Abstract
Anagalidae are extinct primitive Euarchontoglires from Asia, regarded as relatively closely related to basal Glires. So far, the group has been reported only from China and stratigraphically spans from the early Paleocene to the latest Eocene/earliest Oligocene. Anagalids are characterized by a relatively full dental formula featuring slightly enlarged semi-procumbent incisors, prominent canines, and tall cheek teeth with usually heavily worn crowns, indicative of an abrasive diet. Here we report a new genus and species from the late Eocene Ergilin Dzo Formation in southern Mongolia. The first non-Chinese anagalid is also the northernmost record of the family. Zofiagale ergilinensis gen. and sp.nov. is remarkable for its relatively small size (comparable only to the Paleocene genera Huaiyangale and Stenanagale), lack of P1, and molariform teeth showing almost no wear, suggesting a different diet than most Anagalidae. Furthermore, its molars display a strong buccal cingulum, a character in anagalids shared only with Wanogale. Our phylogenetic analysis of representatives of all anagalid genera based on 82 dental characters places Anagale and Anaptogale as the most basal lineages and clusters Zofiagale gen. nov. together with Qipania and Hsiuannania. These results suggest three independent northward dispersal events within the family in the late Eocene.
Similar content being viewed by others
Introduction
Anagalidae is an enigmatic and poorly studied group of primitive members of Euarchontoglires1,2,3 known from the Paleogene of Asia, with its geographic distribution so far restricted to China4,5. The only genus ever considered to be an anagalid from outside of China, namely Khashanagale from the Gashato Formation in Mongolia6, was reassigned later by McKenna and Bell7 to Oxyclaenidae, a condylarth-related group of poorly recognized relationships.
The taxonomic status of anagalid genera is contested and different sources consider true anagalids a different number of genera. The first and only phylogenetic analysis of anagalids was done by Hu4, who considered just seven of the 14 genera to be unquestionable anagalids4,8,9,10,11,12,13,14. The seven genera were Anagale, Anagalopsis, Linnania, Huaiyangale, Hsiuannania, Eosigale, and Qipania. Hu4 classified Stenanagale, Anaptogale, and Diacronus tentatively within Anagalidae, but excluded Khashanagale, Chianshania, and Wanogale from the family.
In a recent work, Li5 considered the three genera that Hu4 removed from the family as tentative anagalids, and also included Interogale (described by Huang and Zheng15) within? Angalidae, a genus that Hu4 did not discuss.
Anagalids were never common, but their fossil record drops substantially after the middle Paleocene (Nongshanian Asian Land Mammal Age), with the only presence of Anagale from the late Eocene (Ulangochuian ALMA) and Anagalopsis, estimated generally in a broader frame of the earliest Oligocene9, (but see Zhang and Wang16 for an earlier estimate). No anagalids have been reported from the early Eocene (Bumbanian and early Arshatan ALMAs) and most of the middle Eocene (recently Li et al.17 described unidentified anagalid remains from Irdinmahan deposits of Wulanhuxia in Nei Mongol). Because of the aforementioned uncertain date of Shanmacheng (=Shih-ehr-ma-ch’eng as reported in Bohlin9), the type locality of Anagalopsis kansuensis, it can be hard to determine the more precise date of extinction of this group of early Euarchontoglires. However, anagalids were certainly present in Asia for nearly 30 million years.
Here we describe a new anagalid from the late Eocene of the Ergilin Dzo Formation in Mongolia (Fig. 1), the first member of Anagalidae found outside of China, and the northernmost representative of the group. It is characterized by a unique dental morphology and relatively small size. We assign this specimen to a new genus and species. Furthermore, we aim at reconstructing the phylogenetic relationships within Anagalidae in only the second cladistic analysis after Hu’s4, using an extended character-data matrix.
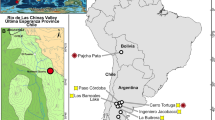
Geography and stratigraphy of new anagalid finding. Map of Mongolia, with the Dornogovi Province (light gray) and the location of Ergilin Dzo (A). Detailed map of the Dornogovi Province with the position of the Ergilin Dzo region (B). Generalized cross section of late Eocene deposits in the Ergil Obo locality and the Novozhilov Hills; SE part of the Ergilin Dzo Promontory (C). Stratigraphic reference section of the Ergilin Dzo Fm. at Ergil Obo, indicating the main beds (I–IV) and sediment layers (1–10; see Supplementary Information for details) (D). Modified from Yanovskaya et al. 1977 (C,D). (The drawings were created in Corel Draw X4 (v. 14.0) by Łucja Fostowicz-Frelik).
Results
Systematic Paleontology
Superorder EUARCHONTOGLIRES Murphy et al. 2001
Order ANAGALIDA Szalay & McKenna, 1971
Family ANAGALIDAE Simpson, 1931
Genus Zofiagale gen. n.
urn:lsid:zoobank.org:act:51CCC038-A454-4217-B849-6242FEE6F84C
Type species: Zofiagale ergilinensis sp. n., monotypic.
