Abstract
A series of chemsensors (1–4) containing fluorobenzene group based on coumarin derivatives have been developed for the selective and sensitive detection of H2S. The advantages of the synthesized fluorescent probe (compound 1) were the low detection limit (4 × 10−6 mol·L−1), good selectivity and high sensitivity which had been demonstrated through UV-vis, fluorescent titration experiments. Besides cytotoxicity test of compounds (1 and 2) was studied and the results indicated that compounds (1 and 2) showed almost no cytotoxicityat at a concentration of 150 μg·mL−1. The interacted mechanism was the thiolysis reaction of dinitrophenyl ether which had been confirmed by fluorescence and HRMS titration experiment. In addition, probe 1 can also detect HS− selectively by naked eye in pure DMSO solvent.
Similar content being viewed by others
Introduction
In the past decade, we has seen a boost of research interest in hydrogen sulfide (H2S), a colorless, flammable, toxic gas with unpleasant smell, which is recognized as a signal gasotransmitter in the body as same as nitric oxide (NO)1,2,3,4,5,6,7,8,9,10 and carbon monoxide (CO)11. Endogenous concentration of H2S is related to some diseases such as Alzheimer’s disease, Down syndrome, liver cirrhosis and diabetes2,9,12,13,14,15,16,17. What’s more, the regulation of H2S levels is also a potential drug development strategy18,19 and the importance of accurate detection of H2S cannot be over-emphasized. Therefore, it presents significant research related to track and quantify H2S inside living cells being crucial in order to understand the biological and pathological roles of H2S. Recently, some methods to determine H2S concentration in biological sample have been developed including the methylene blue, the monobromobimane (MBB), gas chromatography (GC), the sulphide ion selective electrodes (ISE) and fluorescent analysis20,21,22,23. Among these methods, fluorescent analysis has attracted great attention due to the high sensitivity and selectivity for the detection of H2S in many fields such as environment area, pharmacy area and so on24,25,26,27,28,29,30,31.
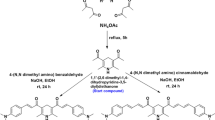
In the present work, a series of “OFF-ON” probes based on coumarin derivatives to detect HS− (Fig. 1). The results of UV-vis titration experiments indicated that the synthesized compounds showed high binding ability for HS− among the tested anions (NaHS (HS−), (n-C4H9)4NAcO (AcO−), (n-C4H9)4NH2PO4 (H2PO4−), (n-C4H9)4NF (F−), (n-C4H9)4NCl (Cl−), (n-C4H9)4NBr (Br−), (n-C4H9)4NI (I−)) and amino acids (Glutathione (GSH), Cysteine (Cys), Homocysteine (Hcy)). Besides, four compounds designed and synthesized could exhibit the probes with strong electron-withdrawing groups located in 2,4-positions of fluorobenzene have strong binding ability for HS− detection which provides a good idea for the design of probe in future.
Results and Discussion
X-ray crystallography
Compound 2 was synthesized according to the route shown in Fig. 1. Fortunately, the crystallographic of compound 2 was obtained by the standing method. The suitable single light yellow crystal was obtained by volatilizing ethyl acetate containing compound 2 at room temperature. The details of the crystallographic determination, selected bond lengths and angles were given in Table 1 and supplementary material respectively.
The crystal of compound 2 suitable for X-ray crystal analysis was obtained and the structure was also confirmed (Fig. 2a). The fluoride atom in benzene cycle forms hydrogen bonds with hydrogen atom (H3) (supplementary material). The overall crystal structure features a chain type joining in through the hydrogen bonds (H….F) along the b axis (Fig. 2b). In the crystal packing of compound 2 (Fig. 2c), there are two stacked forms: (1) π-π stacking of one fluorobenzene ring with another; (2) π-π stacking of one coumarin ring with another, which connected into “chair” conformation along a axis.
