Abstract
Tuleariocaris holthuisi and Arete indicus are two ectocommensal shrimps closely associated with the tropical sea urchin Echinometra mathaei. This study provides a comparison of these two E. mathaei symbiotic crustaceans and particularly focuses on the relationship between T. holthuisi and its host’s pigments (i.e. spinochromes), and its dependency on its host. While all the analyses underline a close association between A. indicus and E. mathaei, they reveal a particularly close interaction between T. holthuisi and its host. Chemical analyses reveal that these shrimps present the same spinochrome composition as E. mathaei, and have similar colouration, allowing camouflage. Isotopic composition and pigment loss after host separation suggest that these pigments are certainly assimilated upon feeding on the urchin. Moreover, symbiont isolation experiments demonstrate the high dependency of T. holthuisi on its host and the importance of the host’s pigments on their survival capacity. Finally, some host recognition mechanisms are investigated for T. holthuisi and show the probable implication of spinochromes in host selection, through chemical recognition. Hence, all the results suggest the essential roles of spinochromes for T. holthuisi, which, in turn, suggests the potential implication of these pigments in the shrimps’ metabolism.
Similar content being viewed by others
Introduction
Symbioses are intimate associations between two heterospecific organisms (commonly called symbiont and host) that are generally classified into three categories: parasitism, commensalism and mutualism (these categories are themselves split into subclasses)1,2,3. According to the degree of dependence, symbioses are facultative or obligate, and the range of host-specificity of a symbiont varies significantly from one species to another. The evolutionary advantage of host-specificity for symbionts is to ensure they live in the appropriate habitat, but the symbiont can also be metabolically dependent on the host. This dependence is well known for many parasites (e.g. internal parasites, such as nematodes and trematodes) where the life cycle cannot be completed without the hosts4. It is also demonstrated in mutualists (e.g. the Symbiodinium algae living in corals) where nutrient exchange occurs5. The fine processes involved in the host-dependence of ectocommensals are, however, not well understood. It is apparent that, when doing a symbiont-host survey, some ectocommensals appear to be host-specific6 while others are more opportunistic7.
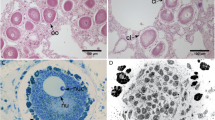
Shrimps are involved in many marine symbioses with varied taxa8. Some shrimp families, like Pontoniine shrimps (Palaemonidae), have up to 60 to 70% of their representatives that live associated with marine organisms like corals, ascidians, gorgonians, sponges or echinoderms9. Among the echinoderms, the sea urchin Echinometra mathaei (Blainville, 1825), which is widespread in the Indo-Pacific Ocean, is the host for two crustacean symbionts: Tuleariocaris holthuisi Hipeau-Jacquotte, 1965 (Palaemonidae) (Fig. 1B,C) and Arete indicus Coutière, 1903 (Alpheidae) (Fig. 1A) (Cimino and Ghiselin 2001; Hipeau-Jacquotte 1965; Gherardi 1991). A. indicus is found on 21% of E. mathaei throughout the Eilat lagoon (Red Sea)6, however, they do not show any evidence of host tissue ingestion6. Omnivorous food habits have been reported in four other species of Arete associated with Japanese sea urchins10. A. indicus is able to recognise the odour produced by E. mathaei, and a conspecific sea urchin Diadema setosum, in their olfactometric systems6. Gherardi (1991) also explored the effects of the shrimps’ separation from the sea urchin host and found that A. indicus is affected by two weeks of isolation, with isolated shrimps being smaller (around 20%) and paler than the controls remaining on the host. Tuleariocaris is a small genus of four shrimp species, with the T. holthuisi type species described the first time in Toliara in Madagascar, where the field work of the present study took place. Unlike A. indicus, the symbiotic relations between T. holthuisi and E. mathaei have not yet been described, and, to date, only the taxonomy has been described for the Tuleariocaris species11,12.
The two symbiotic shrimps, A. indicus and T. holthuisi were common on the E. mathaei sampled in Toliara (Madagascar), and both had a dark colouration, similar to the most common E. mathaei morphotype. T. holthuisi is, however, much more mimetic, and can be visually confused with the spines of the sea urchin. The two species may be found simultaneously on the same individuals, raising the question of the differences in their respective ecological niche, as well as the question of potential competition between the two species. Moreover, it is presently unknown if the two symbionts feed on their host (parasitism) or if they are true commensalists. Carbon and nitrogen stable isotopes have recently been used to study the diets of various echinoderm invertebrate13,14 or vertebrate15 symbionts. These analyses revealed that this method is a powerful tool to determine the food sources that are assimilated over a longer period of life. This method differs from the analysis of the gut contents, which will only reflect recently ingested food. In particular, stable isotope analyses have been performed on a parasitic gastropod of E. mathaei, Vexilla vexillum, which grazes on the spines and the integument covering the tests of sea urchins14,16.