Etymology: Named after Professor Zofia Kielan-Jaworowska (1925–2015), for her exceptional contributions to the understanding of early mammalian fauna from Mongolia; and -gale, γαλῆ (feminine), Greek, meaning “weasel”, a common suffix used for the names of anagalids.
Distribution: As for the type and only species.
Diagnosis: As for the type and only species.
Zofiagale ergilinensis sp. n.
urn:lsid:zoobank.org:act:1CB04445-F10A-40F5-809A-A6F4EE741C95 Fig. 2.
Left mandible body of Zofiagale ergilinensis gen. et sp. nov. The holotype and only specimen (ZPAL MgM-II/100) with complete P4–M3, fragment of P3, and roots of P2 (A–F). Lingual (A), buccal (B,C), anterolingual (D), and occlusal (E,F) views, respectively. Magnification of the buccal sides of P4–M1 showing well-developed cingula (C). Note depth and inclined course of alveolus for semi-procumbent canine (D). Explanatory SEM image for dental loci and morphology (F). Abbreviations: c, canine; co, cristid obliqua; ec, ectoconid; hy, hypoconid; hyd, hypoconulid; mc, mesoconid; me, metaconid; pro, protoconid. (Figure created in Photoshop CS5 and Corel Draw X4 (v. 14.0) by Łucja Fostowicz-Frelik).
Holotype: ZPAL MgM-II/100 fragmentary right dentary with the canine alveolus, P2 roots, and P3–M3 in situ; housed at the Roman Kozłowski Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland (ZPAL).
Type locality and age: Ergilin Dzo locality, Ergilin Dzo Formation, Dornogovi Province, Mongolia (Fig. 1); Ergilian ALMA, late Eocene.
Diagnosis: Smaller than Anagale, Anagalopsis, Hsiuannania, and Qipania; comparable size to Linnania, Huaiyangale, Eosigale, and Interogale; and larger than Stenanagale; based on area of M1. Further differs from Anagalopsis, Qipania, and Interogale in having a smaller canine alveolus. Further differs from all anagalids, except for Interogale, in lacking a P1. Differs from Anagale, Anagalopsis, and Linnania in lacking a diastema between P2 and P3. Further differs from Linnania and Eosigale in lacking a diastema between P3 and P4. Moreover, it differs from all anagalids, except for Eosigale, in lacking a metaconid on P4 and further differs from Anagale and Linnania in lacking a paraconid on P4. From Qipania and Interogale it differs in lacking a cristid obliqua on P4. Further differs from Linnania lofoensis in lacking a paraconid on M1, and differs from L. lofoensis, Interogale, and Wanogale in lacking a paraconid on M2. Further differs from all anagalids, except for L. lofoensis, L. qinglingensis, Interogale, and Wanogale in having a hypoconulid on M1–M2. Further differs from L. lofoensis and Interogale in lacking a paraconid on M3, and differs from all anagalids, except for Wanogale, in having a buccal cingulum on the lower molars. Additionally it differs from Anagalopsis, Linnania, Hsuiannania, and Qipania in having the enamel not extending into the alveolus.
Description and comparisons: The right body of the mandible is broken at the level of the canine alveolus in the rostral end, and at the level of the M3 talonid in the caudal end, exposing the posterior root of the M3. Two mental foramina are visible. The anterior mental foramen is located below the posterior root of the P2, and the posterior mental foramen is smaller and located below the posterior root of P3. The total length of the P4–M3 tooth row is 14.5 mm (the total molar row length is 11.5 mm; see Table 1 for more detailed dental measurements). The buccal height of the body of the mandible at the P4/M1 inter-alveolar septum is 5.7 mm, while at the lingual side it is 6.1 mm.
The lower dental formula for Zofiagale ergilinensis is inferred to be? 0.1.3.3. Zofiagale is characterized by the loss of P1, which is rare in anagalids. Indeed, only Interogale, which has been tentatively classified as an anagalid in Li5 is known to lack a P1 as well15. Another rare characteristic for an anagalid seen in Zofiagale is the presence of a buccal cingulid on M1–M3, whereas most anagalids have a completely smooth buccal surface. There is only one other instance of an anagalid with a buccal cingulid: Wanogale12. Wanogale is known from only an M2, and also has another feature more typical of euarchontans, which is the presence of a precingulid.
