Abstract
Mitochondria play an important role in providing ATP for muscle contraction. Muscle physiology is compromised in Duchenne muscular dystrophy (DMD) and several studies have shown the involvement of bioenergetics. In this work we investigated the mitochondrial physiology in fibers from fast-twitch muscle (EDL) and slow-twitch muscle (soleus) in the mdx mouse model for DMD and in control C57BL/10J mice. In our study, multiple mitochondrial respiratory parameters were investigated in permeabilized muscle fibers from 12-week-old animals, a critical age where muscle regeneration is observed in the mdx mouse. Using substrates of complex I and complex II from the electron transport chain, ADP and mitochondrial inhibitors, we found in the mdx EDL, but not in the mdx soleus, a reduction in coupled respiration suggesting that ATP synthesis is affected. In addition, the oxygen consumption after addition of complex II substrate is reduced in mdx EDL; the maximal consumption rate (measured in the presence of uncoupler) also seems to be reduced. Mitochondria are involved in calcium regulation and we observed, using alizarin stain, calcium deposits in mdx muscles but not in control muscles. Interestingly, more calcium deposits were found in mdx EDL than in mdx soleus. These data provide evidence that in 12-week-old mdx mice, calcium is accumulated and mitochondrial function is disturbed in the fast-twitch muscle EDL, but not in the slow-twitch muscle soleus.
Similar content being viewed by others
Introduction
Duchenne muscular dystrophy (DMD) is a fatal muscular disorder caused by nonsense mutations, large deletions or duplications in the dystrophin gene. DMD is characterized by progressive muscle wasting. The absence of dystrophin, a membrane-associated protein, causes disruption of the dystrophin-glycoprotein complex (DGC), which is critical for maintaining sarcolemma integrity and activity of signaling complexes and ion channels. DGC disruption induces direct calcium influx and/or abnormal cytosolic calcium homeostasis, causing membrane leakage and increased vulnerability of myofibers to necrosis1,2. Calcium is a key regulator of cell signaling and is the main effector of skeletal muscle contraction. The availability of cytoplasmic calcium is regulated by the uptake of calcium by both the sarcoplasmic reticulum and mitochondria. Different muscle fiber types, fast- and slow-twitch, have different mitochondrial function and calcium levels.
The mdx mouse with defective dystrophin expression is one of the most widely used animal models for DMD research. These animals present a mild phenotype and a less severe disease course compared to humans, which is most likely due to the high regenerative capacity of mouse muscles3,4,5. Hence, mdx muscles present cycles of degeneration and regeneration but allow a normal lifespan, contrasting with 75% lifespan reduction in humans5,6.
Muscular dystrophy in mdx mice shows an age-dependent disease severity7,8,9,10. Soon after weaning (21–28 days) mdx mice exhibit intense inflammatory myonecrosis, causing the release of factors that activate the proliferation of quiescent satellite cells important for muscle damage recovery at adulthood. In mature adults at 12 wks, mdx muscles not yet affected by senescence show mild inflammatory reaction and efficient muscular regeneration11,12,13.
During the last decade the involvement of mitochondria in DMD pathogenesis has been identified by different groups9,10,14,15,16,17,18,19. Mitochondria are among the first cell components to be affected in DMD and a decline in mitochondrial activity over time precedes the onset of the disease symptoms17. Nevertheless, in relation to the different phases of the pathology, the physiological function of mitochondria has received very little attention. In particular, mitochondrial physiology in studies of the regeneration phase of the disease was barely mentioned9,10,18. In addition, the studies often used a pool of different muscle samples to analyze mitochondrial physiology14. This is an important issue, since it is well known that one of the determining factors in the study of mitochondrial physiology is the isolation procedure, due to the small tissue mass available. The use of a pool of different muscle samples makes it difficult to relate the results to specific muscle types.
Understanding the mechanisms by which mdx muscles can efficiently regenerate, while human DMD muscles cannot, is of special importance in this field and can open new possibilities for DMD treatment and therapy. Therefore, it is important to assess mitochondrial respiration in skeletal muscles with distinct fiber-type specialization in mdx mice at 12 wks. To address this point, we used permeabilized fibers from fast-twitch extensor digitorum longus (EDL), and slow-twitch soleus from mdx mice at 12 wks. We assessed mitochondrial metabolic states such as coupled and uncoupled respiration and maximal respiration capacity by successive additions of mitochondrial substrates and inhibitors to assess the functioning of the electron transport chain through high-resolution respirometry. We also analyzed the presence of calcium deposits in these muscles (EDL and soleus) in an effort to correlate the deposits with alterations in mitochondrial function.