UV-vis Titration
The UV-vis spectra of probes (1–4) were recorded after addition of amino acids (GSH, Cys, Hcy) and various anions (HS−, AcO−, H2PO4−, F−, Cl− Br− and I−) through UV-vis titration experiments in pure DMSO solution and aqueous solution (DMSO-H2O 4:1, v/v 0.04 mol·L−1 HEPES buffer at pH 7.38) respectively. The data of UV-vis titration experiments manifested only compounds (1, 2) displayed different binding abilities with the above anions and amino acids. The free 1 showed a main absorption at 320 nm, as the HS− increases in pure DMSO solution of probe 1, the absorbance at 320 nm was decreased gradually, along with the simultaneous emergence of a new absorption at 470 nm. In this process, two isosbestic points noted at 330 nm and 348 nm suggesting a clear chemical reaction. Based on the well-establish thiolysis reaction of dinitrophenyl ether, the new absorption at 470 nm could be attributed to coumarin derivative, which was also supported by fluorescence and HRMS titration experiment. Furthermore, the UV-vis spectra of probe 1 with HS− in aqueous solution were also performed (shown in Fig. 3b), however, comparison with DMSO solvent, the probe 1 showed a weak response of UV-vis spectra.
UV-vis spectra of compound 1 (4.0 × 10−5 mol·L−1) with the addition of HS−(0–8 × 10−6 mol·L−1)(a) in DMSO solution; (b) in aqueous solution (DMSO-H2O 4:1, v/v 0.04 mol·L−1 HEPES buffer at pH 7.38). Arrows indicate the direction of increasing HS− concentration; (c) Color changes observed with the addition of 20 equiv. various anions and Cys to DMSO solution of compound 1 respectively; (d) Fluorescent intensity of compound 1 upon the addition of HS−.
The additions of amino acids (Cys, GSH, Hcy) and other anions (AcO−, H2PO4−, F−, Br−, Cl− and I−) to pure DMSO solution of probe 1, only Cys induced similar changes in the UV-vis spectra compared with HS−, which exhibited Cys also interacted with compound 1 (supplementary material). However, the additions of the above amino acids and anions to aqueous solution induced almost no spectra changes of compound 1. The result indicated that compound 1 showed different binding abilities for HS− and Cys in pure DMSO solution and almost no binding abilities with above amino acids and anions in aqueous solution. Therefore, compound 1 could be used as a sensor to detect HS− in aqueous solution.
Subsequently, the colorimetric sensing capabilities of compound 1 were carried out with different anions (Fig. 3c). Obvious color changes from colorless to bright yellow was observed in the presence of HS−, while faint or no color changes happened in the presence of other anions (AcO−, H2PO4−, F−, Cl−, Br−, I− and Cys), which indicated that compound 1 can be used for the detection of HS− as a colorimetric sensor.
Moreover, the detection limit32,33 of compound 1 (4.0 × 10−6 mol·L−1) was implemented through fluorescence titration (Fig. 3d). Further data analysis revealed an excellent linear relationship (r = 0.9963) between the fluorescence signal of the probe 1 at 392 nm and the concentration of HS− (0–8 × 10−6 mol·L−1). Therefore, the detection limit of compound 1 for HS− was determined to be 4.0 × 10−6 mol·L−1.
Next, the UV-vis titration experiments of compound 2 to various anions (HS−, AcO−, H2PO4−, F−, Br−, Cl− and I−) were tested. Upon the addition of increasing amounts of HS− (supplementary material) to DMSO solution of compound 2 a new absorption peak appeared gradually at 485 nm. While, the additions of other anions (AcO−, H2PO4−, F−, Br−, Cl− and I−) to compound 2, almost no spectra changes was observed.
Fluorescence response
The photo-physical responses of four compounds (1–4) in DMSO solvent were also investigated with addition of various amino acids and anions. Just as Fig. 4a showed, an emission peak of free 1 exhibited at about 385 nm. Upon the addition of increasing amounts of HS−, the fluorescence intensity increased obviously at about 392 nm. Similar fluorescence response of compound 1 was observed upon the addition of Cys (supplementary material) compared with HS−. Furthermore, the interactions of probe 1 with amino acids (GSH, Hcy) and other anions (AcO−, H2PO4−, F−, Br−, Cl− and I−) were also investigated. The additions of H2PO4−, AcO− and F− (supplementary material) induced the appearance of fluorescence emission bands centered at about 386 nm, however, nominal changes were induced in the presence of GSH, Hcy, F−, Cl−, Br−, I−.
