Abstract
Photoreceptor (PR) axons project from the retina to the optic lobe in brain and form a precise retinotopic map in the Drosophila visual system. Yet the role of retinal basal glia in the retinotopic map formation is not previously known. We examined the formation of the retinotopic map by marking single PR pairs and following their axonal projections. In addition to confirming previous studies that the spatial information is preserved from the retina to the optic stalk and then to the optic lamina, we found that the young PR R3/4 axons transiently overshoot and then retract to their final destination, the lamina plexus. We then examined the process of wrapping glia (WG) membrane extension in the eye disc and showed that the WG membrane extensions also follow the retinotopic map. We show that the WG is important for the proper spatial distribution of PR axons in the optic stalk and lamina, suggesting an active role of wrapping glia in the retinotopic map formation.
Similar content being viewed by others
Introduction
The visual system of both vertebrates and invertebrates consists of light-sensing photoreceptor (PR) neurons in the retina connected to the inner layers of neurons to form a precise retinotopic map for visual information processing1,2,3. In the vertebrate retina, the PR axons project inward to form synapses with bipolar cells at the outer plexiform layer. The bipolar cells then project inward to make synaptic connections with the retinal ganglion cells (RGC) at the inner plexiform layer. Within each layer, the neurons are connected by interneurons for information integration. The RGCs axons then exit the retina and make connections with the optic tectum or lateral geniculate nucleus (LGN) in the brain in a spatially precise one-to-one retinotopic map. The retinotopic map preserves the spatial information detected by the PR and transmit into the brain for further information processing1,2,3. The formation of the retinotopic map is dependent on gradients of guidance molecules and on activity-dependent interactions among axons1,2,3,4.
The highly regular and repeated structure of the Drosophila visual system makes it an excellent experimental model to study mechanisms regulating the formation of the retinotopic map. The Drosophila compound eye consists of around 800 ommatidia, each with eight photoreceptors (R1-8) plus a number of accessory cells5. The PR axons project into the optic lobe in a spatially precise one-to-one retinotopic map, i.e. maintaining their relative anterioposterior (A-P) and dorsoventral (D-V) order (Fig. 1A). The spatial precision of the PR axonal projections into the optic lobe has been examined in detail in the adult brain of larger insects and of Drosophila6,7,8,9,10,11. The fly eye also provides a great opportunity to study the developmental progression of axonal projections and the formation of the retinotopic map (reviewed by12). The fly compound eye develops from the larval eye imaginal disc, which is connected to the brain via the optic stalk (OS). The eye disc is a single epithelial cell layer, covered with a single apical peripodial membrane that does not contribute to the adult eye. During the third instar larval stage, the eye disc begins to differentiate in a progressive wave moving from the posterior end to the anterior portion. Cells at the front of the wave transiently shorten and form a morphogenetic furrow (MF) along the D-V direction. As the D-V-oriented MF moves anteriorly, cells behind the MF begin to progressively differentiate into rows of ommatidial clusters consisting of photoreceptors5,13. The PRs differentiate in the sequence of R8 - R2/5 - R3/4 - R1/6 - R713. The newly differentiated PRs extend axons basally and then posteriorly along the basal surface, go through the OS and into the optic lobe. The R1-6 axons terminate in the optic lamina and the R7 and R8 axons extend further and enter the medulla14. The R1-6 growth cones terminate between the rows of epithelial glia and marginal glia, forming the lamina plexus15. During the pupal stage, the R1-6 axons defasciculate to undergo extensive rearrangement and make synapses with lamina neurons. The R7 and R8 axons terminate at different layers in the medulla and form synapses with lamina neurons16,17,18. In this study, we will focus on the formation of the first layer of the retinotopic map, i.e. the projection of PR axons to the lamina during the larval stage.