The dentition anterior to P3 is lost, and only two roots and a large alveolus remain. The large alveolus is here considered to have hosted a moderately large, semiprocumbent canine. All the other anagalids for which information about the canines is known (Anagale, Anagalopsis, Eosigale, Qipania, and Interogale) show that they had relatively larger and stronger canines than those of, e.g., zalambdalestids, a group of Cretaceous Eutheria proposed as primitive members of Anagalida sensu lato6. The semiprocumbent canines are known for Anagale and Qipania. No anagalid has a P1 that is significantly larger than the rest of premolars, therefore it is more likely that the large alveolus hosted a canine rather than a P1. The two roots between the canine and the P3 could be interpreted either as a double-rooted P2 with loss of P1, or as a single-rooted P1 and a single-rooted P2. We favor the first interpretation, even taking into account that the loss of P1 is rare in anagalids, and only Interogale seems to have lost this tooth position, as some morphological traits strongly imply a two-rooted condition on P2. The first of the two roots is shifted buccally with respect to the second root, a pattern that coincides with the root positions in P3 (indeed, the anterior root of P3 is displaced more buccally than the posterior root, see Fig. 2E,F). This pattern is also seen in other mammals in which there is crowding of the premolars and no lower diastema, such as in the scandentian Ptilocercus lowii (e.g., USNM 488054). Therefore, we interpret the buccal torsion of the P3 and the lack of diastemata as a clue that the two roots belong to one double-rooted P2, the P1 has been lost, and that there is a fairly high degree of crowding of the premolars in Zofiagale.
The P3 is broken, with most of the trigonid missing, but the two roots are preserved. Because of the crowding of the anterior dentition, there is no diastema distal to P3 in Zofiagale, whereas it is observed in Linnania, Eosigale, and Stenanagale. The remains of P3 indicate presence of a large, tall and presumably single-cusped (protoconid?) trigonid and a small saddle-like talonid (Fig. 2A,B,E,F). The talonid is not basined and its buccal and lingual margins are much lower than the distal one, indicating the presence of a minuscule hypoconulid. Also, the lingual margin of the talonid bears a well pronounced cingulid-like edge.
The P4 has no metaconid, usually present in other anagalids. The only other anagalid known to lack a metaconid is Eosigale. The paraconid is also absent, although a paracristid is clearly visible. The lack of paraconid is more common in anagalids, and only Anagale and Linnania retain one. The protocristid directed linguodistally is strong and slopes lingually to the lingual side of the tooth, in fact somehow replacing functionally the missing metaconid (see Fig. 2F). The talonid is not basined and is similarly saddle-like as in P3, but the distal margin bears a more pronounced eminence, which is most probably a nascent hypoconulid, developed fully in molars.
The mesiodistal length of P4 is smaller compared to that of M1. This feature is shared by most anagalids, except for Eosigale and Qipania, which have similar mesiodistal lengths of the two tooth positions. The P4 does not have a cristid obliqua, which is rarely present in angalids (only found in Qipania and Interogale). The P4 has a buccal cingulid, not seen in any other anagalid.
The M1 does not have a paraconid, although the paracristid is present, and the trigonid basin is deep (Fig. 2F). Many characters of the dental crown, such as small cusps are sometimes difficult to observe in anagalids, as the crowns in most species are worn down very quickly in ontogeny. In any case, the presence of paraconid on M1 has only been found in Linnania. The trigonid and the talonid of M1 have approximately the same area, and the trigonid is less than double of the height of the talonid. The metaconid is higher than the protoconid, and the hypoconid and the entoconid have approximately the same height. The talonid is deeply basined, but the basin area is not very extended. There is a distinct cristid obliqua and small mesoconid at M1. The entoconid is higher than hypoconid but its area is slightly smaller than the latter; it is also placed slightly more mesially than the hypoconid. A cusp-like, rounded hypoconulid is placed centrally at the distal margin of the tooth. The tooth bears a strong buccal cingulid, which causes some extension of the buccal side of the tooth.
The M2 is larger than M1 (Table 1) and more extended buccally. It also does not have a paraconid, but the paracristid is strong and the trigonid basin larger. This contrasts with the expression of the paraconid in Linnania, Interogale, and Wanogale (although there are generic differences in that respect). The metaconid is higher than the protoconid, and the hypoconid and the entoconid are approximately the same height. Both M1 and M2 have similarly developed hypoconulids, only present in a few other anagalids (Linnania, Interogale, and Wanogale). The talonid basin has a greater area than in M1. A weak metaconid and cristid obliqua are present.
The M3 is smaller than M2 and also has no paraconid, although the paracristid is present. This contrasts with Linnania and Interogale, which have a paraconid in this locus. The trigonid and talonid are similar in breadth. This is a rare feature in anagalids, since all other anagalids, except for Anagalopsis, have narrower talonids than trigonids. The talonid is taller than the trigonid, which is due to the upward curvature of the M3 around the area of the hypoconulid lobe. This feature also occurs in Huaiyangale, Eosigale, and Qipania. The hypoconid and the entoconid are approximately the same height, but they are not quite aligned, the entoconid being marginally more distal. The talonid basin is extended and shallower having a relatively flat bottom; it forms a small extension towards a large and distally elongated hypoconulid and invades its surface to some extent (Fig. 2F).
All molars have a distinct hypoflexid. The enamel does not extend into the alveolus, in contrast to a few anagalids (Anagalopsis, Linnania, Hsiuannania, and Qipania; Hu4); furthermore, the molar crowns are relatively wear resistant, a rare trait for anagalids.