Besides, the fluorescence spectral responses of compound 1 with amino acids (Cys, GSH, Hcy) and various anions (HS−, AcO−, H2PO4−, F−, Br−, Cl− and I−) were also examined in aqueous solution. From Fig. 4b, compound 1 showed very weak fluorescence at the absence of anions. After HS− was added, double emission peaks appeared and the fluorescent intensity increased gradually. Results demonstrated that compound 1 showed strong binding ability for HS−. However, weak spectral responses that even could be ignored were induced with addition of amino acids (Cys, GSH, Hcy) and H2PO4−, AcO−, F−, Cl−, Br−, I−.
For compound 2, the fluorescence intensity noted at 434 nm increasing rapidly by titration of HS− (supplementary material). No obvious responses of compound 2 were observed with titration of other anions (AcO−, H2PO4−, F−, Br−, Cl− and I−) and Cys. For compounds (3 and 4), Similar experiments were carried out, however, no significant spectral responses were observed with the addition of other anions (HS−, H2PO4−, AcO−, F−, Br−, Cl− and I−) into DMSO solution of two compounds (3 and 4) which indicated the weak binding abilities of compounds (3 and 4) and other anions (AcO−, H2PO4−, F−, Br−, Cl− and I−) and Cys could be ignored.
Binding constant
The job-pot curves suggested two compounds (1 and 2) interacted with amino acids and various anions as the ratio of 1:1 or 1:2. The UV-vis spectral data was used to calculate the binding constants by non-linear least square method34,35, and the binding constants were listed in the Table 2. Obviously, the binding ability of two compounds (1 and 2) with amino acids and various anions followed the order of HS− ≫ H2PO4−, Cys, AcO−, F−, Br−,Cl−, I−, Hcy and GSH. In general, both compound 1 and compound 2 showed the strongest binding ability for HS− among amino acids and anions. Besides, a theoretical basis and these binding constants were necessary for the optimization of sensor.
The anion binding abilities of compounds (1 and 2) with two electron-withdrawing groups on the fluorobenzene ring were stronger than that of compounds (3 and 4) which had one electron-withdrawing group on the fluorobenzene ring. In addition, the anion binding ability of compound 1 was stronger than that of compound 2 due to the electron-withdrawing ability of nitro group was greater than the trifluoromethyl group36. The above results indicated that strong electron-withdrawing groups located in 2, 4-positions of fluorobenzene provides a easily site for intermolecular HS− attack. Besides, for HS−, the binding ability trend of compounds (1–4) followed the order of 1 > 2 ≫ 3, 4 also could be confirmed by comprehensive analysis of UV-vis, fluorescence titration.
Mechanism
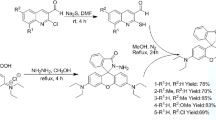
The interacted mechanism was measured by fluorescence and HRMS titration experiment. The broad emission peak of compound 1 appeared at about 396 nm in the absence of HS−. The emission peak shifted to the short wavelength from 396 nm to 380 nm after HS− was added. The emission peak of the single 7-hydroxy-4-methylcoumarin appeared at about 380 nm. The same emission peak observed in the presence of HS− which suggested free 7-hydroxy-4-methylcoumarin was released after compound 1 interacted with HS− (Fig. 5a). Furthermore, the interacted mechanism of host-guest (1-HS−) was also carried by performing MS-HRMS after the addition of 2 equiv. NaHS. The probe 1 itself exhibited a dominant peak at m/z = 341.0551(M-H)− (supplementary material). However, the above peak vanished and a new peak at m/z = 199.0364 (M + Na)+ appeared which was the ion-peak of 7-hydroxy-4-methylcoumarin (Fig. 5b) after the addition of HS−. The MS-HRMS titration experiment suggested the binding ratio of host-guest (1-HS−) was 1:2. The above results indicated the possible interacted mechanism was thiolysis reaction of dinitrophenyl ether (Fig. 5c)37,38,39,40,41,42,43,44.
(a) A comparison of fluorescence spectra in the presence of 3.2 equiv HS− between compound 1 and 7-hydroxy-4-methylcoumarin (4.0 × 10−5 mol·L−1); (b) ESI-HRMS spectrum of compound 2 after addition of 2 equiv of NaHS in DMSO solution. MS-HRMS (m/z): 199.0364 (M + Na)+; (c) The possible interacted mechanism of host-guest.