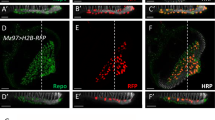
Retinotopic mapping of PR axonal projections in OS and optic lamina. Kaede expression in R3/4 is driven by mδ0.5-GAL4. (A) Schematic drawing summarizes the axon projections of single R3/4 pairs from different A-P and D-V positions in the eye disc into the OS and lamina. Samples were either live discs mounted in low gelling agarose (B–G) or fixed discs mounted on slides (H–J) (N = 7 for live discs and N = 10 for fixed discs). At least 4 single R3/4 pairs were analyzed for each disc. All respected their relative A-P and D-V order. The fixed samples provided better resolution in lamina. Axon projections were traced through different Z-sections. (H-J) Two copies of mδ0.5-GAL4 were used. (B–D), (E–G) and (H–J) are three disc samples. (B,E) Z-projections of XY plane are shown to indicate four photoactivated positions (numbered 1–4) in each disc. (C,F) In the OS, the older axons (#3 and #4) are found in the apical region. In both samples, the most anterior #1 axon has not reached OS and lamina. The anterior-dorsal #2 axon (B) projects to basal-dorsal position (C). The anterior-ventral #2 axon (D) projects to basal-ventral position (E). (D,G) The labeled axon projections (red) in the lamina were 3D-reconstructed by IMARIS and merged with all R3/4 axons (cyan). These axons maintain their relative D-V and A-P positions as in retina. (H) Z-projection shows the position of three photo-activated R3/4 pairs (#1–3) and their axon projections in the lamina, with a higher magnification view of the lamina in the same sample from a different angle (H’). The 3D reconstruction is viewed in two optical cross sections at the red dashed line (I) and white dashed line (J,J’) in H’. (I) The #1–3 axons maintained their relative D-V and A-P positions. (J,J’) In this lateral view of the lamina, the #3 (posterior, older) axon terminate at the lamina plexus (white arrowhead), and the #1 (anterior, young) axon overshoot the lamina plexus. Cyan (green Kaede)/red (red Kaede) presentation is for sample in agarose. Green (green Kaede)/magenta (red Kaede) presentation is for fixed samples. Scale bars are 20 μm for BEH and 10 μm for CDFGH’IJJ’.
The overall organization and design principle of the Drosophila retina/lamina/medulla and the vertebrate retina/inner and outer plexiform layers is very similar19,20,21. Therefore, the study of fly retinotopy can provide useful insight for the understanding of the development of the mammalian visual system. Earlier studies have examined the retinotopic projections of PR axons by electron microscopy (EM) analysis in larger insects8,9,10. The retinotopic map in Drosophila was examined by using the lipophilic dyes DiI and DiO to label the dorsal and ventral PR, respectively, the omb-τlacZ to mark the dorsal- and ventral-most PR axons, and the temporal difference in expression timing of anti-horseradish peroxidase (HRP) and monoclonal antibody 24B10 staining to distinguish the younger and older axons, and followed their axonal projections into OS and optic lobe22,23. The HRP is a neuronal marker24 that stains the core α1-3-fucosylated N-glycan on neuronal cell surface proteins25,26. The 24B10 stains specifically PR cell body and axon27 by recognizing the cell surface glycoprotein Chaoptin28,29. Because the HRP signal appears in differentiating PR about 9 hr. (at 25 °C) before the 24B10 signal, the most anterior (youngest) six or seven rows of ommatidia are labeled by anti-HRP but not by 24B1022. Thus HRP and 24B10 double staining can be used to distinguish the younger and older axons11. Marked PR clones were also analyzed30. The results showed that the PR axons follow the A-P (younger-older) and D-V order in their projection into the OS and optic lamina22. However, because, a group of axons were labelled in these experiments, the spatial resolution of PR axon development is limited. In this study, we used the photoconvertible fluorescent protein Kaede31,32,33 to explore this question. UV irradiation induces a peptide cleavage of Kaede proteins, which results in the rapid and irreversible conversion from green fluorescence to red fluorescence32. By expressing Kaede in specific PRs and selectively photo-activating PR at specific positions, we can follow PR axonal projections into OS and lamina with single axonal pair resolution.
The vertebrate retinal neurons are ensheathed by Müller glia and astrocytes (reviewed by34,35 but the role of these glia in the formation of retinotopic map has not been reported. Glia is involved in the PR axonal projection in Drosophila. In the optic lamina, glia is required for the proper termination of R1-6 axons in the lamina plexus36,37,38. In the eye disc, a group of glia, the retinal basal glia (RBG), migrates from the OS into the basal layer of the eye disc39,40. Their migration into the eye disc follows the PR differentiation and they always lag behind the anterior front of the differentiating PR41. PR axons can project toward ectopic RBGs in the eye disc41, suggesting that RBGs may provide some guidance cues for PR axons. But whether the RBGs are required for PR axon projection is controversial. Expressing the dominant-negative Ras in RBG blocked RBG migration into eye disc and prevented PR axons from entering the OS40. In contrast, knocking down of the αPS2 and βPS integrin in RBG also blocked RBG migration into the eye disc, but the PR axons project normally42.