Chemodetection is a very common process completed by many symbiotic organisms to find their hosts, particularly when the symbiosis is obligatory and concerns a specific host. Evidence of host selections triggered by chemical sensing involving echinoderms as hosts have been shown for polychaetes living on asteroids/holothuroids17,18, shrimps associated with crinoids19, ophiuroids associated with other ophiuroids20, fishes21,22 and crabs23 associated with holothuroids, and bivalves24, gastropods16, crabs25,26, shrimps6,27 and fishes associated with echinoids28. While chemodetection has been proven to play a major role in host selection, it is only recently that it was demonstrated that the symbiotic Harlequin crab Lissocarcinus orbicularis is attracted by the saponins produced by its holothuroid hosts23. Saponins are also considered as toxic molecules protecting sea cucumbers against predators29. Echinoderms develop various molecules for their defence. In echinoids, the polyhydroxynaphthoquinones (PHNQ), also known as spinochromes or echinochromes30,31,32, provide sea urchins with their coloration, and are involved in their protection through antibacterial33,34,35, antioxidant35,36,37,38 and potential immune activities39,40,41,42.
The present paper aims, firstly, to analyse both the level of host-dependence developed by the two shrimp species, and the impact of forced isolation from their sea urchin hosts. Secondly, the “symbiotic addiction” (chemodetection, protective effect) of the shrimps towards the host spinochromes is also investigated.
Results
In situ shrimp population
In Toliara bay, the prevalence of infestation is similar for both symbionts: 36% of E. mathaei are infested by T. holthuisi (Fig. 1B,C) and 43% by A. indicus (Fig. 1A). In most cases, only one individual (both shrimp species included) was observed per sea urchin, with 15 to 20% infested with two shrimps, and between 10 and 15% with three or more shrimps.
Analyses
Spinochrome analyses
The colour similarity between E. mathaei and their symbionts is high, particularly for T. holthuisi (Fig. 1C), rendering them difficult to detect visually. The presence of seven spinochromes were detected in the E. mathaei-conditioned water: three isomers of Spinochrome B and Spinochrome 252, and two isomers of Spinochrome A and Echinochrome A. The compositions were confirmed by accurate mass measurements (ppm < 10). Three spinochromes, Spinochrome B–Iso 2, Spinochrome B–Iso 3 and Spinochrome A–Iso 3, were not detected in the extracts of the E. mathaei tests and spines (Table 1) (Supplementary Fig. S1).
For the symbiotic shrimps, five spinochromes were detected within the extract of T. holthuisi, with confirmation by mass spectrometry (ppm < 10). Echinochrome A was the most abundant spinochrome, followed by Spinochrome A, Spinochrome E, Spinochrome B, and, finally, Spinochrome C (Table 1, Supplementary Fig. S1). For A. indicus extracts, three spinochromes were detected: Spinochrome A–Iso 2 and two isomers of Spinochrome B (Table 1, Supplementary Fig. S1). Spinochrome A–Iso 3 and Spinochrome B–Iso 2 and –Iso 3 were the only spinochromes that did not seem to be present in the host’s tests, but were present in the conditioned water. The compositions were confirmed by accurate mass measurements (ppm < 10) and checked with the literature42.
Symbionts’ diet
Echinometra mathaei has an average isotopic composition (±standard deviation) of 3.8 ± 1.1 for δ15N values, and −10.7 ± 0.7 for δ13C values (Fig. 2). For the isotopic signature of symbionts, T. holthuisi has a value of 6.5 ± 0.7 for δ15N and −11.3 ± 2.9 for δ13C. Finally, A. indicus has a value of 5.4 ± 0.6 for δ15N and −16.1 ± 3.0 for δ13C values. Statistical tests showed no significant differences between the δ13C composition of T. holthuisi and its host E. mathaei (Mann Whitney test, P > 0.05), but revealed significant statistical differences between their δ15N composition (Mann Whitney test, P < 0.0005) with a mean enrichment for T. holthuisi of 3‰. For A. indicus, statistical tests showed significant differences between the δ13C composition of A. indicus and its host E. mathaei (Mann Whitney test, P < 0.05) with a mean difference of 4.5‰, but revealed no significant differences between their δ15N composition (Mann Whitney test, P > 0.05).
Values of δ15N and δ13C of E. mathaei symbionts and their potential food source, their host. Standard ellipse areas corrected for small samples SEAc (solid lines) are estimated for individuals of each species (symbols)43.
The SEAc (Standard Ellipse Areas corrected for small sample)43 calculated for the T. holthuisi (4.8), A. indicus (5.6) and E. mathaei (2.9) populations showed distinct ecological niches for each species, with no overlap (Fig. 2).
Experiments
Host chemodetection
It was found that 66% of the tested T. holthuisi statistically preferred the seawater conditioned by their host than the control seawater (Binomial test, P < 0.001) (Fig. 3). Similarly, 75% of the tested shrimps preferred moving towards the seawater conditioned with the synthetic spinochromes (Binomial test, P < 0.05) rather than the control seawater. When offered the choice between the spinochrome crude extract of E. mathaei and the control seawater, the shrimps were not significantly attracted by the spinochrome crude extract (Binomial test, P > 0.05).