Cytotoxicity Assay
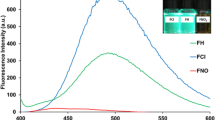
For further biological application point of view, a quantitative cytotoxicity study of two compounds (1 and 2) in vitro was conducted using MTT assay. Owning to Glutathione peroxidase (GPx1) being an important selenoprotein and not found in human breast cancer cells (MCF-7) according to scientific research, therefore, the special cell lines (MCF-7 cell) were selected by us45,46. The MTT assay results indicated that compound 1 and 2 showed very low cototoxicity over a concentration range of 0–150 μg·mL−1 especial probe 2 (Fig. 6). Cellular viability was minimally affected (80%, cellular viability) with the compounds (1 and 2) ( <150 μg·mL−1). In agreement with the binding constants, compounds (1 and 2) showed a high binding capacity and low cytotoxicity, especial probe 1, which indicated that compounds (1 and 2) have a potential application to H2S detection in cells.
Material and Methods
Most staring materials were obtained commercially, all reagents and solvents were of analytical grade and used as received without further purification unless otherwise stated. Sodium hydrolfide and all anions in the form of tetrabutylammonium salts (such as (n-C4H9)4NCl, (n-C4H9)4NBr, (n-C4H9)4NI, (n-C4H9)4NAcO and (n-C4H9)4NH2PO4) and amino acides (Cys, GSH and Hcy) were purchased from Aladdin Chemistry Co. Ltd (Shanghai, China). Dimethyl sulfoxide (DMSO) was distilled in vacuum after being dried with CaH2. 1H NMR spectra were recorded using an Unity Plus-400-MHz spectrometer. HRMS was obtaioned with a Mariner apparatus. Absorption spectra were obtained on UV-vis spectrophotometer (Shimadzu, UV-2600, Japan). Fluorescence emmission spectra were taken on a Cary Eclipse Fluorescence Spectrophotometer (Agilent, USA). The binding constant (Ks) was obtained by non-linear least squares calculation method for data fitting.
The cells that were at logarithmic growth phase were seeded in a 96-well plate at a density of 2.0 × 104 cell peer well for 24 h, followed by treatment different concentration of compound at 37 °C for additional 24 h. Next, cells were collected and washed with PBS three times then 100 µL of culture medium and 20 µL of MTT solution were added to each well respectively, and the cells were incubated for 4 h. The absorbance were detected by microplate reader (Thermo Multiscan MK3, Thermo Fisher Scientific, MA, USA) at 490 nm wavelength measurement. Cell viability (expressed in%) was calculated considering 100% growth at the absence of fluorescence probe, and the viability of other groups were calculated by comparing the optical density reading with the control. The IC50 was defined as the compound concentrations required for 80% inhibition of cell growth.
Synthesis of the probes
The compounds (1–4) were synthesized according to the route shown in Fig. 1.
Synthesis of 7-hydroxy-4-methylcoumarin (1)
7-Hydroxy-4-methylcoumarin was synthesized according to the literature47. The experimental details were described in supplementary material.
Synthesis of compound 1
7-Hydroxy-4-methyl coumarin (2.16 g, 12 mmol) and 2,4-dinitrofluorobenzene (1.5 g, 8 mmol), K2CO3 (3.39 g, 24 mmol) were dissolved in DMF(40 mL) solution with stirring. The above mixture were heated for 4 h at 90 °C at N2 atmosphere and then cooled to room temperature. The reaction was poured into ice-water (400 mL) and extracted with ethyl acetate (3 × 25 mL), dried with MgSO4 overnight. Filtration, purification with column chromatography (CH3COOCH2CH3:CH2Cl2 = 1:40). Stood overnight a yellow precipitate was obtained. Yield: 68%. m.p. 187.9–189.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.94 (s, 1 H), 8.51 (dd, J = 9.2, 2.8 Hz, 1 H), 7.92 (d, J = 8.7 Hz, 1 H), 7.40 (dd, J = 15.6, 5.8 Hz, 2 H), 7.28 (dd, J = 8.7, 2.5 Hz, 1 H), 6.43 (s, 1 H), 2.47 (s, 3 H) (supplementary material). Elemental analysis: Calc. for C16H10N2O7: C, 56.15; H, 2.94; N, 8.18. Found: C, 56.21; H, 2.94; N, 8.16. MS-HRMS (m/z): 341.0551(M-H)- (supplementary material). IR: C-O-C (Diphenyl oxide Phenyl ether Biphenyloxide): 1288 cm−1 (supplementary material).
