Abstract
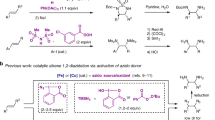
A green, facile and tunable pair electrochemical process was developed for the synthesis of new benzenesulfonamide derivatives by using reductive controlled potential electrolysis of dinitrobenzene (DNB) in the presence of arylsulfinic acids (ASAs). In addition to the usual features associated with paired electrochemical methods, eg high energy efficient, this method has a tunable characteristic, so that, by adjusting the potential, different products can be synthesized. By applying the potential of −0.4 V vs. Ag/AgCl, N-hydroxy-N-(4-nitrophenyl)benzenesulfonamide derivatives are selectively formed, while, by applying the potential of −1.1 V vs. Ag/AgCl, the final products are N-(4-amino-3-(phenylsulfonyl)phenyl) benzenesulfonamide derivatives. This work beautifully shows the potential applications of the electrochemistry as a powerful tool for the synthesis of organic compounds.
Similar content being viewed by others
Introduction
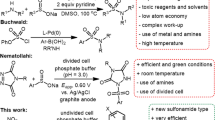
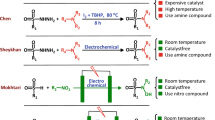
Sulfonamides are an important class of organic compounds with antibacterial activity1 toward gut infections, Pneumocystis jirovecii pneumonia, urinary tract infections, mucous membrane, toxoplasma encephalitis, Kaposi sarcoma herpes virus infection and isospora infections in HIV infection2,3. These antibiotics also used in the treatment of various types of animal diseases4. On the other hand, antibiotic drug resistance is an irrefutable fact that creates a significant problem in public health and is an obstacle to a successful treatment of diseases5,6. These findings indicate that the efforts must be directed toward the synthesis of more potent antibiotics. Accordingly, intense drug discovery programs have led to the synthesis of new sulfonamide derivatives7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. The direct synthesis of N-arylsulfonamides via the formation of N-S band is the most general approach7,8,9,10. Although these methods are efficient, they have two major drawbacks which limit their usefulness. Firstly, they use aromatic amines which are carcinogens11 and secondly, they lead to impure products. Other important methods for the synthesis of N-arylsulfonamide derivatives is the reaction of sulfonamides as nucleophiles with some organic compounds such as halides12,13,14,15, alcohols16,17,18,19,20,21, aryl esters22,23,24 and arylboronicacids25,26,27,28. In these methods although the problem of using aniline has been solved, but some disadvantages such as harsh reaction conditions, tedious workup, using metal catalysts, bases and/or ligands accompany these methods. In another efficient strategy, N-arylsulfonamides were synthesized by the reaction of sulfonyl azides or hydrazides with benzoic acids29 or arylboronic acids30. The main problem of these methods is the use of metal ions such as iridium, copper and palladium which pollute the environment. In this context, we synthesized some new sulfonamide derivatives, via electrochemical oxidation of anilines31,32,33, urazoles34,35, nitroso aromatic compounds36,37,38 and 1,2-dihydropyridazine-3,6-dione39 in the presence of sulfone derivatives. These methods despite their significant advantages over the traditional methods, have used aniline and similar compounds as starting materials. In other efficient methods, nitroarenes have been used instead of anilines in the reaction with arylsulfonyl hydrazides40 and sodium arylsulfinates41. Metal catalysts, unsafe solvents, tedious workup and harsh reaction conditions are disadventages associated with these methods.
In this context, we recently reported the synthesis of some new sulfonamide derivatives, using simple nitroarenes and sodium arylsulfinates as starting materials, under green conditions42. In this study, to extend the scope of our previous work42, we report the synthesis of new benzenesulfonamide derivatives by using the reductive controlled potential electrolysis of dinitrobenzene in the presence of arylsulfinic acids sodium salt. The most important features of this method are safe starting materials, catalyst free condition, safe solvent and easy workup. In addition, the unique features of this method compared to our recent paper42, is its tunable nature for the synthesis of products. In this approach, different products can be obtained just by changing the applied potential.