Depigmentation
The spinochrome solution extracted from T. holthuisi living on E. mathaei showed an average absorbance at 450 nm (±standard deviation) of 31.98 ± 7.23, while the absorbance significantly decreased to 15.29 ± 9.10 after a five-day separation (Mann Whitney test, P < 0.0001) (Fig. 4). The spinochrome solution extracted from A. indicus living on E. mathaei showed an average absorbance (±standard deviation) of 25.86 ± 3.88. There was no significant decrease of absorbance after five days (30.19 ± 10.49) (Mann Whitney test, P > 0.05). Spinochrome concentrations in T. holthuisi and A. indicus were similar when they lived on their host (Mann Whitney test, P > 0.05) but were significantly lower for T. holthuisi than A. indicus when they were isolated (Mann Whitney test, P ≅ 0.01).
Shrimp survival
The survival rate of T. holthuisi decreased rapidly when they were separated from their hosts (Fig. 5). In contrast, after five days, the survival rate for T. holthuisi individuals that were not separated from their hosts was 98%. The two survival curves are statistically different from one another (A Log-rank Mantel-Cox test, P = 0.0001).
The survival rate of T. holthuisi in E. mathaei-conditioned seawater after five days was significantly higher than when individuals were separated and placed in normal seawater (A Log-rank Mantel-Cox test, P ≅ 0.0001). There was no significant difference (A Log-rank Mantel-Cox test, P > 0.05) between the survival rate of T. holthuisi in E. mathaei-conditioned seawater and that of individuals that were not separated from their hosts.
The survival rate of T. holthuisi in seawater with spinochromes was statistically higher compared to the T. holthuisi separated and placed in normal seawater (A Log-rank Mantel-Cox test, P ≅ 0.0002). The survival rate of T. holthuisi in seawater with spinochromes was statistically similar to the survival rate of individuals with E. mathaei-conditioned seawater (A Log-rank Mantel-Cox test, P > 0.05), and was statistically different from the survival rate of individuals on their host (A Log-rank Mantel-Cox test, P ≅ 0.0002).
The survival rates of A. indicus, with or without the host, were respectively 100 and 99%. The survival rates were not statistically significant (A Log-rank Mantel-Cox test, P > 0.05).
Discussion
Echinoderm recognition through chemodetection is performed by many ectocommensals, and is one of the main parameters that ensures the transgenerational sustainability of symbiotic associations6,16,20,23,44,45. Two ectocommensal symbioses associated with echinoderms and decapods had previously been investigated in order to identify the nature of the chemical signals allowing host selection. The saponins of sea cucumber hosts are kairomones that attract the symbiotic Harlequin crabs23, and anthraquinones extracted from crinoid hosts attract the symbiotic Stimpson’s Snapping Shrimp (Caulier et al., submitted). Our data reveal that various host spinochromes are found in the water surrounding sea urchins and that these molecules act as kairomones attracting the symbiotic shrimp T. holthuisi. This was partially shown for A. indicus in a previous study6, where it was found that 68% of shrimps were attracted by the crude extract of E. mathaei. It is possible that molecules other than spinochromes are present in the chemical cue and may disturb symbiont chemodetection, as shown in other recent behavioural experiments (data not published). However, according to previous studies, both saponins and spinochromes are known to act as chemical defence mechanisms for echinoderms: saponins are a toxic predator repellent29,45 and spinochromes are involved in antibacterial and antioxidant processes34,35,37,42,46,47. In both cases, this involves a remarkable co-evolutionary mechanism in which a host’s chemical defences are diverted by the symbionts to their own benefit. Sea urchin tests and spines contain different cocktails of spinochromes that may be species-specific. The species analysed in the present work, E. mathaei, possesses five spinochromes in their tests and spines, with a total of seven spinochromes observed in the water surrounding the sea urchins. The higher number of spinochromes seen in the water may be the result of UV isomerisation.
Spinochromes are kairomones allowing T. holthuisi to recognise their host, and they also allow the shrimp’s colour to perfectly match that of the sea urchin. In a recent study on E. mathaei48, six spinochromes were detected in the tests and spines of the sea urchin: Spinochrome B, Spinochrome E, Spinochrome A–Iso 2, Spinochrome 252, Echinochrome A and Spinochrome C (supplementary Table S1 and Fig. S1). In the present study it was observed that the T. holthuisi pigmentation is certainly due to the presence of spinochromes taken from the host: T. holthuisi possesses five spinochromes that are the same as E. mathaei, and the 13C/12C with the 15N/14N analyses suggest that the sea urchin is the main food source for the shrimp (meaning that shrimp spinochromes would be acquired during feeding). The 13C/12C ratio of T. holthuisi is similar to its host, with a higher 15N/14N ratio, which is typical of organisms that have extremely narrow food sources and, in the case of symbionts, that feed exclusively on their hosts. This was observed for a parasitic gastropod of E. mathaei, Vexilla vexillum which grazes on the spines and the integument covering the tests of sea urchins16. However, while T. holthuisi feed on their host, they do not seem to cause injuries, as there were no lesions observed on E. mathaei (personal observation). Thus, although T. holthuisi could be considered a parasite, it is estimated that the impact on the host’s fitness is limited, and that it is more appropriate to consider this type of symbiosis as commensal1.