Compounds (2–4) were also synthesized according to the above similar procedure.
Compound 2
Suitable single light yellow crystal for X-ray crystal structure analysis was obtained. Yield: 74%. m.p. 139.3–142.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.56 (d, J = 2.8 Hz, 1 H), 8.51 (dd, J = 9.1, 2.8 Hz, 1 H), 7.91 (d, J = 8.7 Hz, 1 H), 7.39–7.30 (m, 2 H), 7.23 (dd, J = 8.7, 2.5 Hz, 1 H), 6.42 (s, 1 H), 2.46 (s, 3 H) (supplementary material). Elemental analysis: Calc. for C17H10FNO5: C, 55.90; H, 2.76; N, 3.83. Found: C, 55.83; H, 2.76; N, 3.84. MS-HRMS (m/z): 366.0589(M + H)+ (supplementary material). IR: C-O-C (Diphenyl oxide Phenyl ether Biphenyloxide): 1274 cm−1 (supplementary material).
Compound 3
Yield: 75%. m.p. 140.9–143.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.16 (dd, J = 8.2, 1.5 Hz, 1 H), 7.86–7.76 (m, 2 H), 7.51 (t, J = 7.8 Hz, 1 H), 7.39 (d, J = 8.3 Hz, 1 H), 7.09–7.01 (m, 2 H), 6.35 (s, 1 H), 2.43 (s, 3 H) (supplementary material). Elemental analysis: Calc. for C16H11NO5: C, 64.65; H, 3.73; N, 4.71. Found: C, 64.72; H, 3.72; N, 4.72. MS-HRMS (m/z): 320.0550(M + Na)+ (supplementary material). IR: C-O-C (Diphenyl oxide Phenyl ether Biphenyloxide): 1273 cm−1 (supplementary material).
Compound 4
Yield: 78%. m.p. 165.5–167.3°C. 1H NMR (400 MHz, DMSO-d6) δ 8.35–8.27 (m, 2 H), 7.88 (d, J = 8.7 Hz, 1 H), 7.28 (dd, J = 10.2, 3.1 Hz, 3 H), 7.19 (dd, J = 8.7, 2.4 Hz, 1 H), 6.40 (s, 1 H), 2.46 (s, 3 H) (supplementary material). Elemental analysis: Calc. for C16H11NO5: C, 64.65; H, 3.73; N, 4.71. Found: C, 64.82; H, 3.74; N, 4.70. MS-HRMS (m/z): 320.0533 (M + Na)+ (supplementary material). IR: C-O-C (Diphenyl oxide Phenyl ether Biphenyloxide): 1267 cm-1 (supplementary material).
A light yellow crystal of compound 2 with dimensions of 0.45 nm × 0.32 nm × 0.23 nm was mounted on a glass fiber. X-ray single-crystal diffraction data was collected on a Rigaku saturn CCD area detector at 293 K with Mo-Ka radiation (λ = 0.71073 Å). The structure was solved by direct methods and refined on F2 by full-matrix least squares methods with SHELXL-9748.
Conclusion
In conclusion, we developed a series of fluorescence probes based on coumarin derivatives for the detection of H2S successively with OFF-ON” fluorescence response. The fluorescence probes, especially compound 1, exhibited remarkable response to H2S against other anions and amino acids in pure DMSO solvent. Otherwise, the probe 1 also showed strong binding ability for HS− in HEPES buffer solution. The interacted mechanism of host-guest was the thiolysis reaction of dinitrophenyl ether. In addition, compound (1 and 2) showed highly sensitivity and low cytotoxicity to MCF-7 cells and probe 1 can also detect HS− selectively by naked eye in pure DMSO solvent. The results of our efforts highlight that the probes, especially compound 1, hold a potential chemical tool for the detection of H2S.
References
Yu, H., Xiao, Y. & Jin, L. A lysosome-targetable and two-photon fluorescent probe for monitoring endogenous and exogenous nitric oxide in living cells. J. Am. Chem. Soc. 134, 17486–17489 (2012).
Szabό, C. Hydrogen sulfide and its therapeutic potential. Nat. Rev. Drug. Discov. 6, 917–935 (2007).
Li, L., Rose, P. & Moore, P. K. Hydrogen sulfide and cell signaling. Ann Rev Pharmacol Toxicol. 51, 169–187 (2011).