Results
Excessive calcium influx and increased membrane permeability are early precursors of damage events in mdx muscular dystrophy1,2. Thus we decided to analyze the presence of calcium deposits in fast and slow muscles of 4- and 12-week-old mdx mice to try to correlate the amount of calcium in the tissue with muscle fiber type and phase of the disease. Muscle sections (EDL and soleus) were stained with Alizarin Red S (which stains calcium specifically) and images of calcium deposits were acquired under polarization microscopy and quantified (Fig. 1). Both soleus and EDL from mdx muscles showed detectable amounts of calcium deposits at both ages (4 and 12 wks; Fig. 1). Conversely, no calcium deposits were detected in C57 control muscles at either age (Fig. 1). Interestingly, both soleus and EDL mdx muscles showed an increase in calcium deposits at 12 wks in comparison with 4 wks. Importantly, we found a ~16% average increase in calcium deposits in EDL from mdx at 12 wks compared to EDL from control at 12 wks (Fig. 1). EDL from mdx at 4 wks showed ~1% increase compared to EDL from control at 4 wks, and soleus at 4 and 12 wks showed ~1% and ~6% increase, respectively, compared to soleus controls (Fig. 1). Wada and colleagues (2014) described ~5% increase in calcium deposits in tibialis anterior muscle from mdx20. These results show that a high accumulation of calcium deposits may be specific to EDL from mdx at 12 wks.
Calcium deposit quantification in soleus and EDL muscles from 4- and 12-wk-old control and mdx animals. Images from alizarin staining were acquired under polarized optical microscopy (A) and the amounts of calcium deposits were quantified in soleus (B) and EDL (C) muscles. The data are expressed as the mean ± SD of 3 animals per experimental group. (B) Soleus: **p = 0.0016 and ***p < 0.0001; (C) EDL: **p = 0.0014 and ***p < 0.0001; n.d - not detected; black bars represent mdx. Scale bars in soleus and in EDL images = 100 µm.
Calcium handling by mitochondria is a key feature of eukaryotic cells: it is involved in energy production, in buffering and shaping cytosolic calcium movements and in the regulation of apoptosis21. Since increased calcium content was a feature of EDL mdx muscle at 12 wks, we decided to analyze whether the increase in calcium was related to mitochondrial function at this stage of the DMD disease.
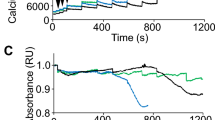
To assess mitochondrial function, we isolated fibers from EDL and soleus muscles from 12 wks mdx dystrophic and control mice and analyzed mitochondrial respiration in permeabilized fibers using commonly used substrates. Substrate combinations of pyruvate/malate (PM) were used to promote reduction of NAD by dehydrogenases to donate electrons to Complex I. In this condition, Complex II (CII) does not contribute to the oxygen consumption observed. Addition of succinate was used to assess complex II by supporting electron flux through CII via flavin adenine dinucleotide (FADH2). The peak that appears prior to the addition of PM in Fig. 2A is an artifact of the electrode measurement. Such alterations are particularly common near the beginning of an experiment, when the electrode is stabilizing the signal after the closure of the oxygraph chamber. The oxygen consumption by EDL muscle fibers from control and mdx mice is shown in Fig. 2. Basically, there was no oxygen consumption before the addition of substrate. Oxygen flux increased after the addition of pyruvate/malate (PM) but no difference was detected on comparing permeabilized EDL fibers derived from control and mdx animals (Fig. 2A,B). As expected, ADP addition promoted an increase in oxygen consumption and allowed us to assess the coupling of the electron transport system (ETS) to ATP synthesis and oxygen consumption. Interestingly, oxygen consumption stimulated by ADP addition was significantly diminished (29% less) in fibers from mdx mice compared with controls (Fig. 2A,B). In coupled mitochondria, there is a direct relationship between oxygen consumption and ADP phosphorylation22. These results suggest that oxygen consumption stimulated by ADP addition is compromised in the EDL from mdx animals and may be associated with observations of loss of muscle function at this stage of the disease (12 wks). However, these results could also occur if there were mitochondrial membrane damage during the preparation of muscle fibers. To rule out this possibility, we added cytochrome c to verify the integrity of the external mitochondrial membrane23. In case of a mitochondrial membrane leak, the addition of cytochrome c would promote an increase in oxygen consumption. However, addition of cytochrome c after ADP caused no change, confirming the viability of the preparations (mitochondrial membrane integrity) during the experiments (Fig. 2A,B). Furthermore, after succinate was included in the reaction medium, we observed a small increase in the oxygen flux (18% and 10% in control and mdx group, respectively), but the differences between animal groups (control vs mdx) were maintained (Fig. 2A,B). The oxygen consumption in permeabilized EDL fibers derived from mdx animals was 33% lower than in those from control animals after succinate addition. The inclusion of oligomycin in the reaction medium decreased the mitochondrial oxygen consumption rate, but control was still higher than mdx fibers. The uncoupled state elicited by FCCP addition allowed us to compare the maximal respiratory capacity and confirm the lower response of fibers derived from mdx mice. These results show a decrease in mitochondrial function in EDL muscle from mdx at 12 wks.