In comparison, Arete indicus are less mimetic and the shrimps can be easily seen on their hosts. A. indicus contains three spinochromes in common with E. mathaei, but stable isotope analyses did not show host-dependent feeding: the large standard deviation observed with 13C/12C values indicates that their food sources are diverse, and the few overlaps with T. holthuisi suggest a different diet. A. indicus could feed on E. mathaei (i.e. in order to ingest naphthoquinones), but not exclusively, and various other food items are probably also eaten by this species (benthic organisms or organic matter).
The analyses also demonstrate that besides spinochromes being involved in T. holthuisi host recognition, and the fact that they provide protection and efficient mimicry, the spinochromes are also involved in much more intimate physiological processes that are essential for the shrimps’ survival. When isolated from its host, T. holthuisi suffers from depigmentation, and most individuals die within five days following host separation. Depigmentation is evident in host-separated T. holthuisi, in comparison with the results obtained for the least host-dependant shrimp, A. indicus. Dependence of T. holthuisi on E. mathaei is also obvious when the survival rate of separated T. holthuisi is compared to that of non-separated individuals or to separated A. indicus. The survival rate of separated T. holthuisi is, moreover, significantly increased when individuals are incubated in E. mathaei-conditioned seawater, or in sea urchin extracted spinochromes. These results suggest that E. mathaei is not only a “house” for T. holthuisi, or its unique food source, but that spinochromes are essential for T. holthuisi survival.
Three hypotheses may be formulated to explain T. holthuisi death in the absence of spinochromes: (i) spinochromes (and other dissolved organic molecules) are the only food source on which the shrimps rely, (ii) the antibacterial and antioxidant properties of spinochromes are essential to protecting the shrimps, or (iii) spinochromes are integral or promote physiological pathways essential for the shrimps’ metabolism. The latter hypothesis may be the most plausible, as spinochromes would almost certainly not provide the necessary nutrients, and it seems unlikely that bacteria or the UV effect of the sun has influenced the survival rates in the experiments (the seawater was filtered and assays were performed in laboratory conditions). The spinochrome “addiction” of T. holthuisi was also observed when the shrimps were separated from their host and placed in the same aquarium as the parasitic gastropod V. vexillum. Under these conditions, it was observed that the shrimps were highly attracted to the gastropod which had ingested spinochromes by feeding on sea urchins.
Evolution has brought about various degrees of host dependence amongst symbionts, some will be loosely dependent, such as in the case of A. indicus, whereas others will be highly dependent, as seen in T. holthuisi. The first group present the advantage of not being exclusively reliant on one host species, and can probably survive to the extinction of a potential host. Conversely, they are certainly less protected, as they are less mimetic and can also use various food sources in their diet. Host-dependent commensals benefit from an “all in one” home, but their evolution and survival are entirely linked to that of their hosts.
Material and Methods
Samples
The sea urchin Echinometra mathaei (Blainville, 1825) and its symbionts, Tuleariocaris holthuisi Hipeau-Jacquotte, 1965 and Arete indicus Coutière, 1903, were collected by scuba diving on the flat of the Great Reef of Toliara, Madagascar (23°23′34″S, 43°38′47″E) in November 2015 and November 2016.
E. mathaei (n = 105) were observed in situ and the number of shrimps of the two species per sea urchin, and the number of infested individuals, were recorded. T. holthuisi (n = 350), A. indicus (n = 130) and E. mathaei (n = 50) were sampled for the behavioural experiments and spinochrome extractions.
Analyses
Extraction and characterisation of spinochromes
The spinochrome cocktails48 that may be present in water conditioned by E. mathaei and those that may be present in T. holthuisi and A. indicus were analysed. For the analysis of the shrimp spinochromes, 30 fresh individuals of each species were dried for 2 hours at 90 °C, pooled and their spinochromes extracted as mentioned below. For the analysis of the spinochromes present in E. mathaei-conditioned seawater, three E. mathaei were placed in three tanks (14 cm × 18 cm × 15 cm) of 1 l each and filled with 1 µm filtered seawater at 25 °C for 24 h. The water was evaporated to dryness at low pressure at 60 °C using a rotary evaporator (Laborota 4001 efficient, Heidolph, Germany).
The spinochrome extraction started with 1 hour of maceration in 6M HCl (10 ml/5 g of samples) before being filtrated under vacuum with a Buchner flask. The solution was partitioned three times with diethyl ether (v/v). The diethyl ether phases were recovered, pooled and partitioned three times with NaCl 5% solution (v/v). Then, the final ethereal phase was recovered and evaporated to dryness at low pressure at 60 °C using a rotary evaporator (Laborota 4001 efficient, Heidolph, Germany), dissolved in 80% methanol, and centrifuged at 10,000 g for 10 minutes. Finally, the supernatant was recovered for analysis.
A Waters Alliance 2695 liquid chromatography device (HPLC) was used to separate the pigments. The system comprises a quaternary pump, a vacuum degasser and an autosampler. The chromatography was performed on a reversed phase column (Kinetex® 5 µm Biphenyl 100 Å, 50 × 4.6 mm, Phenomenex) at 30 °C, with an injected sample volume of 25 µl and a constant flow (1.25 ml/min) of a gradient of eluent A (Water, 0.1% formic acid) and eluent B (Acetonitrile) (supplementary Table S1).