Results and Discussion
Electrochemical reduction of p-dinitrobenzene (DNB)
We report here the electrochemical behavior of DNB. The cyclic voltammograms of DNB (1.0 mM) at glassy carbon electrode, in water solution containing phosphate buffer (c = 0.2 M, pH = 3.5) at scan rate, 100 mV s−1 are shown in Fig. 1. The presence of two nitro groups has caused the creation of two cathodic peaks (CN1 and CN2). The electrochemical behaviour of DNB clearly depends on the switching potential and scan potential direction, so when the potential was scanned from 0.00 to + 0.80 V vs. Ag/AgCl and back, no anodic or cathodic peaks were observed in the sweeping area. However, when the potential direction was reversed and scanned in the negative potential direction (−0.60 V), a well-defined irreversible cathodic peak (CN1) is observed at −0.29 V/Ag/AgCl, correspond to the reduction of one of the nitro groups of DNB to hydroxylamine group. Under these conditions, in the reverse scan, an anodic peak, A1 (EpA1 = 0.42 V), ascribed to the oxidation of N-(4-nitrophenyl)hydroxylamine (NHA) to 1-nitro-4-nitrosobenzene (NNB) and its cathodic counterpart (C1) which is correspond to the reduction of NNB to NHA were observed (Fig. 2)42,43.
However, when the switching potential was changed to −1.1 V/Ag/AgCl, both nitro groups reduced to hydroxylamine groups produces N,N′-(1,4-phenylene)bis(hydroxylamine) (BHA). Under these conditions, in the reverse scan, two successive oxidation processes, makes the anodic peaks A2 and A3. These peaks are related to the oxidation of BHA to N-(4-nitrosophenyl) hydroxylamine (NHA) (peak A2) and oxidation of NHA to 1,4-dinitrosobenzene (DNSB) (peak A3), respectively. Obviously, the cathodic peaks C3 and C2, are related to the reduction of DNSB to NHA and reduction of NHA to BHA (Fig. 2).
Cyclic voltammograms of 1.0 mM of DNB at glassy carbon electrode, in water (with different pH values and same ionic strength)/ethanol (80/20, v/v) mixture. pHs from (a) to (d) are: 2.0, 3.1, 4.5 and 6.5. Scan rate: 100 mV s−1. Temperature: 25 ± 1 °C. Inset: the potential-pH diagram for BHA/NHA (redox couple A2/C2) and NHA/DNSB (redox couple A3/C3).
In addition, we have found that, the change of potential scan rate has no significant effect on the cyclic voltammograms DNB in the range of 10–100 mV s−1(see, Supporting Information). The effect of pH on the cyclic voltammogram of DNB is shown in Fig. 3. As can be seen, all anodic and cathodic peaks are pH-dependent and shift negatively with the increase of pH. The potential-pH diagrams for BHA/NHA (redox couple A2/C2) and NHA/DNSB (redox couple A3/C3) are shown in Fig. 3, inset. As can be seen, in the studied pH range (2.0–6.5), both lines have a slope of ∼60 mV which are consistent with the two-electron/two-proton processes.
The effect of benzenesulfinic acid (BSA) on the cyclic voltammogram of DNB is shown in Fig. 4b. The comparison of this voltammogram with the voltammogram of DNB in the absence of BSA (Fig. 4a), shows three significant changes. (1) The appearance of a new anodic peak (As) at 0.44 V/Ag/AgCl. (2) The disappearance of the cathodic peaks C2 and C3 and (3) the appearance of a new cathodic peak (Cs) at −0.60 V/Ag/AgCl. The disappearance of the cathodic peaks C2 and C3 are evidence that the nitroso compounds NHA and DNSB are removed from the electrode surface by reaction with BSA. In addition, the appearance of the new anodic and cathodic peaks As and Cs confirms the formation of an organic compound with the oxidation potential more than DNB.