Assessment of oxygen flux in high-resolution respirometry experiments using fibers from EDL muscles of 12-wk-old control and mdx animals. (A) Representative trace of the real-time, high-resolution respirometry experiments. A multiple-substrate protocol was used to assess complex I (PM) and complex II (Succ) participation in respiration in permeabilized fibers from extensor digitorum longus (EDL). The additions are shown by vertical lines, with each event identified along the bottom. Pyruvate + Malate (5 mM each); ADP (3 mM); Cytochrome C (10 μM); Succinate (10 mM); Oligomycin (3 μg/mL); FCCP (1 μM); potassium cyanide (KCN, 10 mM). Control animals (black line) and mdx animals (gray line). The continuous lines represent the flux of O2 consumption [pmol/(s*mg)], and the dotted lines represent the O2 concentrations (nmol/mL). In (B–D) the white bars represent control and black bars represent mdx. (B) O2 consumption rates in high-resolution respirometry obtained from permeabilized fibers of control and mdx mice run in parallel. ADP **p = 0.0030, Succ **p = 0.0070, FCCP *p = 0.0379 compared to the indicated group. The graph shown in (C) corresponds to subtraction of the oxygen flux observed after ADP addition and that measured only with PM, **p = 0.0011; The graph shown in (D) corresponds to subtraction of the oxygen flux observed after oligomycin administration from that measured after Succ addition, **p = 0.0047. The data are expressed as the mean ± SD of 9 animals per experimental group.
Next, we investigated whether the changes observed in the fast-twitch muscle tissue were also observed in slow-twitch muscles. We repeated the oxygen consumption experiment using permeabilized fibers from the slow-twitch muscle soleus of control and mdx animals at the same age (12 wks).
As shown in Fig. 3, fibers derived from soleus had a very similar profile compared to fibers from EDL muscle. Importantly, no significant differences in mitochondrial respiration were noted between soleus fibers from mdx and control animals (Fig. 3A,B). However, similarly to the experiment performed with permeabilized EDL fibers, the data show a slight decline in mitochondrial respiration after addition of ADP, succinate and FCCP (Fig. 3A,B) in the fibers isolated from mdx animals [ADP (p = 0.075), Succinate (p = 0.1672) and FCCP (p = 0.0927) comparing mdx with control group].
Assessment of oxygen flux in high-resolution respirometry experiments using fibers from soleus muscle of 12-wk-old control and mdx animals. (A) Representative trace of the real-time, high-resolution respirometry experiments. A multiple substrate protocol was used to assess complex I (PM) and complex II (Succ) participation in respiration by permeabilized fibers from soleus muscle. The additions are shown by vertical lines, with each event identified along the bottom. Pyruvate + Malate (5 mM each); ADP (3 mM); Cytochrome C (10 μM); Succinate (10 mM); Oligomycin (3 μg/mL); FCCP (1 μM); potassium cyanide (KCN, 10 mM). Control animals (black line) and mdx animals (gray line). The continuous lines represent the flux of O2 consumption [pmol/(s*mg)], and the dotted lines represent the O2 concentrations (nmol/mL). In (B-D) the white bars represent control and black bars represent mdx. (B) O2 consumption rates in high-resolution respirometry obtained from permeabilized fibers of control and mdx mice run in parallel. ADP (p = 0.075), Succinate (p = 0.1672) and FCCP (p = 0.0927) comparing mdx with control group. The graph shown in (C) corresponds to subtraction of the oxygen flux observed only with PM from that measured after ADP addition, *p = 0.0206. The graph shown in (D) corresponds to subtraction of the oxygen flux observed after oligomycin administration from that observed after Succ addition, **p = 0.0079. The data are expressed as the mean ± SD of 9 animals per experimental group.
To better identify the differences in mitochondrial function between the two types of muscle (EDL and soleus) at 12 wks, we focused on the oxygen consumption associated with ADP phosphorylation. The results (Figs 2C and 3C) show the values obtained by subtracting the flux measured only with mitochondrial substrates for complex I from the data observed after ADP addition. There was a clear decrease in the ADP phosphorylation capacity in mdx-derived fibers. These data were confirmed by analyzing the values of oxygen flux obtained before and after oligomycin addition (Figs 2D and 3D). The subtraction result represents the oxygen consumption coupled to ADP phosphorylation. As observed in EDL (Fig. 2D) and soleus muscles (Fig. 3D) the coupled respiration was reduced by about 45% in the fibers derived from mdx animals.
