2/Si stacks in a diluted argon flow (Ar/O2 = 20 sccm/5 sccm) with a radio frequency power and process pressure of 100 W and 20 mTorr, respectively. After deposition, the In2O3 and the In2TiO5 films were subjected to a post-deposition CF4 plasma treatment in a plasma-enhanced chemical vapor deposition system with a radio frequency power of 30 W and a pressure of 500 mTorr for 15, 30, and 60 sec. The back-contact was made by depositing a 300 nm-thick aluminium film on the back side of the Si wafer. The sensing area of the deposited In2O3 films was defined by an automatic robot dispenser with an adhesive silicone gel to build the final EIS structure on the printed circuit board using a silver gel to form conductive lines. An epoxy package was used to separate the EIS structure and the copper line. The detailed process flow of the EIS structure fabrication is shown in Fig. 1.
Morphological analyses of the In2O3 and In2TiO5 sensing membranes included XRD, AFM, and SIMS. These analyses were done to investigate the link between structural and electrical characteristics properties. SIMS instruments use an internally yielded beam of either positive or negative ions focused on a sample surface to generate secondary ions. The generated ions are then transferred into a mass spectrometer across a high electrostatic potential. The depth profiling of elemental and molecular species, as well as isotopic ratios of compounds was evaluated by SIMS analysis. The surface morphologies of the In2O3 and In2TiO5 nano-layers were observed using AFM in Bruker Dimension Icon modes with intermittent contact using a silicon tip with a 10 pN/nm spring constant. A sample area of 3 × 3 μm was scanned with actuation rates up to 8 kHz in air and fluid. For XRD analysis of the samples, grazing incidence of X-ray beam CuKa (k = 1.542 Å) radiation was used with an incidence angle step of 0.5° in the diffraction angle range (2θ) from 20° to 60°.
References
Patel, P. D. (Bio)sensors for measurement of analytes implicated in food safety: A review. TrAC Trends Anal. Chem. 21, 96–115 (2002).
Martinoia, S., Massobrio, G. & Lorenzelli, L. Modeling ISFET microsensor and ISFET-based microsystems: A review. Sens. Actuators B. 105, 14–27 (2005).
Surmalyan, A. V. Surface potential behavior in ISFET based bio-(chemical) sensor. Arm J Phys. 5, 194–202 (2012).
Lin, J. C., Huang, B. R. & Yang, Y. K. IGZO nanoparticle-modified silicon nanowires as extended-gate field-effect transistor pH sensors. Sens. Actuators B. 184, 27–32 (2013).
Wei, A., Pan, L. & Huang, W. Recent progress in the ZnO nanostructure-based sensors. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 176, 1409–1421 (2011).
Wang, L. & Wang, E. A novel hydrogen peroxide sensor based on horseradish peroxidase immobilized on colloidal Au modified ITO electrode. Electrochem. Commun. 6, 225–229 (2004).
Bierwagen, O., White, M. E., Tsai, M. Y. & Speck, J. S. Electron transport properties of antimony doped SnO2 single crystalline thin films grown by plasma-assisted molecular beam epitaxy. J. Appl. Phys. 106, 093704 (2010).
Beena, D. et al. Photoluminescence in laser ablated nanostructured indium oxide thin films. J. Alloys Compd. 489, 215–223 (2010).
Quaas, M., Eggs, C. & Wulff, H. Structural studies of ITO thin films with the Rietveld method. Thin Solid Films 332, 277–281 (1998).
Wu, C. H., Chou, T. L. & Wu, R. J. Rapid detection of trace ozone in TiO2–In2O3 materials by using the differential method. Sens. Actuators B. 225, 117–124 (2018).
Babu, S. H., Kaleemulla, S., Rao, N. M. & Krishnamoorthi, C. Indium oxide: A transparent, conducting ferromagnetic semiconductor for spintronic applications. J. Magn. Magn. Mater. 416, 66–74 (2016).
Wang, L. & Xu, X. Semiconducting properties of In2O3 nanoparticle thin films in air and nitrogen. Ceram Int. 41, 7687–7692 (2015).
Pan, T. M., Wang, C. W., Weng, W. H. & Pang, S. T. Impact of titanium content and postdeposition annealing on the structural and sensing properties of TbTixOy sensing membranes. J. Mater. Chem. C. 2, 7575–7582 (2014).
Kao, C. H. et al. Ti-doped Gd2O3 sensing membrane for electrolyte–insulator–semiconductor pH sensor EPOXY. Thin Solid Films 520, 3760–3763 (2012).
Pan, T. M. & Liao, K. M. Comparison of structural and sensing characteristics of Pr2O3 and PrTiO3 sensing membrane for pH-ISFET application. Sens. Actuators B. 133, 97–104 (2018).
Pan, T. M. & Lin, C. W. Structural and sensing characteristics of Dy2O3 and Dy2TiO5 electrolyte−insulator−semiconductor pH sensors. J. Phys. Chem. C. 114, 17914–17919 (2010).
Lai, C. S., Lue, C. E., Yang, C. M. & Pijanowska, D. G. Fluorine incorporation and thermal treatment on single and stacked Si3N4 membranes for ISFET/REFET application. J. Electrochem. Soc. 157, J8–J12 (2010).
Wang, S. D., Lo, W. H. & Lei, T. F. CF4 Plasma treatment for fabricating high-performance and reliable solid-phase-crystallized poly-Si TFTs. J. Electrochem. Soc. 152, G703–G706 (2005).
