Abstract
Errors in identifying the etiology of cardiomyopathy have been described in patients undergoing cardiac transplantation. There are increasing data that cardiovascular magnetic resonance imaging (CMR) provides unique diagnostic information in heart failure. We investigated the association of the performance of CMR prior to cardiac transplantation with rates of errors in identifying the etiology of cardiomyopathy. We compared pre-transplantation clinical diagnoses with post-transplantation pathology diagnoses obtained from the explanted native hearts. Among 338 patients, there were 23 (7%) errors in identifying the etiology of cardiomyopathy. Of these, 22 (96%) occurred in patients with pre-transplantation clinical diagnoses of non-ischemic cardiomyopathy (NICM). Only 61/338 (18%) had CMRs prior to transplantation. There was no significant association between the performance of CMR and errors in the entire study cohort (p = 0.093). Among patients with pre-transplantation clinical diagnoses of NICM, there was a significant inverse association between the performance of CMR and errors (2.4% vs. 14.6% in patients with and without CMR respectively; p = 0.030). In conclusion, CMR was underutilized prior to cardiac transplantation. In patients with pre-transplantation clinical diagnoses of NICM – in whom 96% of errors in identifying the etiology of cardiomyopathy occurred – the performance of CMR was associated with significantly fewer errors.
Similar content being viewed by others
Introduction
Heart failure is associated with significant mortality, morbidity, and healthcare costs. An important prognostic factor in heart failure is the etiology of the underlying cardiomyopathy1. Etiology-specific treatment, in addition to standard heart failure therapies, can slow disease progression, reverse myocardial remodeling, and delay or preclude the need for advanced therapies such as cardiac transplantation2. Examples of such etiology-specific therapies include: coronary revascularization for ischemic cardiomyopathy (ICM), immunosuppression for inflammatory cardiomyopathies such as cardiac sarcoidosis and giant cell myocarditis, and exercise restriction and implantable cardioverter defibrillators for arrhythmogenic cardiomyopathy2. Thus, accurate identification of the etiology of cardiomyopathy may result in improved heart failure outcomes.
Errors in identifying the etiology of cardiomyopathy have been described in patients undergoing cardiac transplantation through comparisons of pre-transplantation clinical diagnoses with post-transplantation diagnoses obtained by pathology examination of the explanted native hearts3,4,5,6,7. The error rates were 8–21%, with the majority of errors involving non-ischemic cardiomyopathies (NICM)3,4,5,6,7.
Cardiovascular magnetic resonance imaging (CMR) has an important role in the evaluation of cardiomyopathy8,9. Tissue characterization of the myocardium using the late gadolinium enhancement technique (LGE CMR) frequently helps identify the etiology of a cardiomyopathy10,11,12,13. Whether CMR could help lower the rates of errors in identifying the etiology of cardiomyopathy is unknown. To investigate the impact of CMR, we studied consecutive patients that underwent cardiac transplantation and examined whether the performance of CMR any time prior to transplantation (hereon noted as pre-transplantation CMR) was associated with fewer errors in identifying the etiology of cardiomyopathy.
Methods
Overview
We performed a retrospective, observational study using the University of Minnesota’s Cardiovascular Magnetic Resonance Registry14 and the Cardiac Transplantation Registry. We hypothesized that performance of pre-transplantation CMR would be associated with fewer errors. The study was approved by University of Minnesota’s Institutional Review Board with a waiver of informed consent. It was performed in accordance with relevant guidelines and regulations.
Study cohort
Consecutive adult patients who underwent cardiac transplantation at the University of Minnesota from January 1, 2004 through December 31, 2017 were included. This time period was selected to match that of ready clinical availability of CMR at our institution.
Methods
CMRs were performed on a Siemens 1.5T scanner as per standardized protocols15,16 and included cine CMR for the assessment of left and right ventricular structure and function, and tissue characterization of the myocardium using the late gadolinium enhancement technique (LGE CMR).
Demographic and clinical data, and pre- and post-transplantation cardiomyopathy diagnoses were independently extracted from patient records by two investigators (L.Q.L. and C.S.). Differences between the investigators were resolved by consensus. Data collected on pre-transplantation diagnostic testing included the performance of: echocardiography, coronary angiography, endomyocardial biopsy, pathology evaluation of the apex of the left ventricle (LV) obtained at the time of left ventricular assist device (LVAD) implantation, and CMR.
Since patients are often referred to our institution for cardiac transplantation, referral records were also reviewed to identify diagnostic testing performed outside of our institution prior to cardiac transplantation. Five (1.5%) patients with CMRs did not have LGE CMR and were excluded from the final analysis.