Since we found a decrease in mitochondrial function together with an increase in calcium deposits in EDL and soleus muscles from mdx, we decided to analyze whether these changes could be correlated with alterations in the expression of sarcoendoplasmic reticulum (SR) calcium transport ATPases (SERCAs). SERCAs are pumps that transport calcium ions from the cytoplasm into the SR24 and therefore could be related to the increased calcium found in mdx muscles. We analyzed the expression of Atp2a1 (SERCA1) and Atp2a2 (SERCA2a) mRNAs in EDL and soleus muscles from 4- and 12-wks mdx dystrophic and C57 control mice using real-time PCR (qPCR). There was no significant difference between control and mdx soleus muscle in Atp2a1 (SERCA1) mRNA levels from either 4- or 12-wks mice, nor in EDL muscle from 12-wks mice (Fig. 4A,B,F). However, expression of SERCA1 in EDL from 4-wks mdx mice was significantly lower than the control (Fig. 4E). In addition, there was a significant decrease in the expression of Atp2a2 (SERCA2a) mRNA levels in soleus muscle from both 4- and 12-wks mdx (Fig. 4C,D) and an increase in SERCA2a mRNA from EDL of mdx muscle at 12 wks (Fig. 4H); and no significant differences were found in EDL of mdx muscle at 4 wks (Fig. 4G). These results show alterations in the expression of Atp2a1 (SERCA1) and Atp2a2 (SERCA2a) mRNAs from mdx EDL and soleus muscles.
Expression of Atp2a1 (SERCA1) and Atp2a2 (SERCA2a) mRNA in EDL and soleus muscles from 4- and 12-week-old mdx and C57 control mice. Total RNA was extracted from muscles for qPCR analysis of the expression of Atp2a1 (SERCA1) and Atp2a2 (SERCA2a). Soleus (A–D) and EDL (E–H) muscle Atp2a1 (SERCA1 - A,B,E,F) and Atp2a2 (SERCA2a - C,D,G,H) mRNA expression from 4- and 12-week-old mdx (grey bars) and C57 control mice (white bars). N = 3–5 qPCR. Values expressed as mean ± SD. *p < 0.05.
Discussion
Duchenne muscular dystrophy (DMD) is the most common and lethal form of human muscle disease25. Even with intense ongoing research, there is yet no known cure or effective treatment for DMD. The mdx mouse with defective dystrophin expression is one of the most widely used animal models for DMD research5,6, but different from human DMD, mdx adults (12 wks) present a mild phenotype and a less severe disease course. Despite histological signs of muscle degeneration, necrosis and inflammation, most of the body muscles are efficiently regenerated in adult mdx mice5,6,11. Therefore, understanding the mechanisms by which 12-wk-old mdx muscles can efficiently regenerate, while human DMD muscles cannot, is of special importance in this field and can open new possibilities for DMD treatment and therapy.
Here, we focused our efforts on the study of mitochondrial function and its possible correlation with calcium deposits and SERCA expression in slow- and fast-twitch muscles from mdx mice at 12 wks.
Taken together, our results show decreased mitochondrial function (respiration), increased calcium deposits and increased Atp2a2 (SERCA2a) RNA expression in fast-twitch muscle (EDL) in the mdx mice at 12 wks. Conversely, slow-twitch muscle (soleus) from mdx mice at 12 wks did not show significant differences in mitochondrial function, presented more modest calcium deposits compared to mdx EDL muscle, and showed decreased Atp2a2 (SERCA2a) RNA expression when compared to control soleus muscle (12 wks). It is important to point out that despite the lack of alterations in mitochondrial respiration, a decrease in oxygen consumption associated with ADP phosphorylation capacity was found in mdx-derived fibers from soleus (12 wks).
The drugs and substrates that we used in High-Resolution Respirometry, and in the sequence that they were employed, allowed an overview of the most important mitochondrial parameters in respiration: respiration with substrates and ADP, coupled respiration fraction, proton leak respiration fraction and maximal respiration. In our study we used: (i) ADP and oligomycin, which interfere with ATP synthase/complex V activity; (ii) factors that directly interfere with the membrane potential, such as FCCP; and (iii) pyruvate + malate and succinate, which are substrates for complexes I and II, respectively. Complex I and complex II are commonly the main ports of entry for electrons in the mitochondrial respiratory chain and interruptions in their supply usually lead the oxygen consumption to zero. Although we focus our studies on complexes I and II, alterations in their function have a direct impact on the remaining members of the respiratory chain. Changes in ADP phosphorylation lead to alterations in the mitochondrial membrane potential, and as a result cause a reduction in the whole chain activity.
This is the first description of mitochondrial respiration in muscles from 12-wk-old mdx mice using multiple mitochondrial function parameters for the comparison of EDL (fast-twitch muscle) and soleus (slow-twitch muscle). It is important to point out that even with a pronounced reduction in mitochondrial respiration, fast-twitch muscles (EDL) from 12-wk-old mdx mice undergo a regeneration process11. One possible explanation comes from previous work showing that satellite cells from dystrophic mdx mice display accelerated differentiation26, and this process may overcome the mdx deficiency in mitochondrial function.