The effect of potential scan rate on the cyclic voltammograms of DNB in the presence of BSA is shown in Fig. 5. The significant change is the reappearance of C2 and C3 peaks at higher potential scan rates. When the potential scan rate increases, the reaction time for the reaction of BSA with electrochemically generated NHA and DNSB decreased so that, the unreacted NHA and DNSB produce cathodic peaks C2 and C3 in the reverse scan.
Since DNB have two cathodic peaks, controlled-potential method was used for its selective reduction. Two electrolysis experiments were carried out in the presence of ASAs, at cathodic potentials of −0.4 V and −1.1 V vs. Ag/AgCl, respectively and electrolysis progress was monitored by cyclic voltammetry (Fig. 6). At cathodic potential of −0.4 V, the current of cathodic peak CN1 (IpCN1) decreased with the charge passed and finally disappeared when the charge passed was about 4e− per molecule of DNB. Under these conditions, IpCN2 does not change. At cathodic potential of −1.1 V, however, both cathodic peak currents, IpCN1 and IpCN2 decreased with the charge passed and disappeared when the charge passed was about 12e− per molecule of DNB.
Cyclic voltammograms of DNB during controlled-potential coulometry: I) DNB (1.0 mmol) in the presence of BSA (1.0 mmol) at −0.4. II) DNB (2.0 mmol) in the presence of BSA (4.0 mmol) at −1.1 V. Working electrode; glassy carbon. Solvent; aqueous solution containing phosphate buffer (c = 0.2 M, pH = 3.5). Scan rate: 100 mV s−1; Temperature: 25 ± 1 °C.
The reaction scheme for the synthesis of benzenesulfonamides SA and DS is given in Figs 7 and 8. Holding the electrode potential at −0.4 V vs. Ag/AgCl is the essential condition for the synthesis of the benzenesulfonamides SA1-SA3 (Fig. 7). However, when the electrode potential reached −1.1 V/Ag/AgCl, the main product, benzenesulfonamides NS1-NS3 are produced after reduction of DNB to p-diaminobenzene (Fig. 8). As shown in Fig. 7, the cathodically formed NHA is oxidized at the anode to 1-nitro-4-nitrosobenzene (NNB). The reaction of NNB with ASAs as a nucleophile produces the benzenesulfonamides SA1-SA3.
Figure 8, shows the possible mechanistic pathway for the synthesis of NS derivatives. When the applied potential is −1.1 V/Ag/AgCl, two nitro groups after consumption of 12e− per molecule of DNB, converts to amine groups. In the next stage, the electrochemically generated 1,4-diaminobenzene (DAB), at the anode after passing 2e−, is converted to p-quinonediimine (QDI)44,45. The reaction of ASAs with QDI, along with aromatization, leading to sulfonamide Int1 (or Int2)31. It should be noted that QDI can be attacked by ASAs either form C or N atoms to form two types of intermediates (Int1 or Int2). Another oxidation step along with a chemical reaction converts Int1 (or Int2) to N-arylsulfonamide derivatives (NS1-NS3) as the final products. According to Fig. 8, the anodic peak As (Fig. 4) is related to the oxidation of Int1 (or Int2) to the corresponding quinonediimine (Int3) (or Int4). The presence of an electron withdrawing sulfone group in the structure of Int1 (or Int2) makes its oxidation harder than that of BHA (peak A2) and NHA (peak A3).
Conclusions
The results of this work have three important implications. (a) This work has led to the synthesis of new benzenesulfonamide derivatives that may have medicinal applications. (b) This work has used a simple cell and electrodes, safe starting materials, catalyst free condition, safe solvent, easy workup room temperature conditions and low energy consumption (because of pair strategy) for the synthesis of title compounds SA1-SA3 and NS1-NS3.(c) This work reports a tunable electrochemical method for the synthesis of SA1-SA3 and NS1-NS3. In this method, the applied potential for the synthesis of benzenesulfonamides SA1-SA3, is −0.4 V, however benzenesulfonamides NS1-NS3 can be synthesized only by changing the applied potential from −0.4 V to −1.1 V/Ag/AgCl.