Pre-transplantation diagnoses were defined as those made by the cardiac transplantation team using clinical, hemodynamic and diagnostic testing data, and documented in the patients’ records prior to transplantation. Post-transplantation diagnoses were defined as those made by pathologists on routine pathology analyses of explanted hearts. ICM by pathology was recognized by the presence of ischemic-pattern (transmural or subendocardial, in a coronary artery distribution) replacement fibrosis, and the absence of findings suggestive of a non-ischemic etiology (inflammation, granulomas, etc.).
As in previous studies3,4,5,6,7, errors were defined as discordances between pre-transplantation clinical diagnoses and post-transplantation pathology diagnoses. For patients with NICM diagnoses, the specific etiology of NICM was compared.
Statistics
Statistical analyses were performed using Stata 13 (StataCorp LP, College Station, Texas, USA). Parametric continuous variables were expressed as means with standard deviation (SD). Categorical variables were expressed as counts with percentages. Comparison between groups was performed with a 2-sample Student t test for continuous, normal variables, and Wilcoxon rank sum test for continuous, non-normal data. Categorical data were compared using either chi-squared test or Fisher’s exact test. All tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
During the study period, 341 patients underwent cardiac transplantation. Of these, three (0.9%) patients were excluded because their pathology reports for explanted hearts were not available. The pre-transplantation clinical diagnoses for the excluded patients were: idiopathic dilated cardiomyopathy, idiopathic restrictive cardiomyopathy and complex congenital heart disease. The remaining 338 patients comprised the study cohort.
Errors in identifying the etiology of cardiomyopathy
Details of pre- and post-transplantation cardiomyopathy diagnoses are provided in Table 1. Of 338 study patients, 152 (45%) had pre-transplantation clinical diagnoses of ICM. Overall, there were errors in identifying the etiology of cardiomyopathy in 23 (7%) study patients. Details of individual patients with errors are listed in Table 2.
Among patients with pre-transplantation clinical diagnoses of ICM, one (0.5%) had a post-transplantation diagnosis of idiopathic dilated cardiomyopathy. Among the 186 (55%) patients with pre-transplantation clinical diagnoses of NICM, there were errors in identifying the etiology of cardiomyopathy in 22 (12%). Thus, 22/23 (96%) of all errors occurred in patients with clinical diagnoses of NICM.
Among patients with errors after clinical diagnoses of NICM, ICM was the most common missed diagnosis, accounting for 30% (7/23) of errors. NICM diagnoses that were missed include: three cases each of myocarditis, idiopathic dilated cardiomyopathy and arrhythmogenic cardiomyopathy, two cases each of cardiac amyloidosis and hypertrophic cardiomyopathy, and one case each of cardiac sarcoidosis, giant cell myocarditis and left ventricular non-compaction cardiomyopathy.
Comparison of patients with and without pre-transplantation CMRs
Patient characteristics stratified by performance of pre-transplantation CMR are listed in Table 3. CMR was performed pre-transplantation in 61 (18%) patients, of which, 19 (31%) were performed in patients with pre-transplantation clinical diagnoses of ICM. Patients with pre-transplantation CMR were younger, more likely to be female, and more likely to have had an endomyocardial biopsy.
Associations of errors with the performance of pre-transplantation CMRs
There was no significant association between the performance of pre-transplantation CMR and errors overall – 1.6% in patients with pre-transplantation CMR vs. 7.9% in those without transplantation CMR; p = 0.093. Among patients with pre-transplantation clinical diagnoses of ICM, there was no association between the performance of pre-transplantation CMR and errors. Among patients with pre-transplantation clinical diagnoses of NICM, there was a significant inverse association between the performance of pre-transplantation CMR and errors – 2.4% in patients with pre-transplantation CMR vs. 14.6% in those without transplantation CMR; p = 0.030. Thus, patients with pre-transplantation clinical diagnoses of NICM and pre-transplantation CMRs had 86% lower odds of having an error in identifying the etiology of cardiomyopathy compared with those with pre-transplantation clinical diagnoses of NICM and no pre-transplantation CMRs.
Discussion
In a contemporary comparison of pre-transplantation clinical diagnoses of cardiomyopathy with post-transplantation pathology diagnoses, there were errors in 7% of the study cohort. While the performance of CMR pre-transplantation was not associated with fewer errors overall, it was associated with significantly fewer errors in the subgroup with pre-transplantation clinical diagnoses of NICM. The latter accounted for 96% of all errors.