Different muscles from mdx mice present characteristic ultrastructure, physiology and pathology27, partly dependent upon fiber composition (slow- or fast-twitch fibers). EDL is a homogenous fast-contracting muscle and soleus is a slow-contracting fatigue-resistant muscle. Mouse muscles can present different physiologies depending on their specific anatomic location28: in the limbs, anterior muscles (EDL) are faster than posterior muscles (soleus). Fast-twitch muscles from dystrophic mdx mice are especially susceptible to contraction-induced injury29,30, which could contribute to an increased calcium influx in EDL from mdx. This may explain our results showing that the fast-twitch muscle EDL is more affected (than soleus) in mdx, with increased calcium deposits and reduced mitochondrial respiration. High calcium concentrations are necessary for the activation of proteases, such as calpains, thus suggesting that fast-twitch fibers (EDL) are more susceptible to calcium-dependent proteolysis than slow-twitch fibers (soleus).
It has been shown by different groups that mitochondria play a role in DMD9,10,14,15,16,17,18,19. Calcium entry owing to sarcolemmal tears caused by the fragility of the dystrophin-deficient sarcolemma and by the calcium leak channels results in calcium overload in the dystrophic muscle17. Mitochondria serve as a buffer for part of this calcium overload. This causes a decline in mitochondrial respiratory function which in turn affects muscle function. Interestingly, it has been shown that sarcolemmal injury causes mitochondria to cluster at the site of muscle repair31.
We also analyzed the expression of Atp2a1 (SERCA1) and Atp2a2 (SERCA2a) mRNAs in EDL and soleus muscles from control and mdx animals, aiming to correlate it with mitochondrial function and calcium deposits. It has been reported that DMD muscles present alterations in SERCA activity32,33,34, associated with higher cytoplasmic calcium levels and alterations in mitochondrial permeability transition pore formation35,36. Here we found an increase in Atp2a2 (SERCA2a) mRNA levels in EDL muscle from 12-wk mdx compared to age-matched controls, which may be correlated with the decrease in mitochondrial function and the increase in calcium deposits found in this muscle. While both EDL and soleus from mdx at 12 wks showed calcium deposits (compared to control), we found 3-fold more deposits in EDL than in soleus. We hypothesize that the abnormal calcium overload found in EDL muscles from mdx mice leads to an increase in SERCA2a expression to reestablish normal cytoplasmic calcium levels, since SERCA pumps transport calcium ions from the cytoplasm into the SR. In agreement with our results, Schertzer and colleagues (2008) found that transcript levels of SERCA2a are higher in EDL muscles from 20-week-old mdx mice compared with C57 control mice37.
Interestingly, a decrease in Atp2a1 (SERCA1) RNA expression was found in fast-twitch muscle (EDL) during the myonecrosis stage in the mdx mice (4 wks) compared to control, whereas no significant differences were found in its expression in EDL during the regeneration stage (12 wks). These results suggest a recovery of the expression of SERCA1, the main SERCA found in EDL muscle, during the regeneration stage of the disease.
In contrast with the observed increase in the expression of Atp2a2 (SERCA2a) in EDL from mdx at 12 wks (compared to control), there was a decrease in the expression of Atp2a2 (SERCA2a) at 12 wks in soleus muscles from mdx animals (compared to control). Furthermore, we found a significant decrease in the expression of Atp2a1 (SERCA1) in EDL from mdx mice at 4 wks, compared to the control at 4 wks; while no differences were detected in SERCA1 expression in soleus from mdx mice at 4 wks in relation to control. Since slow-twitch muscles (soleus) from dystrophic mdx mice are not especially susceptible to contraction-induced injury29,30 compared to fast-twitch muscles (EDL), our data suggest that the expression of SERCA1 and SERCA2a could be modulated in mdx muscles by the levels of calcium deposits and contraction-induced injuries. Furthermore, type I muscle fibers are less sensitive to the mdx injury29,30, thus the increase in SERCA2a expression in EDL from mdx mice at 12 wks could be associated with the regeneration process observed in the mdx mice at this age14.
It has been shown that different DMD muscles have different abilities to regulate calcium homeostasis38, and they also differ in mechanical properties that can offer resistance to damage39. Fast-twitch muscles are generally the most susceptible to muscular dystrophy and ageing in all species40. Our data are in accordance with the concept that the fast-twitch muscle EDL from mdx is more sensitive to alterations in calcium homeostasis than the slow-twitch muscle soleus.
Our work suggests a role for calcium in the regulation of mitochondrial respiration. It has been reported that increases in cytoplasmic calcium result in changes in the activity of mitochondrial dehydrogenases, such as FAD-glycerol phosphate dehydrogenase, pyruvate dehydrogenase phosphatase, NAD+-isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase41. The activity of mitochondrial dehydrogenases drives the respiratory chain and ATP synthesis.
Taken together, our data show that EDL from mdx mice at 12 wks is altered to a greater extent than soleus from mdx mice at 12 wks. These alterations include: increased calcium deposits, reduced mitochondrial activity, and increased expression of both SERCA1 and SERCA2a. These results show that fast-twitch muscles from mdx are more susceptible to injury than slow-twitch muscles.