Zhou, Y., Cai, Y., Lau, K. M. & Chen, K. J. Control of threshold voltage of AlGaN/GaN HEMTs by fluoride-based plasma treatment: From depletion mode to enhancement mode. IEEE Trans. Electron Devices 53, 2207–2215 (2006).
Lai, C. S. et al. Suppression of interfacial reaction for HfO2 on silicon by pre-CF4 plasma treatment. Appl. Phys. Lett. 89, 11–14 (2006).
Yousif, A. A. & Mahdi, Z. S. Effect of laser irradiation on structure, morphology and gas sensor properties of chemical spray pyrolysis deposited nanostructured In2O3 films. International Journal of Scientific & Engineering Research. 7, 116–134 (2016).
Kao, C. H., Chen, H. & Huang, C. Y. Effects of Ti addition and annealing on high-k Gd2O3 sensing membranes on polycrystalline silicon for extended-gate field-effect transistor applications. Appl. Surf. Sci. 286, 328–333 (2013).
Isabel, A. P. S. et al. Sensing and structural properties of Ti-doped tin oxide (SnO2) membrane for bio-sensor applications. Ceram. Int. 43, 10386–10391 (2017).
Pan, T. M. & Yu, T. Y. & Wang, C. C. High-k Nd2O3 and NdTiO3 charge trapping layers for nonvolatile memory metal-SiO2-high-k-SiO2-silicon devices. J. Electrochem. Soc. 155, G218–G223 (2008).
Van Hal, R. E. G., Eijkel, J. C. T. & Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuators B. 24, 201–205 (1995).
Lai, C. S., Wu, W. C., Fan, K. M., Wang, J. C. & Lin, S. J. Effects of post CF4 plasma treatment on the HfO2 thin film. Jpn. J. Appl. Phys. 44, 2307–2310 (2005).
Jamasb, S., Collins, S. D. & Smith, R. L. A physical model for threshold voltage instability in Si3N4- gate H+- sensitive FET’s (pH ISFET’s). IEEE Trans. Electron Devices 45, 1239–1245 (1998).
Schlodder, E. & Witt, H. T. Stoichiometry of proton release from the catalytic center in photosynthetic water oxidation. J. Biol. Chem. 274, 30387–30392 (1999).
Lisco, F. et al. Pinhole free thin film CdS deposited by chemical bath using a substrate reactive plasma treatment. J. Renewable Sustainable Energy 6, 011202 (2014).
Seo, H. II. et al. ISFET glucose sensor based on a new principle using the electrolysis of hydrogen peroxide. Sens. Actuators B. 40, 1–5 (1997).
Castillo, J., Gáspár, S., Leth, S. & Niculescu, M. A. Mortari, I. Bontidean, V. Soukharev, S. A. Dorneanu, A. D. Ryabov, and E. Csöregi. Biosensors for life quality - Design, development and applications. Sens. Actuators B. 102, 179–194 (2004).
Matysik, F. M. Trends in Bioelectroanalysis. 20–42 (Springer, 2016).
Weng, W. H., Wang, C. W., Pang, C. T. & Pan, T. M. Enzymatic glucose biosensor based on TbYxOy electrolyte-insulator-semiconductor. J. Electrochem. Soc. 163, B445–B452 (2016).
Kao, C. H. et al. Electrical, material and multianalyte-sensitive characteristics of thermal CeO2/SiO2-stacked oxide capacitors. Thin Solid Films 570, 552–557 (2014).
Kao, C. H. et al. Multianalyte biosensor based on pH-sensitive ZnO electrolyte–insulator–semiconductor structures. J. Appl. Phys. 115, 184701 (2014).
Wu, M. H., Cheng, C. H., Lai, C. S. & Pan, T. M. Structural properties and sensing performance of high-k Sm2O3 membrane-based electrolyte–insulator–semiconductor for pH and urea detection. Sens. Actuators B. 138, 221–227 (2009).
Lee, M. L. et al. Comparison of ZnO and Ti-doped ZnO sensing membrane applied in electrolyte-insulator-semiconductor structure. Ceram. Int. 44, 6081–6088 (2018).
Kao, C. H. et al. Fabrication of multianalyte CeO2 nanograin electrolyte–insulator–semiconductor biosensors by using CF4 plasma treatment. Sensing and Bio-Sensing Research. 5, 71–77 (2015).
Acknowledgements
This work was supported by the Ministry of Science and Technology under the contract of MOST 107–2221-E-182-036-. This work was supported by the Chang Gung University under contract CMRPD2H0181.
Author information
Authors and Affiliations
Contributions
Chun-Fu Lin, Chyuan-Haur Kao, and Chan-Yu Lin, designed the research. Chun-Fu Lin and Chia Shao Liu graphed the device structure. Chun-Fu Lin and Yi Wen Liu devised the crystal growth method, and grew and characterized the materials. Chun-Fu Lin, Chyuan-Haur Kao, and Chan-Yu Lin analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, C.F., Kao, C.H., Lin, C.Y. et al. Comparison Between Performances of In2O3 and In2TiO5-Based EIS Biosensors Using Post Plasma CF4 Treatment Applied in Glucose and Urea Sensing. Sci Rep 9, 3078 (2019). https://doi.org/10.1038/s41598-019-39012-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-39012-9
This article is cited by
-
Ti-doped indium gallium oxide electrolyte–insulator–semiconductor membranes for multiple ions and solutes detectors
Journal of Materials Science: Materials in Electronics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.