Phylogenetic analysis
In order to assess the phylogenetic relationships of Zofiagale with other anagalid genera, we conducted a cladistic analysis. A list of anagalid-specific characters was created based on character diagnoses from Chow et al.10, Xu11,12, Ting and Tong13, Ting and Zhang14, and Li5, in addition to some of Hu’s4 characters from his original phylogenetic analysis of the family. These characters have been complemented by characters taken from phylogenies of other groups of Euarchontoglires1,18,19 (see Supplementary Material, Table S1). The primitive eutherian Zalambdalestes lechei was chosen as the outgroup for Anagalidae following some broader analyses on Glires1,2,20,21.
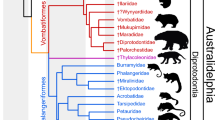
To correctly place Zofiagale in a phylogenetic context, a representative for each of the 14 anagalid genera were included. A total of 82 dental characters were scored for 15 taxa (see Supplementary Information 1). The analysis yielded a single most parsimonious tree (Fig. 3). Anaptogale and Anagale are the most basal anagalids in this tree, and constitute two separate primitive lineages (Fig. 3). The rest of anagalid taxa are evenly distributed between two distict clades. Zofiagale is a sister taxon to Hsiuannania and Qipania, and, in turn, this clade is the sister group to a clade composed of Chianshania and Eosigale, and Huaiyangale at a more basal position. The second major clade contains a clade grouping most genera presumed tentative anagalids (as per Hu4), such as Stenanagale, Wanogale, and Interogale, with Linnania being the most basally nested, and Anagalopsis clustered with Diacronus.
Hypothesis of relationship of known anagalid genera, with listed synapomorphies. The figured tree is the most parsimonious and only resulting tree of the analysis. For further details of the phylogenetic analysis see text and Supplementary Information (Table S1, and Dataset S1). Color code for character states: yellow (0), red (1), blue (2), green (3), and purple (4). (Figure created by Sergi López-Torres with Corel Draw X4 (v. 14.0)).
Discussion
Anagalidae are a poorly known group of placental mammals with no extant representatives. The type and best known genus is Anagale gobiensis. By virtue of its almost complete skull and some postcranial remains8, Anagale has been often used in phylogenetic analyses of Eutheria where it has appeared well nested within Euarchontoglires1,2,22. Our results challenge the general topology of Hu’s4 hypothesis of relationships among anagalids, relegating Anagale to a basal position on our tree, and separating Linnania and Anagalopsis from Eosigale, Huaiyangale, and Hsiuannania–Qipania cluster. Our tree, however, agrees with Hu’s4 on the close relationship between Hsiunnania and Qipania, and Huaiyangale and Eosigale. Our tree shows a split between two major groups of anagalids. One of these clades includes Hsiuannania, Qipania, Zofiagale, Huaiyangale, Chianshania, and Eosigale. This clade appears well supported by a few synapomorphies: a P3 smaller than P4, presence of a paracingulum on M1, absence of a paraconule and a metaconule on M1-2, absence of a diastema distal to P2, a talonid taller than the trigonid on M3, and a smaller area of M1 compared to M2. The clade includes four out of seven taxa of Hu’s4 unambiguous anagalids. The other major group of anagalids is composed of Interogale, Wanogale, Stenanagale, Linnania, Anagalopsis, and Diacronus. This clade is well supported by the following synapomorphies: presence of a minute parastyle on M2, a distinctive paraconule and metaconule on M1–2, an erect rather than semi-procumbent lower canine, a large root of the lower canine, and a trigonid taller than the talonid on M3.
A new genus Zofiagale is well nested within the anagalid tree; in fact, it represents one of the most derived taxa, closely related to Hsiunnania and Qipania. It shows some unique dental characters which stress its independent phylogenetic heritage and imply a long evolutionary history. Interestingly, Zofiagale shares its two most characteristic features with species which do not show the most typical anagalid dental morphology and whose anagalid status has been questioned. With Wanogale it shares a strong buccal cingulum on lower molars and crowns that do not heavily wear down, and with Interogale, a lack of P1. Although Hu4 did not consider Wanogale to be an anagalid, Li5 placed it as a tentative member of the family. On the other hand, Interogale, also of questionable status, was considered as belonging to Tillodontia by Wang and Jin23.
The taxon sample for our tree does not allow us to decide on what is and what is not an anagalid. In order to do so, other non-anagalid euarchontogliran representatives (i.e., primates, scandentians, lagomorphs, rodents, etc.) should be included in a broader phylogenetic analysis; of course, this raises the question if anagalids are paraphyletic, especially because many tentative anagalids according to Hu4 and Li5 are well nested within our sample. Such phylogenetic analysis including representatives of euarchontan and Glires lineages is necessary also to properly assess the position of anagalids within the broader framework of Euarchontoglires. However, an analysis of this magnitude is beyond the scope of this study.