The HPLC analysis device was coupled with a mass spectrometer, allowing the pigments to be determined. The mass spectrometry spectra were obtained on a Waters Quattro Ultima by scanning between m/z 50 and 1500 using an Electrospray ionisation (ESI) source operated in the negative ionisation mode. The ESI conditions were as follows: capillary voltage of 3.1 kV, cone voltage of 40 V, source temperature at 120 °C and desolvation temperature at 300 °C. Dry nitrogen was used as the ESI gas with a flow rate of 50 l/h for the gas cone and 500 l/h for the desolvation gas.
The accurate mass measurements and molecular formula of PHNQ ion predictions were performed on a Waters Q-ToF Premier using an Electrospray ionisation source in the negative ionisation mode, by scanning between m/z 50 and 600 with scan durations of 1 s and an inter-scan time of 0.1 s. The ESI conditions were as follows: capillary voltage of 3.1 kV, cone voltage of 40 V, source temperature at 120 °C and desolvation temperature 300 °C. Dry nitrogen was used as the ESI gas with a flow rate of 50 l/h for the gas cone and 600 l/h for the desolvation gas. The mass spectrometer was equipped with a lockspray setup to obtain high mass accuracy of PHNQ ions. Sodium iodide was used as a reference sample with m/z 126.9045 as the lock mass (iodide anion). Mass spectra analyses were performed on MassLynx 4.1. mass spectrometry software (Waters, Milford, MA, USA) and compared to the literature42. PHNQ were drawn using “ChemDraw 15.0.0.106” (PerkinElmer Informatics. Inc.) software and annotated with the “Affinity Designer” software.
Host-dependent feeding
The diets of symbiotic organisms can be diversified; they can be dependent on the host if they feed on their tissues or divert food caught by the hosts14. As spinochromes are partly in the body wall of sea urchins, it was necessary to determine whether T. holthuisi and A. indicus fed on E. mathaei tissue. For this, 10 T. holthuisi, 10 A. indicus and 10 E. mathaei were collected to perform stable isotope analyses. Integument tissues were scraped from the sea urchins. The samples were dried at 60 °C for 24 h before being crushed. All samples were acidified using an HCL fumigation technique (fuming HCL, 37%, Merck) for 48 h in order to remove the calcium carbonate. Indeed, inorganic carbon does not reflect the isotopic composition of an animal’s diet. Isotopic ratios and elemental content measurements were performed using an isotopic ratio mass spectrometer (IsoPrime100, Isoprime, UK) interfaced in continuous flow with an elemental analyser (vario MICRO cube, Elementar, Germany). Isotope ratios for C and N were reported conventionally49 in per mil (‰) using standard delta (δ) notation relative to their respective international standards, Vienna-Pee Dee Belemnite (V-PDB) and atmospheric N2:
where X = 13C or 15N, R = 13C/12C or 15N/14N. Analytical precision was assessed by procedural blanks, internal replicates (i.e., glycine, in-house crustacean and seagrass reference material) and isotopic certified material: sucrose (IAEA-C6; δ13C = −10.8 ± 0.3‰) and ammonium sulfate (IAEA-N2; δ15N = 20.3 ± 0.3‰), obtained from the International Atomic Energy Agency (IAEA, Vienna, Austria). Standard deviations on replicated measurements presented hereafter were 0.1‰ for δ13C and 0.2‰ for δ15N. Neither chemical lipid extractions nor a posteriori lipid corrections were performed, due to the often limited relevance of a posteriori corrections for aquatic invertebrates containing high proportions of chitin in addition to lipids and proteins50. The isotopic compositions were analysed with the SIBER package compared using the Mann Whitney test with the “Prism 6” (GraphPad) software. The isotopic ecological niches were compared using the Stable Isotope Bayesian Ellipses package (SIBER) in R version 2.2.2.
Host selection and symbiont isolation
Host chemodetection
The experiments were carried out to study host chemical recognition using T. holthuisi with three differently conditioned seawaters, each as a potential chemical stimulus. First, the test was performed on 30 individuals with conditioned seawater. Conditioned seawater was obtained by submerging two individuals of E. mathaei per litre of filtered (1 µm) seawater for 2 hours. Secondly, 20 individuals were tested with synthetic pure molecules: 2-hydroxynaphtoquinone (Sigma Aldrich St. Louis, MO, USA) at a concentration of 0.1 g/l of seawater. This concentration was chosen in accordance with Caulier et al23., and recent behavioural experiments using another symbiosis between an urchin and a crab (non-published data). Lower concentrations were also partially tested (1 mg/l, 100 µg/l) and provided similar results. Finally, 25 individuals were tested with the E. mathaei crude extract at a concentration of 1 mg per litre of seawater. The experiments were performed with a Davenport olfactometer23. The latter is composed of a Y-shaped glass tube of 2 cm diameter, 20 cm length and a 10 cm region where the paired branches are connected to two opaque tanks of 1 l each. One tank was filled with the test seawater and the second contained the control seawater. Both experimental seawaters were filtered and controlled to 25 °C and 35 for salinity, and filtered at 1 µm. The seawaters flowed from the two tanks through the Y-tube and were evacuated at the base of the unpaired branch at a speed of 2-3 cm/s. Fluorescein was used prior to the experiments in order to control the flow turbulence inside the olfactometer. The tested shrimp was first introduced at the base of the unpaired branch. If the shrimp was stimulated, it moved into the unpaired branch to the junction and potentially chose one of the paired branches. The orientation of the shrimp was recorded. The position of the test seawater and control seawater was inverted after every 5 replicates after a complete washing of the Davenport system. The shrimps were considered to have made a choice when they entered one of the two tanks. After more than 5 minutes of testing without any movement, the trials were considered as “No choice”. Each shrimp was tested only once. The shrimps’ orientation preferences were analysed using a binomial test relative to a random distribution (50/50) with the “Prism 6” (GraphPad) software.