Materials and Methods
Apparatus and Reagents
All voltammetric and coulometric experiments were done using an Autolab model PGSTAT 20 potentiostat/galvanostat. A glassy carbon disc (1.8 mm diameter) and a platinum wire were used as the working and counter electrodes, respectively. The glassy carbon electrode was polished using alumina slurry followed by washing with water and acetone. The reference electrode used was Ag/AgCl (saturated KCl). All electrodes are prepared from AZAR Electrodes. These electrodes are used in voltammetric experiments. An assembly of two carbon plates (each one, 10 cm lenght, 7 cm width and 1 cm thickness) were used as the anode and cathode in controlled-potential coulometry.
p-Dinitrobenzene (DNB), arylsulfinic acids sodium salt (ASAs), phosphate, acetate salts and ethanol were obtained from commercial sources and were used without further purification. The purity of products has been checked by TLC, and characterization has been done using 1H NMR, 13C NMR, IR spectroscopic techniques and mass spectrometry.
Electroorganic synthesis of benzenesulfonamide derivatives
Electroorganic synthesis of SA and NS derivatives were performed under controlled-potential conditions. In a typical procedure, a solution (80 mL) of water (phosphate buffer, pH = 3.5, c = 0.2 M)/ethanol mixture (80/20, v/v) containing p-dinitrobenzene (DNB) and arylsulfinic acids sodium salt (ASAs) was electrolyzed at optimum conditions according (Table 1).
The electrolysis was terminated when the decay of the current became more than 95%. Since, the products are insoluble in water (phosphate buffer, pH = 3.5, c = 0.2 M)/ethanol mixture (80/20, v/v), separation is carried out only by filtration. The collected solids were washed on the filter with distilled water (several times).
N-hydroxy-N-(4-nitrophenyl)benzenesulfonamide (SA1) (Fig. 9). Isolated yield: 76%. Mp = 139–142 °C. IR (KBr, cm−1): 3336, 3115, 1614, 1591, 1520, 1448, 1348, 1178, 1161, 858, 755, 689, 609. 1H NMR, δ ppm (400 MHz, acetone-d6): 7.48 (d, J = 11.5 Hz, 2 H), 7.51–7.58 (m, 4H), 7.71–7.74 (m, 1H), 8.18 (d, J = 11.0 Hz, 2H), 10.7 (s, 1H, OH). 13C NMR, δ ppm (100 MHz, acetone-d6): 122.1, 123.8. 128.8, 129.3, 131.1, 132.9, 134.4, 145.8, 148.4, 148.5. MS (EI) m/z (%): 77 (100), 141 (87), 278 (27), 294 [44, (M+)].
N-hydroxy-4-methyl-N-(4-nitrophenyl)benzenesulfonamide (SA2) (Fig. 10). Isolated yield: 73%. Mp = 137–139 °C. 1H NMR (400 MHz, DMSO-d6): δ ppm 2.37 (s, 3H, CH3), 7.37 (d, J = 3.6 Hz, 3H), 7.44 (t, J = 6 Hz, 2H), 7.68–7.71 (m, 1H), 8.20 (d, J = 9.2 Hz, 2H), 11.49 (d, J = 3.2 Hz, 1H, OH). 13C NMR (100 MHz, DMSO-d6): δ ppm 21.06 CH3, 122.0, 124.1, 128.6, 129.0, 129.5, 131.5, 131.7, 145.0, 145.2, 148.3. IR (KBr): 3346, 2959, 2930, 1611, 1522, 1344, 1289, 1166, 857, 669, 556 cm−1. MS (EI, 70 eV) m/z (relative intensity) 77 (100), 141 (31), 278 (26), 308 [50, (M+)].