Predmore, B. L., Lefer, D. J. & Gojon, G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid Redox Signaling. 17, 119–140 (2012).
Yang, G. et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-lyase. Science. 322, 587–590 (2008).
Kimura, H. Hydrogen sulfide:its production, release and functions. Amino Acides. 41, 113–121 (2011).
Whiteman, M. & Moore, P. K. Hydrogen sulfide and the vasculature:a novel vasculoprotective entity and regulator of nitric oxide bioavailability. J. Cell. Mol. Med. 13, 488–507 (2009).
Kimura, H. Hydrogen sulfide:its production and fuctions. Exp. Physiol. 96, 833–835 (2011).
Gadalla, M. M. & Snyder, S. H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 113, 14–26 (2010).
Sen, N. et al. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell. 45, 13–14 (2012).
Hong, P. K., Ryter, S. W. & Choi, A. M. K. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. 46, 411–449 (2006).
Kamoun, P., Belardinelli, M.-C., Chabli, A., Lallouchi, K. & Chadefaux-Vekemans, B. Endogenous hydrogen sulfide overproduction in down syndrom. Am. J. Med. Genet. 116, 310–311 (2003).
Takano, Y., Shimamoto, K. & Hanaoka, K. Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. J. Clin. Biochem. Nutr. 58, 7–15 (2016).
Yan, Y. et al. Sensitive detection of sulfide based on the self-assembly of fluorescent silver nanoclusters on the surface of silica nanospheres. Talant. 174, 387–393 (2017).
Eto, K. et al. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Bio. Chem. Bioph. Res. Co. 293, 1485–1488 (2002).
Kashfi, K. The role of hydrogen sulfide in health and disease. Biochem. Pharmacol. 149, 1–4 (2018).
Cao, X. et al. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 149, 20–28 (2018).
Martelli, A. et al. Hydrogen sulphide: novel opportunity for drug discovery. Med. Res. Rev. 32, 1093–1130 (2012).
Vandiver, M. S. & Snyder, S. H. Hydrogen sulfide: a gasotransmitter of clinical relevance. J. M. M. 90, 255–263 (2012).
Li, H. et al. Simultaneous removal and measurement of sulfide on the basis of turn-on fluorimetry. Int. J. Environ. Sci. Technol., https://doi.org/10.1007/s13762-017-1483-z (2017).
Hughes, M. N., Centelles, M. N. & Moore, K. P. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo. a review. Free Radicals Biol. Med. 47, 1346–1353 (2009).
Shen, X. G. et al. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radicals Biol. Med. 50, 1021–1031 (2011).
Chen, Y. M. et al. Highly selective probes of copper(II) complexes for sulfide detection and cytotoxicity assay. J. Sulfur Chem. 39, 308–321 (2018).
Thomas, S. C., Samuel, M. R. & Xue, Z. L. Quantitative, colorimetric paper probe for hydrogen sulfide gas. Sensors Actuat. B-Chem. 253, 846–851 (2017).
Xu, Q. C., Gu, Z. Y. & Xing, G. W. Design and synthesis of a Cu(II)-complex-based carbazole-hemicyanine hybrid for fluorescent sensing of H2S in SDS micellar solution. Tetrahedron. 73, 2123–2130 (2017).
Zhang, D. & Jin, W. Highly selective and sensitive colorimetric probe for hydrogen sulfide by a copper (II) complex of azo-dye based on chemosensing ensemble approach. Spectrochim Acta. A. 90, 35–39 (2012).
Sun, M. et al. Fluorescence signaling of hydrogen sulfide in broad pH range using a copper complex based on BINOL-benzimidazole ligands. Inorg. Chem. 54, 3766–3772 (2015).
Ding, S., Feng, W. & Feng, G. Rapid and highly selective detection of H2S by nitrobenzofurazan (NBD) ether-based fluorescent probes with an aldehyde group. Sensors Actuat. B-Chem. 238, 619–625 (2016).
Park, C. S. et al. A near-infrared “turn-on” fluorescent probe with a self-immolative linker for the in vivo quantitative detection and imaging of hydrogen sulfide. Biosens. Bioelectron. 89, 919–92 (2016).