Depigmentation
Some observations during the survival tests suggested a pigmentation loss after the shrimps were separated from their host. In order to quantify this depigmentation, a comparison test was performed under two conditions:
-
(i)
Five individuals from each shrimp species were placed on one E. mathaei (number of tanks = 2; number of shrimps = 10). The seawater was replaced every day with fresh filtered (1 µm) seawater.
-
(ii)
Five individuals from each shrimp species were placed without their host (number of tanks = 3 for T. holthuisi and 1 for A. indicus; number of shrimps = 15 for T. holthuisi and 5 for A. indicus). The seawater was replaced every day with fresh filtered (1 µm) seawater.
All the experiments were performed for five days in tanks of 14 × 18 × 15 cm containing 1 l of filtered (1 µm) seawater at 25 °C. Live shrimps were collected at the end of the tests and dried at 90 °C for 2 hours. Each shrimp was then weighed, placed in 1 ml of 100% ethanol to extract the pigments and stored in the dark at 5 °C before being measured. Later, the absorbance of the ethanol solution was measured at 450 nm cm−1. Pigment concentrations are expressed as optical density (O.D.) divided by dried weight. The pigment concentrations were compared using the Mann Whitney test with the “Prism 6” (GraphPad) software.
Shrimp survival
Tuleariocaris holthuisi survival times and rates (i.e. number of shrimps that survived per day) were recorded under four treatments:
-
(i)
Five individuals were placed on one E. mathaei (number of replicates = 7; number of shrimps = 35). The seawater was replaced every day with fresh filtered (1 µm) seawater.
-
(ii)
Five individuals were placed without their host (number of replicates = 25; number of shrimps = 120). The seawater was replaced every day with fresh filtered (1 µm) seawater.
-
(iii)
Five individuals were incubated in E. mathaei-conditioned sea water (number of replicates = 5; number of shrimps = 25). The seawater was replaced everyday with fresh filtered (1 µm) seawater conditioned for 24 h by one E. mathaei.
-
(iv)
Five individuals were incubated in sea water with 0.5 mg/l of E. mathaei spinochromes crude extract from tests and spines (number of replicates = 6; number of shrimps = 30). The seawater was replaced every day with fresh filtered seawater (1 µm) including 0.5 mg/l of host crude extract.
As the host-dependence shown by Arete indicus was much lower, the survival times and rates were only estimated under the first and second conditions:
-
(i)
Five individuals were placed on one E. mathaei in the same conditions as the T. holthuisi experiment (number of replicates = 7; number of shrimps = 35)
-
(ii)
Five individuals without their host were placed in the same conditions as the T. holthuisi experiment (number of replicates = 8; number of shrimps = 40).
All the experiments were performed in tanks of 14 × 18 × 15 cm containing 1 l of filtered seawater at 25 °C. The number of live shrimps was verified every day at the same time for five days. The survival curves were calculated with survival analysis tools on the “Prism 6” (GraphPad) software. Their comparisons were performed with the Log-rank (Mantel-Cox) test.
Ethics Statement
The animals used in our experiments were maintained and treated in compliance with the guidelines specified by the Belgian Ministry of Trade and Agriculture.
References
Parmentier, E. & Michel, L. Boundary lines in symbiosis forms. Symbiosis 60, 1–5 (2013).
De Bary, A. Die Erscheinung der Symbiose (ed. Trübner, V. V. K. J.) (Strassburg, 1879).
Paracer, S. & Ahmadjian, V. Symbiosis: An Introduction to Biological Associations (Oxford University Press, 2000).
Olsen Wilford, O. Animal Parasites: Their Life Cycles and Ecology (Dover Publications, 1986).
Baker, A. C. Flexibility and specificity in coral-algal symbiosis: Diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34, 661–689 (2003).
Gherardi, F. Eco-ethological aspects of the symbiosis between the shrimp Athanas indicus (Coutière 1903) and the sea urchin Echinometra mathaei (de Blainville 1825). Trop. Zool. 4, 107–128 (1991).
Moyses, C. R. S., Spadacci-Morena, D. D., Xavier, J. G., Antonucci, A. M. & Lallo, M. A. Ectocommensal and ectoparasites in goldfish Carassius auratus (Linnaeus, 1758) in farmed in the State of São Paulo. Rev. Bras. Parasitol. Veterinária 24, 283–289 (2015).
Ross, D. M. The biology of Crustacea. (ed. Bliss, D.) 163–212 (Academic Press Inc., 1983).