Coronary angiography is conventionally used to identify ICM versus NICM. However, this approach is limited because the absence of obstructive coronary artery disease (CAD) does not always rule out ICM; in a recent systematic review of myocardial infarction with non-obstructive coronary arteries, 24% of patients had late gadolinium enhancement (LGE) in an ischemic pattern, possibly due to spontaneous healing of acute thrombotic occlusion from atherosclerotic CAD or thromboembolic disorders, or coronary vasospasm17. This may explain errors in the six patients with pre-transplantation diagnoses of NICM after coronary angiography, but with post-transplantation diagnoses of ICM. Conversely, the presence of obstructive CAD in a patient with cardiomyopathy does not always signify ICM and may simply be incidental. This may explain the error in the patient with pre-transplantation clinical diagnosis of ICM. Through identification of the ischemic pattern of LGE characterized by involvement of the subendocardium (i.e. subendocardial or transmural) and location in the perfusion territory of an epicardial coronary artery, CMR can accurately distinguish between ICM and NICM12,18.
Although multiple studies have previously demonstrated the role of CMR for the identification of the etiology of cardiomyopathy, they have all defined ICM based on the presence of significant CAD by coronary angiography10,19,20,21. Additionally, all these studies investigated the role of CMR in distinguishing ICM from NICM, and none have systematically investigated its role in identifying the specific etiology of NICM.
Identification of the specific etiology of NICM by CMR relies on the recognition of specific LGE phenotypes based on locations and patterns12. Examples include diffuse transmural LGE for cardiac amyloidosis, and multifocal, epicardial LGE with involvement of the right ventricular aspect of the basal interventricular septum for cardiac sarcoidosis (Fig. 1). Absence of LGE narrows the differential diagnosis to idiopathic dilated cardiomyopathy, familial cardiomyopathy, stress cardiomyopathy, peripartum cardiomyopathy, and toxic (alcoholic or anthracycline-related) cardiomyopathy. Frequently, interpretation of a patient’s LGE phenotype in the context of other clinical data yields a specific etiology for the NICM. Newer techniques such as T1 mapping can also help in identifying etiologies of NICM such as cardiac amyloidosis and Fabry cardiomyopathy.
Examples of Study Patients with CMRs and No Errors in Identifying the Etiology of Cardiomyopathy. Panel A is a two-chamber LGE CMR image of a patient with ischemic cardiomyopathy. Yellow arrows point to transmural LGE in the distribution of the left anterior descending coronary artery. Panel B is a four-chamber LGE CMR image of a patient with cardiac amyloidosis. Yellow arrows point to diffuse transmural LGE; also note the low signal intensity of the blood in the cardiac chambers, which is also typical for cardiac amyloidosis. Panel C demonstrates a basal short-axis LGE CMR image of a patient with cardiac sarcoidosis. Yellow arrows point to LGE involving multiple segments in epicardial, transmural and near-transmural patterns; also note involvement of the right side of the interventricular septum, which is typical for cardiac sarcoidosis. Panel D is a pathology image from the same patient as in Panel A showing subendocardial myocyte loss and replacement fibrosis (yellow arrows) consistent with a healed ischemic myocardial infarction (magnification 10x; hematoxylin and eosin stain). Panel E is a pathology image from the same patient as in Panel B showing apple-green birefringence of amyloid protein (yellow arrows) in polarized light (magnification 20x; Congo red stain). Panel F is a pathology image of the interventricular septum from the same patient as in Panel C showing foci of perivascular inflammation comprising predominantly of histiocytes and lymphocytes. Although this pattern of inflammation is non-specific, it is consistent with treated sarcoidosis. Also seen are foci of replacement fibrosis (magnification 50x; hematoxylin and eosin stain). Non-caseating granulomas were previously seen on endomyocardial biopsy performed after the CMR identified cardiac sarcoidosis.
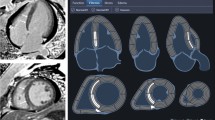
The lone NICM case missed despite a pre-transplantation CMR had a pre-transplantation clinical diagnosis of left ventricular non-compaction cardiomyopathy and on post-transplantation pathology examination was found to have arrhythmogenic cardiomyopathy with biventricular involvement, related to a mutation in the LMNA gene (Fig. 2). The patient met criteria for the diagnosis of left ventricular non-compaction cardiomyopathy; the noncompaction/compaction ratio measured on a short axis image at end-systole was 2.2 (>2 denotes the presence of left ventricular non-compaction cardiomyopathy)22. Patients fulfilling criteria for left ventricular non-compaction cardiomyopathy have previously been described to have left-dominant arrhythmogenic cardiomyopathy23.