Ryu, H. G. et al. Two-photon fluorescent probe for hydrogen sulfide based on a red-emitting benzocoumarin dye. Tetrahedron Lett. 59, 49–53 (2018).
Fei, Q. et al. Design of BODIPY-based near-infrared fluorescent probes for H2S. J. Photoch. Photobio. A. 355, 305–310 (2017).
Zhu, M. et al. Visible near-infrared chemosensor for mercury ion. Org. Lett. 10, 1481–1484 (2008).
Isaad, J. & Achari, A. E. Biosourced 3-formyl chromenyl-azo dye as Michael acceptor type of chemodosimeter for cyanide in aqueous environment. Tetrahedron. 67, 5678–85 (2011).
Liu, Y., You, C. C. & Zhang, H. Y. Supramolecular Chemistry., (Nankai University Publication, Tianjin, 2001).
Bourson, J., Pouget, J. & Valeur, B. Ion-responsive fluorescent compounds. 4. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza- and diaza-crown ethers. J. Phys. Chem., 97, 4552–4557 (1993).
Hansch, C., Leo, A. & Taft, R. W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 97, 165–195 (1991).
Das, A. K. et al. Neighbouring group participation of thiol through aldehyde group assisted thiolysis of active ether: ratiometric and vapor phase fast detection of hydrogen sulfide in mixed aqueous media. New J. Chem. 39, 5669–5675 (2015).
Huang, Z. et al. Aldehyde group assisted thiolysis of dinitrophenyl ether: a new promising approach for efficient hydrogen sulfide probes. Chem. Commun. 50, 9185–9187 (2014).
Liu, T. et al. A lysosome-targetable fluorescent probe for imaging hydrogen sulfide in living cells. Org. Lett. 15, 2310–2313 (2013).
Zheng, K. et al. A two-photon fluorescent probe with a large turn-on signal for imaging hydrogen sulfide in living tissues. Anal. Chim. Acta. 853, 548–554 (2015).
Zhang, C. et al. A FRET-ICT dual-quenching fluorescent probe with large off-on response for H2S: synthesis, spectra and bioimaging. Chem. Commun. 51, 7505–7508 (2015).
Wang, J. et al. A BODIPY-based turn-on fluorescent probe for the selective detection of hydrogen sulfide in solution and in cells. Talanta. 144, 763–768 (2015).
Zhao, Q. et al. “Turn-on” fluorescent probe for detection of H2S and its applications in bioimaging. S.A.P:M&B.S. 189, 8–12 (2018).
Chen, Q. et al. Sensitive and rapid detection of endogenous hydrogen sulfide distributing in different mouse viscera via a two-photon fluorescent probe. Anal. Chim. Acta. 896, 128–136 (2015).
Vibet, S. et al. Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radical Bio. Med. 44, 1483–1491 (2008).
Shang, X. et al. Development and cytotoxicity of Schiff base derivative as a fluorescence probe for the detection of L-Arginine. J. Mol. Struct. 1134, 369–373 (2017).
Ahluwalia, V. K., Bhagat, P., Aggarwal, R. & Chandra, R. Intermediates for Organic Synthesis, I. K. International Pvt. Ltd, Delhi (2005).
Sheldrick G. M. SHELX97. Programs for crystal structure analysis. University of Gottingen, Germany (1997).
Acknowledgements
This work was supported by the Fund of Program for Science & Technology Innovation Talents in Universities of Henan Province (15HASTIT039); Fund of Fluorescence Probe and Biomedical Detection Research Team of Xinxiang City (CXTD16001), Scientific and Technological Research Projects of Henan Province, China (182102311124), Science and Technology Project of Education department of Henan Province (14A150012), and Science and Technology Project of Xinxiang City (15SF08).
Author information
Authors and Affiliations
Contributions
X.S., H.W., T.W. directed the work. Y.C. conceived the idea and designed the experiments. Y.C. and Z.X. performed the synthesis. C.L., H.C. performed the cytotoxicity Assay. Y.C. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Shang, X., Li, C. et al. The synthesis, crystal, hydrogen sulfide detection and cell assement of novel chemsensors based on coumarin derivatives. Sci Rep 8, 16159 (2018). https://doi.org/10.1038/s41598-018-34331-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34331-9
Keywords
This article is cited by
-
Detection of hydrogen sulfide using BODIPY based colorimetric and fluorescent on-off chemosensor
Journal of Chemical Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.