The time bracket for the anagalid evolution spans from the early Paleocene to late Eocene/earliest Oligocene, with the timing of Anagalopsis ocurrence still a matter of debate5,9,16. Nevertheless, it is one of the last occurring anagalids, together with Anagale and Zofiagale (Fig. 4). The fact that all three northern anagalids belong to three distinct lineages within the family suggests that disperal events between southern and northern China (including the Mongolian Plateau) were probably common sometime between the late Paleocene and late Eocene, and these happened at least three times within Anaglidae.
Phylogeny of anagalids with a temporal context. Tree from Fig. 3 plotted against Asian Land Mammal Ages (ALMA). Abbreviations: Olig. = Oligocene; K/Pg = Cretaceous-Paleogene boundary. (Figure created by Sergi López-Torres with Corel Draw X4 (v. 14.0)).
Most anagalids have significantly worn-down cheek teeth, which has led McKenna24 to suggest that they may have obtained fairly abrasive food below the surface of the ground. This view endorsed Bohlin’s9 idea that anagalids were potentially fossorial. However, back in 1963, the only anagalids known were Anagale and Anagalopsis, and the variety of dental shape and body masses reported in anagalids have increased greatly (Fig. 5). The present record of anagalids shows taxa with non-worn or lightly worn cheek teeth, such as Zofiagale and Wanogale. This suggests that their diet could not be primarily composed of abrasive foods, and most probably would differ from that suggested for Anagale and Anagalopsis. It is, however, worth noting that this inference derives from a sample size of a single specimen and should therefore be taken with caution. Alternatively, ZPAL MgM-II/100 could belong to a very young adult (the M3 is erupted) who has not started wearing down the crowns. However, if that was the case and this animal had an abrasive diet, it would be expected to find differential wear between M1 and M3 (i.e., M3 erupts later), and there is not.
Zofiagale is one of the smallest anagalids, with an estimated body mass of 516 g, Kay’s25 threshold sets a minimum of 500 g for primates to acquire their protein mainly from leaves based on observations of modern primates, with species below that mass being mostly insectivorous. Besides Eocene euprimates, this threshold has been used in Paleocene and Eocene plesiadapiforms and non-primate euarchontans as well26,27,28,29 and could be tentatively informative in other early Paleogene mammals of generalized morphotypes similar to those of plesiadapiforms. The estimated body mass for Zofiagale sits closely to the 500 g threshold, suggesting that an animal this size could easily acquire protein from both insects and leaves, without relying solely on any of the two. Given the blunt cusps, it is likely that the diet of Zofiagale might have been largely enriched with fruits. Whereas the presence of a buccal cingulid (rare in anagalids) helps providing gingival protection from the opposing upper tooth and/or food30,31,32, it also effectively broadens the tooth, generating more surface for pressing a bolus of fruit.
Methods
The specimen was photographed with a scanning microscope Hitachi S−3400 N without coating, in a natural mode (low vacuum) at the Museum and Institute of Zoology, Polish Academy of Sciences, Warsaw, Poland. Furthermore, we documented the specimen using a Keyence digital microscope VHX 5000, and a Nikon (SMZ-800) stereo-microscope at the Institute of Paleobiology (IPAL), Polish Academy of Sciences, Warsaw. The measurements (Table 1) were taken using SYLVAC digital caliper and Keyence digital microscope software with an accuracy to the nearest 0.01 mm. Dental terminology generally follows Meng and Wyss22.
Geological settings
The specimen described here comes from the Ergilin Dzo ridge (“Promontory Bluff”), the locality discovered in 1922 by one of the Central Asiatic Expeditions of the AMNH33,34, in the Dornogovi Province, southeastern Mongolia. The specimen was collected in 1963 by a Polish field party during the first Polish-Mongolian Paleontological Expedition35. The Polish team prospected only the southeastern part of the ridge of Ergilin Dzo, in the vicinity of Ergil Obo (=Ardyn Obo as reported in Andrews34) and Ergil Ula35. The Ergilin Dzo ridge is an eminent promontory, ca. 50 km in length, stretching in the EW direction (at ca. 43° 29’ N latitude and 109° E longitude36), composed of late Eocene sediment beds, which comprise the Ergilin Dzo Formation36,37,38. The faunal list of fossil vertebrates recovered from Ergilin Dzo currently includes approximately 60 taxa37 (see also Supplementary Information), with more than 40 mammals assigned to Eulipotyphla, Lagomorpha, Rodentia, Carnivora, ‘Condylarthra’, Artiodactyla, and Perissodactyla37,39,40,41,42,43,44,45 (see Supplementary Information for a detailed list and additional references), the last two groups being the most diverse.