4-Chloro-N-hydroxy-N-(4-nitrophenyl)benzenesulfonamide (SA3) (Fig. 11). Isolated yield: 78%. Mp = 142–143 °C. IR (KBr, cm−1): 3404, 1609, 1522, 1589, 1522, 1347, 1183, 1169, 762, 588. 1H NMR, δ ppm (400 MHz, acetone-d6): 7.50 (d, J = 9.2 Hz, 2H), 7.55–7.60 (m, 4H), 8.20 (d, J = 9.2 Hz, 2H), 10.86 (s, 1H, OH). 13C NMR, δ ppm (100 MHz, acetone-d6): 122.3, 124.0, 128.8, 129.1, 131.0, 131.4, 140.4, 145.9, 148.1, 148.2. MS (EI) m/z (%): 75 (100), 149 (65), 279 (5), 328 [30, (M+)].
N-(4-amino-3-(phenylsulfonyl)phenyl)benzenesulfonamide (NS1) (Fig. 12). Isolated yield: 60%. Mp = 174–176 °C. (Lit. 175–176 °C)46. IR (KBr, cm−1): 3466, 3360, 1623, 1499, 1466, 1143, 800, 734, 607. 1H NMR, δ ppm (400 MHz, DMSO-d6): 4.22 (s, 1H, NH), 5.68 (s, 1H, NH2), 6.02 (s, 1H), 6.52 (s, 1H), 6.71 (t, J = 16.4 Hz, 1H), 7.19 (s, 1H), 7.35 (s, 1H), 7.68–7.79 (m, 6H), 7.94 (d, J = 10.8 Hz 2H). 13C NMR, δ ppm (125 MHz DMSO-d6): 117.6, 119.0, 121.0, 125.1, 126.1, 126.6, 128.8, 129.1, 129.5, 130.4, 132.0, 133.9, 139.4, 140.2. MS (EI) m/z (%): 77 (100), 110 (80), 149 (86), 170 (20), 279 (6), 387[20, (M+ − 1)].
N-(4-amino-3-tosylphenyl)-4-methylbenzenesulfonamide) (NS2) (Fig. 13). Isolated yield: 68%. Mp = 172–175 °C.IR (KBr): 3446, 3365, 2958, 1728, 1500, 1285, 1140, 658, 589 cm−1. 1H NMR (400 MHz, DMSO-d6): δ ppm 2.38 (s, 6H, CH3), 4.20 (s, 1H, NH), 5.65 (s, 1H, NH2), 6.62 (s, 1H), 6.64–6.81 (m, 2H), 7.11 (d, J = 10.4 Hz, 1H), 7.37 (d, J = 7.6 Hz, 3H), 7.65–7.85 (m, 4H). 13C NMR (100 MHz, acetone-d6): δ ppm 20.52 CH3, 113.1, 117.3, 118.1, 118.9, 120.2, 123.1, 124.9, 126.8, 127.7, 128.3, 128.9, 129.2, 129.4, 131.1.MS (EI) m/z (%): 77 (100), 141 (27), 278 (15), 418[12, (M+ + 2)].
N-(4-amino-3-(4-chlorophenylsulfonyl)phenyl)-4-chlorobenzenesulfonamide (NS3) (Fig. 14). Isolated yield: 66%. Mp = 165–168 °C. IR (KBr): 3460, 3376, 1628, 1500, 1315, 1145, 757, 629, 557 cm−1. 1H NMR (400 MHz, acetone-d6): δ ppm 7.30 (t, J = 7.6 Hz, 1H), 7.35–7.40 (m, 3H), 7.45–7.54 (m, 4H), 7.57 (d, J = 8.0 Hz, 2H), 7.64 (d, J = 8.0 Hz, 2H), 7.76–7.81 (m, 2H). 13C NMR (100 MHz, acetone-d6): δ ppm 118.8, 124.3, 126.1, 128.2, 128.3, 128.8, 129.3, 130.6, 131.0, 132.6, 132.7, 134.0, 136.2, 142.1.MS (EI) m/z (%): 51 (36), 77 (100), 141 (87), 155 (48), 278 (31), 292(12), 455 [25, (M+ – 1)].