Although our study did not answer the question of why 12-wk mdx muscles can efficiently regenerate while human DMD muscles cannot, we did find a decrease in the overall mitochondrial function in 12-wk mdx muscle, which is in contrast with data from human DMD muscles. A significantly higher activity of mitochondrial complex I was observed in patients with DMD42. The difference in the activity of complex I in DMD muscles from humans and mice could be related to the differences in their regenerative capacities. More studies are necessary to sort out the exact role of complex I in DMD pathophysiology in humans and in different animal models.
Our results show important physiological differences between fast (EDL) and slow-twitch (soleus) muscles from 12-wk-old mdx mice. Our data could have an important impact on the understanding of the pathophysiology of DMD.
Methods
Ethics statement
This study followed the principles of good laboratory animal care and experimentation in compliance with ethical recommendations in the guidelines of the Brazilian College for Animal Experimentation. The study protocol for handling of animals was approved (protocol number: CEPA 00174/09) by the Ethics Committee for Animal Research (Comitê de Ética em Pesquisa Animal, CEPA) of the Universidade Federal Fluminense (UFF, Niterói, Rio de Janeiro, Brazil).
Animal and muscle handling
Isogenic male 4 and 12 week-old-mice mdx dystrophic and age-matched C57BL/10J (C57) control non-dystrophic mice were kept in the animal housing facilities at the Institute of Biology in the Universidade Federal Fluminense (Rio de Janeiro, Brazil). Mice were housed in a ventilated rack (Alesco, São Paulo, Brazil) in autoclaved cages and kept at a constant 12 h/12 h light-dark cycle and temperature (24 °C) with free access to food and water. Mice were sacrificed by cervical dislocation and extensor digitorum longus (EDL) and soleus skeletal muscles were collected.
Alizarin Red staining
At least 4 pieces of soleus and EDL muscles from each mouse were fixed in 10% neutral buffered formalin and further processed into paraffin-embedded cassettes. Sections of 5 μm in thickness were placed on poly L-lysine-coated slides and stained with Alizarin Red S 2% (ARS) solution for 3 to 5 minutes. Then, sections were washed 5 times with acetone, acetone-xylol (1:1), and xylol, for 20 seconds each step and mounted in glycerol43. Images of calcium deposits birefringence were observed and acquired under bright-field and polarization microscopy on an Axiovert 100 microscope (Zeiss, Germany). Images of calcium deposits were acquired under polarized light microscopy since this contrast-enhancing technique improves the quality of the image obtained with birefringent materials such as calcium deposits. Quantification of the area occupied by calcium deposits from Alizarin Red S staining was performed using Image J software.
Acquisition of permeabilized skeletal muscle fibers
Nine independent experiments were carried out (one permeabilized fiber bundle for each group) with mdx dystrophic and age-matched C57 control non-dystrophic mice at 12 wks. Animals were subjected to euthanasia and EDL or soleus muscles from each group were harvested. Fiber bundles (~2 mg) were isolated in ice-cold BIOPS buffer (containing 2.77 mM CaK2EGTA, 7.23 mM K2EGTA, 20 mM imidazole, 20 mM taurine, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, 0.5 mM dithiothreitol, and 50 mM K-MES, pH 7.1) and permeabilized using saponin at 50 µg/mL according to an established protocol44. After 30 min of saponin exposure, muscle fibers were washed for 10 min in respiration medium (see details under oxygen consumption measurement) and then used for respirometry analysis.
Oxygen consumption measurement
O2 consumption experiments were conducted using a high-resolution Oxygraph-2k system (Oroboros Instruments GmbH, Innsbruck, Austria)23. The composition of respiration medium (MiR05) was: 110 mM sucrose, 60 mM K-MES, 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM K-HEPES, pH 7.145. Fibers were placed in the oxygraph chamber containing 2 mL of respiration medium and experiments were performed at 37 °C. Sequential addition of substrates and drugs to the indicated final concentrations was as follows: pyruvate/malate (5 mM/5 mM), ADP (3 mM), cytochrome c (10 µM), succinate (10 mM), oligomycin (3 μg/mL), carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone) (FCCP, 0.6–1 μM) and potassium cyanide (KCN, 10 mM). Oxygen consumption was allowed to stabilize before proceeding to a new substrate/drug injection. The software Datlab5 (Oroboros Instruments GmbH, Innsbruck, Austria) was used to extract specific values of oxygen flux from chosen intervals (the areas corresponding for the addition of substrates or drugs) to plot the oxygen consumption data.