The age of the Ergilin Dzo Formation was originally established as pertaining to the Oligocene36,37, and the Ergilian ALMA was proposed as an early Oligocene ALMA36; it was further correlated with the Chadronian NALMA (North American Land Mammal Age). Because the Chadronian, long considered equivalent to early Oligocene is now placed in the late Eocene as a result of the revised Eocene-Oligocene Boundary46, consequently the Ergilin Dzo Fm. also moved into the latest Eocene in age (most probably the middle to late Chadronian; 35.7–33.7 Ma approximate). At present, Ergilin Dzo is a reference section for the Ergilian ALMA, which precedes the earliest Oligocene Shandgolian ALMA47. The Ergilin Dzo area during the late Eocene witnessed an increasing aridification48 towards forest-steppe, which agrees with paleoenvironmental conditions at the Eocene-Oligocene transition in central Asia49.
Cladistic analysis
A parsimony analysis was performed using TNT 1.550 with all characters equally weighted. Multiple character states were set to be interpreted as polymorphisms, instead of uncertainties. Forty-one of the 82 characters (1, 7, 12–13, 20–23, 26–31, 33–34, 37–38, 40, 43, 46, 48, 53, 57, 59–63, 65–72, 75, 79–82) were ordered based on a morphoclinal criterion, and the rest were left unordered. A traditional search was implemented with 1000 repetitions. The tree-bisection-reconnection (TBR) algorithm was used for branch swapping, with 1000 trees to save per replication.
Size estimation
We estimated the body mass of Zofiagale and comparative anagalid taxa using Conroy’s51 equation for prosimians. Although Zofiagale is not a primate, Conroy’s51 equations have been used to estimate body mass for plesiadapiforms28,29,51,52,53,54,55, and anagalids have a more similar generalized morphotype to plesiadapiforms than to modern Glires. Therefore, an equation based on a prosimian subset would be preferable for anagalids, rather than equations based on living rodents56 or lagomorphs57.
Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the Official Register of for the International Code of Zoological Nomenclature (IZCN), mandatory for electronic-only publications. The ZooBank LSIDs (Life Science Identifiers) can be resolved by appending the LSID to the prefix “http://zoobank.org”. The LSID for this publication is urn:lsid:zoobank.org:pub:65C21FB2-3E88-4A00-8900-6380A4661E81. The identifiers for taxa appear following each new name (see Systematic Paleontology).
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Meng, J., Hu, Y.-M. & Li, C.-K. The osteology of Rhombomylus (Mammalia: Glires): implications for phylogeny and evolution of Glires. Bull. Am. Mus. Nat. Hist. 275, 1–247 (2003).
Asher, R. J. et al. Stem Lagomorpha and the antiquity of Glires. Science 307, 1091–1094 (2005).
Fostowicz-Frelik, Ł. Convergent and parallel evolution in early Glires (Mammalia) in Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolution, Methods and Concepts (ed. Pontarotti, P.) 199–216 (Springer Verlag, 2017).
Hu, Y. Two new genera of Anagalidae (Anagalida, Mammalia) from the Paleocene of Qianshan, Anhui and the phylogeny of anagalids. Vert. PalAsiat 31, 153–182 (1993).
Li, C.-K. Order Anagalida in Basal Synapsids and Mammals, Volume III, Eulipotyphlans, Proteutheres, Chiropterans, Euarchontans, and Anagalids (eds. Li, C.-K. & Qiu, Z.) Fascicle 3 (Serial no. 16), Palaeovertebrata Sinica 390–411 (Science Press, 2016).
Szalay, F.S. & McKenna, M.C. Beginning of the age of mammals in Asia: the late Paleocene Gashato fauna, Mongolia. Bull. Am. Mus. Nat. Hist. 144, 273–317 (1971).
McKenna, M. C & Bell, S. Classification of Mammals Above the Species Level. (Columbia University Press, New York, 1997).
Simpson, G. G. A new insectivore from the Oligocene, Ulan Gochu Horizon, of Mongolia. Am. Mus. Novit. 505, 1–22 (1931).
Bohlin, B. Some mammalian remains from Shih-ehr-ma-ch’eng, Hui-hui-p’u area, Western Kansu. Reports from the Scientific Expedition to the North-Western Provinces of China under Leadership of Dr Sven Hedin. The Sino-Swedish Expedition Publication 35, VI. Vertebrate Paleontology 5, 1–48 + 7 pl (1951).
Chow, M. M., Chang, Y., Wang, B. & Ting, S. New mammalian genera and species from the Paleocene of Nanhsiung, N. Kwangtung. Vert. PalAsiat. 11, 31–35 (1973).
Xu, Q. New materials of Anagalidae from the Palaeocene of Anhui (A). Vert. PalAsiat. 14, 174–184 (1976).
Xu, Q. New materials of Anagalidae from the Palaeocene of Anhui (B). Vert. PalAsiat. 14, 242–251 (1976).
Ting, S. & Tong, Y. Some Paleocene anagalids from Nanxiong, Guangdong. Vert. PalAsiat. 17, 137–145 (1979).
Ting, S. & Zhang, Y. Insectivora and Anagalida from the Chijiang Basin of Jiangxi Province: Mesozoic and Cenozoic Red Beds of South China. Selected Papers from the Cretaceous-Tertiary Worshop. Institute of Vertebrate Paleontology, Paleoanthropology and Nanjing Institute of Paleontology Science Press, Nanxiong, Guangdong Province, 354–358 (1979).