References
Patrick, G. In An Introduction to Medicinal Chemistry. 2nd ed (Oxford University press, 2001).
Wong, Y. Y., Johnson, B., Friedrich, C. T. & Lauren, T. A. Trepania, Hepatic expression profiles in retroviral infection: relevance to drug hypersensitivity risk. Pharma Res. Per. 5, 1–16 (2017).
Angius, F. et al. Antimicrobial sulfonamides clear latent Kaposi sarcoma herpesvirus infection and impair MDM2–p53 complex formation. J. Antibiot. 70, 962–966 (2017).
Riviere, J. E. & Papich, M. G. Veterinary Pharmacology and Therapeutics 9th ed (Wiley-Blackwell, 2009).
Sköld, O. Resistance to trimethoprim and sulfonamides. Vet. Res. 32, 261–273 (2001).
Takahashi, T. et al. Relationship between mutations in dihydropteroate synthase of Pneumocystis carinii f. sp. hominis isolates in Japan and resistance to sulfonamide therapy. J. Clin. Microbiol. 38, 3161–3164 (2000).
Tang, X. D. et al. Copper-catalyzed sulfonamides formation from sodium sulfinates and amines. Chem. Commun. 49, 6102–6104 (2013).
Caddick, S. J., Wilden, D. & Judd, D. B. Direct synthesis of sulfonamides and activated sulfonate esters from sulfonic acids. J. Am. Chem. Soc. 126, 1024–1025 (2004).
Nishikawa, M., Inaba, Y. & Furukawa, M. Behavior of sulfinic acid toward N-chloramines and related compounds. Chem. Pharm. Bull. 31, 1374–1377 (1983).
DeBergh, J. R., Niljianskul, N. S. & Buchwald, L. Synthesis of aryl sulfonamides via palladium-catalyzed chlorosulfonylation of arylboronic acids. J. Am. Chem. Soc. 135, 10638–10641 (2013).
Skipper, P. L., Kim, M. Y., Sun, H. L. P., Wogan, G. N. & Tannenbaum, S. R. Behavior of sulfinic acid toward N-chloramines and related compounds. Carcinogenesis 31, 50–58 (2010).
Yin, J. & Buchwald, S. L. Synthesis of aryl sulfonamides via palladium-catalyzed chlorosulfonylation of arylboronic acids. J. Am. Chem. Soc. 124, 6043–6048 (2002).
Baffoe, J., Hoe, M. Y. & Touré, B. B. Copper-mediated N-heteroarylation of primary sulfonamides: Synthesis of mono-n-heteroaryl sulfonamides. Org. Lett. 12, 1532–1535 (2010).
Rosen, B. R., Ruble, J. C., Beauchamp, T. J. & Navarro, A. Mild Pd-catalyzed N-arylation of methanesulfonamide and related nucleophiles: avoiding potentially genotoxic reagents and byproducts. Org. Lett. 13, 2564–2567 (2011).
Tan, B. Y. H., Teo, Y. C. & Seow, A. H. Low catalyst loadings for ligand‐free copper (I)‐oxide‐catalyzed N‐arylation of methanesulfonamide in water. Eur. J. Org. Chem. 2014, 1541–1546 (2014).
Qin, H., Yamagiwa, N., Matsunaga, S. & Shibasaki, M. Bismuth‐catalyzed direct substitution of the hydroxy group in alcohols with sulfonamides, carbamates, and carboxamides. Angew. Chem. 119, 413–417 (2007).
Shi, F. et al. Green and efficient synthesis of sulfonamides catalyzed by nano-Ru/Fe3O4. J. Am. Chem. Soc. 131, 1775–1779 (2009).
Shi, F. et al. Copper‐catalyzed alkylation of sulfonamides with alcohols. Angew. Chem. 121, 6026–6029 (2009).