Study Patient with CMR and Error in Identifying the Etiology of Cardiomyopathy. Panel A is a short axis cine-CMR image showing excessive trabeculations with a noncompaction/compaction ratio at end-systole of 2.2. Panel B is a four-chamber LGE CMR image of the same patient showing epicardial LGE in the mid-lateral wall (yellow arrows) and mid-myocardial LGE in the basal septum (yellow arrows). Panel C shows a pathology image from the same patient demonstrating extensive fatty infiltration of the myocardium (black arrows).
Our overall error rate is on the lower end of the range seen in prior studies of errors in identifying the etiology of cardiomyopathy in patients undergoing cardiac transplantation3,4,5,6,7 (Table 4). This may be due to the availability of CMR during our entire study period, and its use in 18%. Previous studies included patients that underwent cardiac transplantation prior to the pioneering publications of LGE CMR in 1999–200124,25,26.
While our study findings are based on patients with end-stage cardiomyopathy that subsequently underwent cardiac transplantation, they carry a greater implication for patients with newly-diagnosed cardiomyopathy. CMR was only performed in 18% of our study cohort, highlighting that it is greatly underutilized for the identification of the etiology of cardiomyopathy. Early and accurate diagnosis of the etiology of cardiomyopathy can guide etiology-specific treatment, with the potential for reversal of myocardial remodeling and recovery of systolic function, and avoidance of advanced treatments such as implantable cardioverter defibrillator therapy, cardiac resynchronization therapy, LVAD therapy and cardiac transplantation2.
Limitations
Our study is limited by its retrospective, single-center design. Pre-transplantation diagnostic testing was performed at the discretion of the transplantation team. CMR was performed infrequently; many patients did not receive CMR because they were referred for cardiac transplantation from hospitals without CMR programs with implantable cardioverter-defibrillators in place, limiting the use of CMR. Newer CMR techniques such as T1 mapping were performed only in a recent minority of CMRs since the study period spanned 14 years. Patients with CMRs had a higher rate of endomyocardial biopsies; however, whether these were a consequence of CMR findings is not known. Post-transplantation pathology interpretations were not blinded to pre-transplantation clinical diagnoses. With regard to the implications, it may not be feasible to perform CMR in patients that present in a hemodynamically unstable state and require ventricular assist devices or cardiac transplantation before they are stable enough to undergo a CMR.
Conclusions
Comparing pre-transplantation clinical diagnoses of cardiomyopathy with post-transplantation pathology diagnoses, there were errors in 7% of the study cohort. Ninety-six percent of all errors occurred in patients with pre-transplantation clinical diagnoses of NICM. In these patients, the performance of CMR pre-transplantation was associated with 86% lower odds of having an error. Prospective studies are needed to investigate whether routine use of CMR in patients with clinical diagnoses of NICM could improve clinical outcomes by decreasing errors in identifying the etiology of cardiomyopathy.
References
Felker, G. M. et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342, 1077–1084, https://doi.org/10.1056/NEJM200004133421502 (2000).
Bozkurt, B. et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 134, e579–e646, https://doi.org/10.1161/CIR.0000000000000455 (2016).
Bortman, G., Sellanes, M., Odell, D. S., Ring, W. S. & Olivari, M. T. Discrepancy between pre- and post-transplant diagnosis of end-stage dilated cardiomyopathy. Am J Cardiol 74, 921–924 (1994).
Angelini, A. et al. Discordance between pre and post cardiac transplant diagnosis: implications for pre- and postoperative decision making. Cardiovasc Pathol 8, 17–23 (1999).
Luk, A. et al. Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients? The importance of endomyocardial biopsy. Can J Cardiol 25, e48–54 (2009).
Mehra, L. M. & Uber, P. A. Preponderance and implications of etiologic misclassification in advanced heart failure: a clinical-pathologic investigation. J Heart Lung Transplant 32, 268–269, https://doi.org/10.1016/j.healun.2012.12.003 (2013).
Roberts, W. C. et al. Morphologic features of the recipient heart in patients having cardiac transplantation and analysis of the congruence or incongruence between the clinical and morphologic diagnoses. Medicine (Baltimore) 93, 211–235, https://doi.org/10.1097/MD.0000000000000038 (2014).
American College of Cardiology Foundation Task Force on Expert Consensus, D. et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 55, 2614–2662, https://doi.org/10.1016/j.jacc.2009.11.011 (2010).