Huang, X. & Zheng, J. A new anagalid from Upper Paleocene of Nanxiong Basin, Guangdong. Vert. PalAsiat. 21, 59–63 (1983).
Zhang, Z.-Q. & Wang, J. On the geological age of mammalian fossils from Shanmacheng, Gansu Province. Vert. PalAsiat. 54, 351–357 (2016).
Li, Q. & Wang, Y. & Fostowicz-Frelik, Ł. Small mammal fauna from Wulanhuxiu (Nei Mongol, China) implies faunal turnover across the Irdinmanhan–Sharamurunian boundary. Acta Palaeontol. Pol. 61, 759–776 (2016).
Silcox, M. T. A phylogenetic analysis of the Plesiadapiformes and their relationship to Euprimates and other Archonta [Ph.D. thesis]. Baltimore, (Johns Hopkins University, Baltimore, 2001).
Olson, L. E., Sargis, E. J. & Martin, R. D. Phylogenetic relationships among treeshrews (Scandentia): a review and critique of the morphogical evidence:. J. Mamm. Evol. 11, 49–71 (2004).
Archibald, J. D., Averianov, A. O. & Ekdale, E. G. Late Cretaceous relatives of rabbits, rodents, and other extant eutherian mammals. Nature 414, 62–65 (2001).
Meng, J. Phylogeny and divergence of basal Glires. Bull. Am. Mus. Nat. Hist. 285, 93–109 (2004).
Meng, J. & Wyss, A. R. The morphology of Tribosphenomys (Rodentiaformes, Mammalia): Phylogenetic implications for basal Glires:. J. Mamm. Evol. 8, 1–71 (2001).
Wang, Y.-Q. & Jin, X. A new Paleocene tillodont (Tillodontia, Mammalia) from Qianshan, Anhui, with a review of Paleocene tillodonts from China. Vert. PalAsiat. 42, 13–26 (2004).
McKenna, M. C. New evidence against tupaioid affinities of the mammalian family Anagalidae. Am. Mus. Novit. 2158, 1–16 (1963).
Kay, R. F. On the use of anatomical features to infer foraging behavior in extinct primates in Adaptations for Foraging in Non-Human Primates (eds Rodman, P. S. & Cant, C. G.H.) 21–53 (Columbia University Press, 1984).
Seiffert, E. R., Costeur, L. & Boyer, D. M. Primate tarsal bones from Egerkingen, Switzerland, attributable to the middle Eocene adapiform Caenopithecus lemuroides. PeerJ 3, e1036 (2015).
López-Torres, S., Selig, K. R., Prufrock, K. A., Lin, D. & Silcox, M. T. Dental topographic analysis of paromomyid (Plesiadapiformes, Primates) cheek teeth: more than 15 million years of changing surfaces and shifting ecologies. Hist. Biol. 30, 76–88 (2017).
Silcox, M. T., Bloch, J. I., Boyer, D. M., Chester, S. G. B. & López-Torres, S. The evolutionary radiation of plesiadapiforms. Evol. Anthropol. 26, 74–94 (2017).
Silcox, M. T. & López-Torres, S. Major questions in the the study of primate origins. Annu. Rev. Earth Pl. Sc. 45, 113–137 (2017).
Kermack, D. M., Kermack, K. A. & Mussett, F. The Welsh pantothere Kuehneotherium praecursoris. Zool. J. Linn. Soc–Lond. 47, 407–423 (1968).
Hillson, S. Teeth. Manuals in Archaeology. 2nd ed. (Cambridge University Press, Cambridge, UK 2005).
Anderson, P. S. L., Gill, P. G. & Rayfield, E. J. Modeling the effects of cingula structure on strain patterns and potential fracture in tooth enamel. J. Morphol. 272, 50–65 (2011).
Berkey, C. P. & Morris, F. K. Geology of Mongolia. Nat. Hist. C. Asia. Am. Mus. Nat. Hist 2, 1–475 (1927).
Andrews, R. C. The new conquest of Central Asia. Nat. Hist. C. Asia. Am. Mus. Nat. Hist 1, 1–687 (1932).
Kielan-Jaworowska, Z. & Dovchin, N. Narrative of the Polish-Mongolian Palaeontological Expeditions 1963–1965. Palaeontol. Pol. 19, 7–30 (1968).
Russell, D. E. & Zhai, R.-J. The Paleogene ofAsia: mammals and stratigraphy. Mém. Mus. Natl. Hist. Nat. Série C Sci. Terre. 52, 1–488 (1987).
Yanovskaya, N. M., Kurochkin, E. N. & Devyatkin, E. V. Ergilin-Dzo locality – the stratotype of the lower Oligocene in South-East Mongolia. Trans. Joint Soviet-Mongol. Paleont. Exped. 4, 1–104 (1977).