Watson, A. J., Maxwell, A. C. & Williams, J. M. Borrowing hydrogen methodology for amine synthesis under solvent-free microwave conditions. J. Org. Chem. 76, 2328–2331 (2011).
Yu, X., Liu, C., Jiang, L. & Xu, Q. Manganese dioxide catalyzed N-alkylation of sulfonamides and amines with alcohols under air. Org. Lett. 13, 6184–6187 (2011).
Qu, P., Sun, C., Ma, J. & Li, F. The N‐alkylation of sulfonamides with alcohols in water catalyzed by the water‐soluble iridium complex {Cp* Ir [6, 6′‐(OH) 2bpy](H2O)}[OTf] 2. Adv. Synth. Catal. 356, 447–459 (2014).
Hicks, J. D., Hyde, A. M., Cuezva, A. M. & Buchwald, S. L. Pd-catalyzed N-arylation of secondary acyclic amides: catalyst development, scope, and computational study. J. Am. Chem. Soc. 131, 16720–16734 (2009).
Shekhar, S., Dunn, T. B., Kotecki, B. J., Montavon, D. K. & Cullen, S. C. A general method for palladium-catalyzed reactions of primary sulfonamides with aryl nonaflates. J. Org. Chem. 76, 4552–4563 (2011).
Lam, P. Y. S., Vincent, G., Clark, C. G., Deudon, S. & Jadhav, P. K. Copper-catalyzed general C=N and C=O bond cross-coupling with arylboronic acid. Tetrahedron Lett. 42, 3415–3418 (2001).
Pan, C., Cheng, J., Wu, H., Ding, J. & Liu, M. Cu(OAc)2-catalyzed N-arylation of sulfonamides with arylboronic acids or trimethoxy(phenyl)silane. Synth.Commun. 39, 2082–2092 (2009).
Moon, S. Y., Nam, J., Rathwell, K. & Kim, W. S. Copper-catalyzed Chan–Lam coupling between sulfonylazides and boronic acids at room temperature. Org. Lett. 16, 338–341 (2014).
Ouyang, B. et al. Synthesis of N-arylsulfonamides by a copper-catalyzed reaction of chloramine-T and arylboronic acids at room temperature. Synlett. 29, 111–115 (2018).
Loukrakpam, D. C. & Phukan, P. CuI catalyzed sulfamidation of arylboronic acid using TsNBr2 at room temperature. Tetrahedron Lett. 58, 4855–4858 (2017).
Lee, D. & Chang, S. Direct C-H amidation of benzoic acids to introduce meta‐and para‐amino groups by tandem decarboxylation. Chem. Eur. J. 21, 5364–5368 (2015).
You, C., Yao, F., Yan, T. & Mingzhong, C. A highly efficient heterogeneous copper-catalyzed Chan–Lam coupling reaction of sulfonylazides with arylboronic acids leading to N-arylsulfonamides. RSC Adv. 6, 43605–43612 (2016).
Nematollahi., D. & Maleki, A. Electrochemical oxidaton of N,N-dialkyl-p-phenylenediamines in the presence of arylsulfinic acids. An efficient method for the synthesis of new sulfonamide. Electrochem. Commun. 11, 488–491 (2009).
Beiginejad, H. & Nematollahi, D. Electrochemical synthesis of sulfonamide derivatives based on the oxidation of 2,5-diethoxy-4-morpholinoaniline in the presence of arylsulfinicacids. J. Org. Chem. 79, 6326–6329 (2014).
Nematollahi, D., Khazalpour, S., Ranjbar, M. & Momeni, S. A. green strategy for the synthesis of sulfone derivatives of p-methylaminophenol: Kinetic evaluation and antibacterial susceptibility. Sci. Rep. 7, 1–11 (2017).
Varmaghani, F., Nematollahi, D., Mallakpour, S. & Esmaili, R. Electrochemical oxidation of 4-substituted urazoles in the presence of arylsulfinic acids: an efficient method for the synthesis of new sulfonamide derivatives. Green Chem. 14, 963–967 (2012).