De Grave, S. Biogeography of lndo-Pacific Pontoniinae (Crustacea, Decapoda): a PAE analysis. J. Biogeogr. 28, 1239–1253 (2007).
Suzuki, H. Taxonomic review of four alpheid shrimps belonging to the genus Athanas with reference to their sexual phenomena. Sci. Reports Yokohama Natl. Univ. Sect. II 1–52 (1970).
Hipeau-Jacquotte, R. Notes de faunistique et de biologie marines de Madagascar. III. Un nouveau decapode nageur (Pontoniinae) associé aux oursins dans la région de Tulear: Tuleariocaris holthuisi nov. gen. et nov. sp. Recl. des Trav. la Stn. Mar. d’Endoume 37, 247–259 (1965).
Marin, I. & Anker, A. On the Presence of the Pontoniine Shrimp, Tuleariocaris Holthuisi Hipeau-Jacquotte, 1965 (Decapoda, Pontoniinae) on the Pacific Coast of Panama. Crustaceana 82, 505–508 (2009).
Fourgon, D., Lepoint, G. & Eeckhaut, I. Assessment of trophic relationships between symbiotic tropical ophiuroids using C and N stable isotope analysis. J. Mar. Biol. Assoc. UK 86, 1443 (2006).
Caulier, G., Lepoint, G., Van Nedervelde, F. & Eeckhaut, I. The diet of the Harlequin crab Lissocarcinus orbicularis, an obligate symbiont of sea cucumbers (holothuroids) belonging to the genera Thelenota, Bohadschia and Holothuria. Symbiosis 62, 91–99 (2014).
Parmentier, E. & Das, K. Commensal vs. parasitic relationship between Carapini fish and their hosts: some further insight through δ13C and δ15N measurements. J. Exp. Mar. Bio. Ecol. 310, 47–58 (2004).
Vaïtilingon, D., Eeckhaut, I., Fourgon, D. & Jangoux, M. Population dynamics, infestation and host selection of Vexilla vexillum, an ectoparasitic muricid of echinoids, in Madagascar. Dis. Aquat. Organ. 61, 241–255 (2004).
Davenport, D., Camougis, G. & Hickok, J. F. Analyses of the behaviour of commensals in host-factor. 1. A hesioned polychaete and a pinnotherid crab. Anim. Behav. 8, 209–218 (1960).
Dimock, R. V. J. & Davenport, D. Behavioral Specificity and the Induction of Host Recognition in a Symbiotic Polychaete. Biol. Bull. 141, 472–484 (1971).
Vandenspiegel, D., Eeckhaut, I. & Jangoux, M. Host selection by Synalpheus stimpsoni (De Man), an ectosymbiotic shrimp of comatulid crinoids, inferred by a field survey and laboratory experiments. J. Exp. Mar. Bio. Ecol. 225, 185–196 (1998).
Fourgon, D., Jangoux, M. & Eeckhaut, I. Biology of a ‘babysitting’ symbiosis in brittle stars: analysis of the interactions between Ophiomastix venosa and Ophiocoma scolopendrina. Invertebr. Biol. 126, 385–395 (2007).
Miyazaki, S., Ichiba, T., Reimer, J. D. & Tanaka, J. Chemoattraction of the pearlfish Encheliophis vermicularis to the sea cucumber Holothuria leucospilota. Chemoecology 24, 121–126 (2014).
Van Meter, V. B. & Ache, B. W. Host location by the pearlfish Carapus bermudensis. Mar. Biol. 26, 379–383 (1974).
Caulier, G., Flammang, P., Gerbaux, P. & Eeckhaut, I. When a repellent becomes an attractant: harmful saponins are kairomones attracting the symbiotic Harlequin crab. Sci. Rep. 3, 2639 (2013).
Gage, J. Experiments with the behaviour of the bivalves Montacuta substriata and M. ferruginosa, ‘commensals’ with spatangoids. J. Mar. Biol. Assoc. United Kingdom 46, 71 (1966).
Gray, I. E., McCloskey, L. R. & Weihe, S. C. The Commensal Crab Dissodactylus mellitae and Its Reaction to Sand Dollar Host-Factor. J. Elisha Mitchell Sci. Soc. 84, 472–481 (1968).
De Bruyn, C., De Ridder, C., Rigaud, T. & David, B. Chemical host detection and differential attraction in a parasitic pea crab infecting two echinoids. J. Exp. Mar. Bio. Ecol. 397, 173–178 (2011).
Ache, B. W. & Davenport, D. The Sensory Basis of Host Recognition by Symbiotic Shrimps, Genus Betaeus. Biol. Bull. 143, 94–111 (1972).
Dix, T. G. Association between the echinoid Evechinus chloroticus (Val.) and the clingfish Dellichthys morelandi Briggs. Pacific Sci. 23, 332–336 (1969).
Van Dyck, S. et al. The triterpene glycosides of Holothuria forskali: usefulness and efficiency as a chemical defense mechanism against predatory fish. J. Exp. Biol. 214, 1347–1356 (2011).
Kornprobst, J. -M. Substances naturelles d’origine marine - Tome 2 (Lavoisier S.A.S., 2005).