qPCR
For the analysis of the expression of Atp2a1 (SERCA1) and Atp2a2 (SERCA2a) mRNA in EDL and soleus muscles from 4 and 12-week-old mdx and C57 control mice, total RNA was extracted using the TRIzol Reagent (Invitrogen, Carlsbad, USA) and Machery Nagel kit (Macherey Nagel, Düren, DE) according to manufacturers’ protocols. Briefly, the aqueous phase from the initial TRIzol protocol was transferred into the Machery Nagel blue column and followed downstream as in the manufacturer’s protocols. The cDNA synthesis was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) according to the manufacturer’s protocols with 750 ng total RNA for soleus and EDL. Followed cDNA synthesis, mRNA expressions were evaluated by qPCR using the HOT FIREPol® Evagreen® qPCR Supermix (Solis Biodyne, Denmark) in the Master Cycler Realplex system (Eppendorf, Germany). Primer pair sequences are shown in Table 1. Quantification of the samples’ mRNA expression was calculated from the standard curve method and their expression corrected by the geometric mean of the reference genes (for soleus: Rpl0, Pol2a, Ppib; for EDL: Rpl0, G6pdh, Hprt1). The best reference gene combinations were chosen according to their Cq values, using the smallest variance between the groups. The PCR program was as follows: denaturation 12 min 95 °C, 40 cycles of 15 sec 95 °C, 30 sec 60 °C, 30 sec 72 °C, following melting program. Quality of qPCR and genomic DNA contamination were checked using intron-spanning primers, reverse transcriptase-negative samples from cDNA synthesis and melting curve analysis obtained from each reaction.
Statistical analysis
Statistical analyses were performed using the GraphPad prism 7 software (GraphPad Software, Inc., San Diego, CA, USA), and to assess the level of statistical significance a nonparametric Student’s t-test (Mann-Whitney test) was used with p < 0.05. The results were expressed as mean values ± standard deviation (SD). For Serca1 and Serca2a qPCR data, differences between groups were analyzed using an unpaired, one-tailed t test. Outliers were identified by Grubbs’ test and excluded. Results are shown as mean values ± SD of at least 3 animals per group.
References
Allen, D. G., Gervasio, O. L., Yeung, E. W. & Whitehead, N. P. Calcium and the damage pathways in muscular dystrophy. Can. J. Physiol. Pharmacol. 88, 83–91 (2010).
Allen, D. G., Whitehead, N. P. & Froehner, S. C. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev. 96, 253–305 (2016).
Rodrigues, M., Echigoya, Y., Fukada, S.-I. & Yokota, T. Current translational research and murine models for Duchenne muscular dystrophy. J Neuromuscul Dis. 3, 29–48 (2016).
Yucel, N., Chang, A. C., Day, J. W., Rosenthal, N. & Blau, H. M. Humanizing the mdx mouse model of DMD: the long and the short of it. NPJ Regen Med. 3, 4 (2018).
McGreevy, J. W., Hakim, C. H., McIntosh, M. A. & Duan, D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis. Model Mech. 8, 195–213 (2015).
Duddy, W. et al. T. Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia. Skeletal Muscle. 5, 16 (2015).
Grounds, M. D. Two-tiered hypotheses for Duchenne muscular dystrophy. Cell Mol. Life Sci. 65, 1621–1625 (2008).
Bani, C. et al. Pattern of metalloprotease activity and myofiber regeneration in skeletal muscles of mdx mice. Muscle Nerve. 37, 583–592 (2008).
Kuznetsov, A. V. et al. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol. Cell Biochem. 183, 87–96 (1998).
Schuh, R. A., Jackson, K. C., Khairallah, R. J., Ward, C. W. & Spangenburg, E. E. Measuring mitochondrial respiration in intact single muscle fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R712–719 (2012).
Grounds, M. D., Radley, H. G., Lynch, G. S., Nagaraju, K. & De Luca, A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol. Dis. 31, 1–19 (2008).
Turk, R. et al. Muscle regeneration in dystrophin-deficient mdx mice studied by gene expression profiling. BMC Genomics. 6, 98–113 (2005).
Carnwath, J. W. & Shotton, D. M. Muscular dystrophy in the mdx mouse histopathology of the soleus and extensor longus muscles. J. Neurol. Sci. 80, 39–54 (1987).
Percival, J. M., Siegel, M. P., Knowels, G. & Marcinek, D. J. Defects in mitochondrial localization and ATP synthesis in the mdx mouse model of Duchenne muscular dystrophy are not alleviated by PDE5 inhibition. Hum. Mol. Genet. 22, 153–167 (2013).
Gannoun-Zaki, L. et al. Down-regulation of mitochondrial mRNAs in the mdx mouse model for Duchenne muscular dystrophy. FEBS Lett. 375, 268–272 (1995).
Kelly-Worden, M. & Thomas, E. Mitochondrial dysfunction in Duchenne muscular dystrophy. Open J. Endocr. Metab. Dis. 4, 211–218 (2014).
Vila, M. C. et al. Mitochondria mediate cell membrane repair and contribute to Duchenne muscular dystrophy. Cell Death Differ. 24, 330–342 (2017).
Rybalka, E., Timpani, C. A., Cooke, M. B., Williams, A. D. & Hayes, A. Defects in mitochondrial ATP synthesis in dystrophin-deficient mdx skeletal muscles may be caused by complex I insufficiency. Plos One. 9, e115763 (2014).
Lucas-Heron, B., Schmitt, N. & Ollivier, B. Muscular dystrophy: possible role of mitochondrial deficiency in muscle degeneration processes. J. Neurol. Sci. 95, 327–334 (1990).