Varmaghani, F., Nematollahi, D. & Mallakpour, S. Electrochemical oxidative coupling of hexamethylene-bis-urazole and arylsulfinic acids: synthesis of bis-sulfonamide derivatives. J. Electrochem. Lett. 1, H14–H16 (2012).
Khazalpour, S. & Nematollahi, D. Electrochemical and chemical synthesis of different types of sulfonamide derivatives of N,N-dimethyl-1,4-benzenediamine using 4-nitroso-N,N-dimethylaniline. Green Chem. 17, 3508–3514 (2015).
Khazalpour, S., Nematollahi, D., Ahmad, A. & Dowlati, B. Electroreductive nucleophile acceptor generation. Electrochemical synthesis of N-(4-(dimethylamino) phenyl) benzenesulfonamide. Electrochim. Acta 180, 909–913 (2015).
Nematollahi, D., Namdar, A. & Momeni, Sh. Cyclic voltammetry-assisted mechanistic evaluation of sulfonamide synthesis. A simple and green method for the synthesis of N-(1-hydroxynaphthalen-2-yl). J. Electroanal. Chem. 810, 161–170 (2018).
Nematollahi, D., Varmaghani, F. & Saremi, M. Electrochemical oxidation of 1,2-dihydropyridazine-3,6-dione in the presence of arylsulfinic acids: a green method for the synthesis of new sulfonamides. J. Electrochem. Soc. 160, G93–G95 (2013).
Zhaoa, F., Li, B., Huanga, H. & Deng, G. J. Palladium-catalyzed N-arylsulfonamide formation from arylsulfonylhydrazides and nitroarenes. RSC Adv. 6, 13010–13013 (2016).
Zhang, W., Xie, J., Rao, B. & Luo, M. Iron-catalyzed N-arylsulfonamide formation through directly using nitroarenes as nitrogen sources. J. Org. Chem. 80, 3504–3511 (2015).
Mokhtari, B., Nematollahi, D. & Salehzadeh, H. Paired electrochemical conversion of nitroarenes to sulfonamides, diarylsulfones and bis (arylsulfonyl) aminophenols. Green Chem. 20, 1499–1505 (2018).
Cougnon, C., Gohier, F., Bélanger, D. & Mauzeroll, J. In situ formation of diazonium salts from nitro precursors for scanning electrochemical microscopy patterning of surfaces. Angew. Chem. 121, 4066–4068 (2009).
Maleki, A. & Nematollahi, D. Electrochemical synthesis and mechanestic study of quinone imines exploiting the dual character of N,N-dialkyl-p-phenylenediamines. Org. Lett. 13, 1928–1931 (2011).
Maleki, A. & Nematollahi, D. An efficient electrochemical method for the synthesis of methylene blue. Electrochem. Commun. 11, 2261–2264 (2009).
Adams, R. & Samuels, W. P. J. R. Quinone imides. XXXVIII. Adducts of p-quinonedimethanesulfonimide and their hydrolysis products. J. Am. Chem. Soc. 77, 5383–5385 (1955).
Acknowledgements
We acknowledge the Bu-Ali Sina University Research Council and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for their support of this work.
Author information
Authors and Affiliations
Contributions
B.M. and D.N. and H.S. conceived and designed the study. B.M. did the experiments. B.M. and D.N. wrote the manuscript. D.N. directed the research.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mokhtari, B., Nematollahi, D. & Salehzadeh, H. A tunable pair electrochemical strategy for the synthesis of new benzenesulfonamide derivatives. Sci Rep 9, 4537 (2019). https://doi.org/10.1038/s41598-019-38544-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38544-4
This article is cited by
-
A new type of convergent paired electrochemical synthesis of sulfonamides under green and catalyst-free conditions
Scientific Reports (2023)
-
A new electrochemical strategy for the synthesis of a new type of sulfonamide derivatives
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.