Saneyoshi, M., Tsubamoto, T., Watabe, M. & Tsogtbaatar, K. Lithostratigraphic and sedimentological analysis of the upper Eocene Ergilin Dzo Formation of Ergilin Dzo locality, Mongolia. Hayashibara Mus. Nat. Sci. Res. Bull. 3, 149–153 (2010).
Matthew, W. D. & Granger, W. The fauna of the Ardyn Obo formation. Am. Mus. Nov. 98, 1–5 (1923).
Osborn, H. F. Cadurcotherium from Mongolia. Am. Mus. Nov. 92, 1–2 (1923).
Matthew, W. D. & Granger, W. New creodonts and rodents from the Ardyn Obo formation of Mongolia. Am. Mus. Nov. 193, 1–7 (1925).
Matthew, W. D. & Granger, W. New ungulates from the Ardyn Obo formation of Mongolia with faunal list and remarks on correlation. Am. Mus. Nov. 195, 1–12 (1925).
Burke, J. J. New fossil Leporidae from Mongolia. Am. Mus. Nov. 1117, 1–23 (1941).
Dashzeveg, D. On two Oligocene Hyaenodontidae from Erghilyin-Dzo (Mongolian People’s Republic). Acta Palaeontol. Pol. 9, 263–274 (1964).
Shevyreva, N. S. New rodents from the Paleogene of Mongolia and Kazakhstan. Paleont. J. 3, 134–145 (1972).
Prothero, D. R & Emry, R. J. The Chadronian, Orellan, and Whitneyan North American Land Mammal Ages in Late Cretaceous and Mesozoic Mammals of North America (ed. Woodburne, M.O.) 156–168 (Columbia University Press, 2004).
Lucas, S. G. Chinese Fossil Vertebrates. (Columbia University Press, New York, 2001).
Dill, H. G. et al. Facies-related diagenetic alteration in lacustrine–deltaic red beds of the Paleogene Ergeliin Zoo Formation (Erdene Sum area, S. Gobi, Mongolia). Sediment. Geol. 181, 1–24 (2005).
Sun, J. et al. Synchronous turnover of flora, fauna, and climate at the Eocene–Oligocene Boundary in Asia. Sci. Rep. 4, 7463 (2014).
Goloboff, P. A. & Catalano, S. A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Conroy, G. C. Problems of body-weight estimation in fossil primates. Int. J. Primatol. 8, 115–137 (1987).
Rose, K. D., Beard, K. C. & Houde, P. Exceptional new dentitions of the diminutive plesiadapiforms Tinimomys and Niptomomys (Mammalia), with comments on the upper incisors of plesiadapiforms. Ann. Carnegie Mus. 62, 351–361 (1993).
Bloch, J. I. & Gingerich, P. D. Carpolestes simpsoni, new species (Mammalia, Proprimates) from the late Paleocene of the Clarks Fork Basin, Wyoming. Contrib. Mus. Paleontol. Univ. Mich. 30, 131–162 (1998).
Gingerich, P. D. & Gunnell, G. F. Brain of Plesiadapis cookei (Mammalia, Proprimates): surface morphology and encephalization compared to those of Primates and Dermoptera. Contrib. Mus. Paleontol. Univ. Mich. 31, 185–195 (2005).
Fox, R. C., Scott, C. S. & Buckley, G. A. A. ‘giant’ purgatoriid (Plesiadapiformes) from the Paleocene of Montana, USA: mosaic evolution in the earliest primates. Palaeontology 58, 277–291 (2015).
Moncunill-Solé, B., Jordana, X., Marín-Moratalla, N., Moyà-Solà, S. & Köhler, M. How large are the extinct giant insular rodents? New body mass estimations from teeth and bones. Integr. Zool. 9, 197–212 (2014).
Moncunill-Solé, B., Quintana, J., Jordana, X., Engelbrektsson, P. & Köhler, M. The weight of fossil leporids and ochotonids: body mass estimation models for the order Lagomorpha. J. Zool. 295, 269–278 (2015).
Acknowledgements
We would like to thank Li Q. for access to anagalid material at the Institute of Vertebrate Paleontology and Paleoanthropology in Beijing, China. We also thank M. Kowalewska-Groszkowska for assistance in taking pictures using the scanning electron microscope at the Museum and Institute of Zoology of the Polish Academy of Sciences and M. Dziewiński (IPAL) for the help with some photographs. TNT is made freely available with the sponsorship of the Willi Hennig Society. This research was supported by a National Science Centre (Cracow, Poland) grant No. 2015/18/E/NZ8/00637 to Ł.F.-F.
Author information
Authors and Affiliations
Contributions
Ł.F.F. designed the project. Both authors performed the research, analyzed data, discussed results, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Torres, S., Fostowicz-Frelik, Ł. A new Eocene anagalid (Mammalia: Euarchontoglires) from Mongolia and its implications for the group’s phylogeny and dispersal. Sci Rep 8, 13955 (2018). https://doi.org/10.1038/s41598-018-32086-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32086-x
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.