Wada, E. et al. Dietary phosphorus overload aggravates the phenotype of the dystrophin-deficient mdx mouse. Am J Pathol. 184, 3094–3104 (2014).
Contreras, L., Drago, I., Zampese, E. & Pozzan, T. Mitochondria: the calcium connection. Biochim. Biophys. Acta. 1797, 607–618 (2010).
Chance, B. & Williams, G. R. Respiratory enzymes in oxidative phosphorylation: I. Kinetics of oxygen utilization. J. Biol. Chem. 217, 383–394 (1955).
Gnaiger, E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 128, 277–297 (2001).
Periasamy, M. & Kalyanasundaram, A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 35, 430–442 (2007).
Hoffman, E. P. & Dressman, D. Molecular pathophysiology and targeted therapeutics for muscular dystrophy. Trends Pharmacol. Sci. 22, 465–470 (2001).
Yablonka-Reuveni, Z. & Anderson, J. E. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev. Dyn. 235, 203–212 (2006).
Turner, P. R., Westwood, T., Regen, C. M. & Steinhardt, R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 335, 735–738 (1988).
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 (2011).
Moens, P., Baatsen, P. & Maréchal, G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J. Muscle Res. Cell Motil. 14, 446–451 (1993).
Consolino, C. M. & Brooks, S. V. Susceptibility to sarcomere injury induced by single stretches of maximally activated muscles of mdx mice. J. Appl. Physiol. 96, 633–638 (2004).
Sharma, N. et al. Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles. J. Biol. Chem. 287, 30455–30467 (2012).
Divet, A. & Huchet-Cadiou, C. Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflugers Arch. 444, 634–643 (2002).
Divet, A., Lompre, A. M. & Huchet-Cadiou, C. Effect of cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca-ATPase, on skeletal muscles from normal and mdx mice. Acta Physiol. Scand. 184, 173–186 (2005).
Kargacin, M. E. & Kargacin, G. J. The sarcoplasmic reticulum calcium pump is functionally altered in dystrophic muscle. Biochim. Biophys. Acta. 1290, 4–8 (1996).
Millay, D. P. et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 14, 442–447 (2008).
Alderton, J. M. & Steinhardt, R. A. How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc. Med. 10, 268–272 (2000).
Schertzer, J. D. et al. Muscle-specific overexpression of IGF-I improves E-C coupling in skeletal muscle fibers from dystrophic mdx mice. Am. J. Physiol. Cell Physiol. 294, C161–188 (2008).
Khurana, T. S. et al. Absence of extraocularmuscle pathology in Duchenne’s muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J. Exp. Med. 182, 467–475 (1995).
Wiesen, M. H., Bogdanovich, S., Agarkova, I., Perriard, J. C. & Khurana, T. S. Identification and characterization of layer-specific differences in extraocular muscle m-bands. Invest. Ophthalmol. Vis. Sci. 48, 1119–1127 (2007).
Lynch, G. S., Hinkle, R. T., Chamberlain, J. S., Brooks, S. V. & Faulkner, J. A. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J. Physiol. 535, 591–600 (2001).
Tarasov, A. I., Griffiths, E. J. & Ruttera, G. A. Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 52, 28–35 (2012).
Jongpiputvanich, S., Sueblinvong, T. & Norapucsunton, T. Mitochondrial respiratory chain dysfunction in various neuromuscular diseases. J Clin Neurosci. 12, 426–428 (2005).
McGee-Russell, S. Histochemical methods for calcium. J. Histochem. Cytochem. 6, 22–42 (1958).
Kuznetsov, A. V. et al. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 3, 965–976 (2008).
Gnaiger, E. et al. Mitochondria in the cold. Life in the cold (eds Heldmaier, G. & Klingenspor, M.), 431–442 (Springer, 2000).
Acknowledgements
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This work is part of RBG’s master thesis in the Programa de Pós-Graduação em Ciências Morfológicas (PCM/UFRJ). The authors would like to thank Dr Martha Sorenson (UFRJ) for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
A.T.S., C.M., F.F.B., M.L.C., R.B.G., T.M.O.C., T.Q.-S. and W.S.S. conceived the study, designed the experiments and participated in the interpretation of data; A.T.S., F.F.B. and R.B.G. performed the experiments; C.M., M.L.C. and T.Q.S. supervised the master’s thesis of R.B.G. and W.S.S. supervised the PhD thesis of A.T.S. All authors were responsible for writing the manuscript and they all approved its final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gaglianone, R.B., Santos, A.T., Bloise, F.F. et al. Reduced mitochondrial respiration and increased calcium deposits in the EDL muscle, but not in soleus, from 12-week-old dystrophic mdx mice. Sci Rep 9, 1986 (2019). https://doi.org/10.1038/s41598-019-38609-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38609-4
This article is cited by
-
LED therapy plus idebenone treatment targeting calcium and mitochondrial signaling pathways in dystrophic muscle cells
Cell Stress and Chaperones (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.