McMurray, J. J. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33, 1787–1847, https://doi.org/10.1093/eurheartj/ehs104 (2012).
McCrohon, J. A. et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 108, 54–59, https://doi.org/10.1161/01.CIR.0000078641.19365.4C (2003).
Mahrholdt, H., Wagner, A., Judd, R. M., Sechtem, U. & Kim, R. J. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 26, 1461–1474, https://doi.org/10.1093/eurheartj/ehi258 (2005).
Senthilkumar, A., Majmudar, M. D., Shenoy, C., Kim, H. W. & Kim, R. J. Identifying the etiology: a systematic approach using delayed-enhancement cardiovascular magnetic resonance. Heart Fail Clin 5, 349-367, vi, https://doi.org/10.1016/j.hfc.2009.02.009 (2009).
Parsai, C., O’Hanlon, R., Prasad, S. K. & Mohiaddin, R. H. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson 14, 54, https://doi.org/10.1186/1532-429X-14-54 (2012).
Huang, H. et al. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 19, 34, https://doi.org/10.1186/s12968-017-0348-4 (2017).
Kramer, C. M., Barkhausen, J., Flamm, S. D., Kim, R. J. & Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 10, https://doi.org/10.1186/1532-429X-10-35 (2008).
Kramer, C. M., Barkhausen, J., Flamm, S. D., Kim, R. J. & Nagel, E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 15, https://doi.org/10.1186/1532-429X-15-91 (2013).
Pasupathy, S., Air, T., Dreyer, R. P., Tavella, R. & Beltrame, J. F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 131, 861–870, https://doi.org/10.1161/CIRCULATIONAHA.114.011201 (2015).
Mahrholdt, H. et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 109, 1250–1258, https://doi.org/10.1161/01.CIR.0000118493.13323.81 (2004).
Soriano, C. J. et al. Noninvasive diagnosis of coronary artery disease in patients with heart failure and systolic dysfunction of uncertain etiology, using late gadolinium-enhanced cardiovascular magnetic resonance. J Am Coll Cardiol 45, 743–748, https://doi.org/10.1016/j.jacc.2004.11.037 (2005).
Casolo, G. et al. Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: diagnostic accuracy of late gadolinium enhancement. Am Heart J 151, 101–108, https://doi.org/10.1016/j.ahj.2005.03.068 (2006).
Valle-Munoz, A. et al. Late gadolinium enhancement-cardiovascular magnetic resonance identifies coronary artery disease as the aetiology of left ventricular dysfunction in acute new-onset congestive heart failure. Eur J Echocardiogr 10, 968–974, https://doi.org/10.1093/ejechocard/jep115 (2009).
Stacey, R. B., Andersen, M. M. St, Clair, M., Hundley, W. G. & Thohan, V. Comparison of systolic and diastolic criteria for isolated LV noncompaction in CMR. JACC Cardiovasc Imaging 6, 931–940, https://doi.org/10.1016/j.jcmg.2013.01.014 (2013).
Sen-Chowdhry, S. et al. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol 52, 2175–2187, https://doi.org/10.1016/j.jacc.2008.09.019 (2008).
Kim, R. J. et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100, 1992–2002 (1999).
Kim, R. J. et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343, 1445–1453, https://doi.org/10.1056/NEJM200011163432003 (2000).
Simonetti, O. P. et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 218, 215–223, https://doi.org/10.1148/radiology.218.1.r01ja50215 (2001).
Acknowledgements
Mehmet Akçakaya was supported by NIH grant R00HL111410. Chetan Shenoy was supported by NIH grant K23HL132011, University of Minnesota Clinical and Translational Science Institute KL2 Scholars Career Development Program Award (NIH grant KL2TR000113-05) and NIH grant UL1TR000114.
Author information
Authors and Affiliations
Contributions
L.Q.L. acquired the data and drafted the manuscript. F.K. acquired data. K.-H.C., O.O., P.S.N., C.M.M., M.A., A.F.-F. revised the manuscript critically for important intellectual content. CS conceived and designed the study, acquired the data, drafted the manuscript, and revised the manuscript critically for important intellectual content. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, L.Q., Kazmirczak, F., Chen, KH.A. et al. Impact of Cardiovascular Magnetic Resonance Imaging on Identifying the Etiology of Cardiomyopathy in Patients Undergoing Cardiac Transplantation. Sci Rep 8, 16212 (2018). https://doi.org/10.1038/s41598-018-34648-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34648-5
Keywords
This article is cited by
-
Extracellular volume is an independent predictor of arrhythmic burden in dilated cardiomyopathy
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.