Anderson, H. A., Mathieson, J. W. & Thomson, R. H. Distribution of spinochrome pigments in echinoids. Comp. Biochem. Physiol. 28, 333–345 (1969).
Thomson, R. H. Naturally Occuring Quinones (Academic Press Inc., 1971).
Stekhova, S. I., Shentsova, E. B., Kol’tsova, E. B. & Kulesh, N. I. Antimicrobial activity of polyhydroxynaphthoquinones from sea urchins. Antibiot. Khimioter. 33, 831–833 (1988).
Haug, T. et al. Antibacterial activity in Strongylocentrotus droebachiensis (Echinoidea), Cucumaria frondosa (Holothuroidea), and Asterias rubens (Asteroidea). J. Invertebr. Pathol. 81, 94–102 (2002).
Shankarlal, S., Prabu, K. & Natarajan, E. Antimicrobial and Antioxidant Activity of Purple Sea Urchin Shell (Salmacis virgulata L. Agassiz and Desor 1846). Am. J. Sci. Res. 6, 178–181 (2011).
Zhou, D.-Y. et al. Extraction and antioxidant property of polyhydroxylated naphthoquinone pigments from spines of purple sea urchin Strongylocentrotus nudus. Food Chem. 129, 1591–1597 (2011).
Li, D.-M. et al. Extraction, structural characterization and antioxidant activity of polyhydroxylated 1,4-naphthoquinone pigments from spines of sea urchin Glyptocidaris crenularis and Strongylocentrotus intermedius. Eur. Food Res. Technol. 237, 331–339 (2013).
Powell, C., Hughes, A. D., Kelly, M. S., Conner, S. & McDougall, G. J. Extraction and identification of antioxidant polyhydroxynaphthoquinone pigments from the sea urchin, Psammechinus miliaris. LWT - Food Sci. Technol. 59, 455–460 (2014).
Sciani, J. M. et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp. Biol. Med. 236, 277–280 (2011).
Gonzalez-Aravena, M. et al. Immune response of the Antarctic sea urchin Sterechinus neumayeri: cellular, molecular and physiological approach. Rev. Biol. Trop. 63, 309–320 (2015).
Majeske, A. J., Bayne, C. J. & Smith, L. C. Aggregation of Sea Urchin Phagocytes Is Augmented In Vitro by Lipopolysaccharide. PLoS One 8, e61419 (2013).
Shikov, A. N., Pozharitskaya, O. N., Krishtopina, A. S. & Makarov, V. G. Naphthoquinone pigments from sea urchins: chemistry and pharmacology. Phytochem. Rev. 17, 509–534 (2018).
Pasotti, F. et al. Benthic Trophic Interactions in an Antarctic Shallow Water Ecosystem Affected by Recent Glacier Retreat. PloS ONE 10, e0141742 (2015).
Caulier, G., Brasseur, L., Gerbaux, P., Flammang, P. & Eeckhaut, I. Crinoid anthraquinones are kairomones allowing host selection for the symbiotic snapping shrimp Synalpheus stimpsoni. submitted (2018).
Eeckhaut, I. et al. Effects of Holothuroid Ichtyotoxic Saponins on the Gills of Free-Living Fishes and Symbiotic Pearlfishes. Biol. Bull. 228, 253–65 (2015).
Kuwahara, R. et al. Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT - Food Sci. Technol. 42, 1296–1300 (2009).
Egorov, E. A. et al. Histochrome, a new antioxidant, in the treatment of ocular diseases. Vestn. Oftalmol. 115, 34–35 (1999).
Brasseur, L. et al. Identification and quantification of spinochromes in body compartments of Echinometra mathaei’s colored types. R. Soc. Open Sci. 5, 171213 (2018).
Logan, J. M. et al. Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J. Anim. Ecol. 77, 838–846 (2008).
Coplen, T. B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 25, 2538–2560 (2011).
Acknowledgements
The authors would like to thank the Institut Halieutique et des Sciences Marines (IH.SM) (Toliara, Madagascar) for having allowed them to carry out their sampling within their institute. This work was supported by an FRFC project no. 14603427 and the CDR project n°29121385 (Marine Symbiosis) (F.R.S.-FNRS, Fonds National de la Recherche Scientifique). G.L. is Research Associate appointed by the F.R.S.-FNRS. This study is a contribution by the Centre Interuniversitaire de Biologie Marine (CIBIM). L.B. would like to thank the Franeau fund (UMONS), the Leopold III fund and the Agathon de Potter grant for their financial support. The MS lab is grateful to the F.R.S.-FNRS for their financial support for the acquisition of the Waters QToF Premier mass spectrometer and for their continuing support.
Author information
Authors and Affiliations
Contributions
L.B. and G.C. conceived and designed the experiments; L.B. and G.L. performed the experiments; L.B., G.L. and G.C. analysed the data; G.L., P.G. and I.E. contributed reagents/materials/analysis/tools; L.B. and G.C. wrote the paper; all authors reviewed the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brasseur, L., Caulier, G., Lepoint, G. et al. Echinometra mathaei and its ectocommensal shrimps: the role of sea urchin spinochrome pigments in the symbiotic association. Sci Rep 8, 17540 (2018). https://doi.org/10.1038/s41598-018-36079-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36079-8
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.