The PR axons do not project into optic lobe as naked axons; they are enwrapped by glial membrane. The RBG consist of three major glia cell types, namely carpet glia (CG), surface glia (SG) and wrapping glia (WG) that are distinguished by morphological and molecular characteristics41,43,44. The WGs differentiate from SG44,45 in response to FGF signaling46 and extend membrane to wrap around the PR axons. This wrapping process is dependent on FGFR signaling and on the interaction of two cell surface proteins: Borderless (Bdl) expressed in WG and Turtle (Tutl) expressed in PR47,48. However, whether the glial wrapping of PR axons follow the same retinotopic principle and whether the glial wrapping plays any role in PR axonal projection have not been studied. In this study, we examined the temporal and spatial progression of WG membrane wrapping of PR axons and addressed whether the wrapping affects PR axonal projection. Our results demonstrated axonal ensheathment by wrapping glia is critical for the retinotopic map formation.
Results
Retinotopic mapping of PR axonal projections in OS and optic lamina
To achieve better spatial and temporal resolution in the retinotopic map analysis, we photo-activated Kaede in multiple single PR axon pairs at different A-P and D-V positions in the eye disc and then followed their axonal projections into OS and optic lamina. Kaede was expressed in the R3/4 PR by mδ0.5-GAL4 (mδ0.5-GAL4 + UAS-Kaede, abbreviated as mδ0.5 > Kaede). We tested different laser intensities and different sizes of laser illumination areas to identify the optimal condition for specifically photo-converting Kaede in a single R3/4 PR pair to follow their axonal projections (Supplementary Fig. 1). Several R3/4 pairs at different locations in the mδ0.5 > Kaede eye disc were labelled and examined in live or fixed discs (Fig. 1; Supplementary Fig. 2). The PR axons maintained their relative D-V and A-P positions from the eye disc to lamina (Fig. 1B–G). For example, the anterior-dorsal axon (Fig. 1B) projects to basal-dorsal position (Fig. 1C). The anterior-ventral axon (Fig. 1D) projects to basal-ventral position (Fig. 1E). In the OS, the younger (from anterior PR) axons are located at the basal position and the older (from posterior PR) axons are located at the apical positions (Fig. 1C,C’,F,F’). This order is further supported by monitoring axons in OS of the ex vivo cultured eye disc over time (Supplementary Fig. 3). In TEM analysis of anterior or posterior sections of eye disc (Fig. 2C,D), axons without clear fasciculation, representing young axons, are located at the basal layer, just above the basal glia layer. Apical to this are fascicles with 7 axons, representing axons from ommatidial clusters containing R8 and R1-6 but without the last differentiating R7. Further apical are fascicles with 8 axons, representing axons from mature ommatidial clusters with the full R1-8 axons. In summary, the PR axons project basally to the top of basal glia layer and the more mature axonal bundles are progressively pushed toward more apical positions in the eye disc, as well as in the OS. During this process and when projecting into lamina, their relative D-V and A-P positions are preserved.
Young-old PR axons follow basal-apical order in the basal layer of eye disc. (A) Schematic diagram shows that the eye-antenna disc was sectioned at different A-P positions along the D-V axis. (B) The cross sections (basal-apical surface) were examined by TEM. The photoreceptor (PR) and glia layers can be distinguished. (C–E) The apical side is up and basal side is at bottom. In both anterior and posterior sections, the youngest PR axons (not yet fasciculated; purple) are at the bottom (basal). The 7-axons fascicles (without the R7 axons; light green) lie just above the non-fasciculated axons. The mature 8-axons fascicles (light orange) lie at more apical positions. Scale bars are 20 μm (B) or 1 μm (C–E).
Young R3/4 axons transiently overshoot in lamina
To achieve better spatial resolution of PR axons in the lamina, we used another method to analyze the projection pattern of young versus older PR axons. PR differentiate in the order of R8 = >R2/5 = >R3/4 = >R1/6 = >R713. We labeled R3/4 and R7 by expressing mCD8GFP and Kaede driven by R3/4-specific mδ0.5-GAL4 and R7-specific sev181-GAL4, respectively. Since R3/4 differentiates earlier than R7, there is an anterior region (6-7 rows of ommatidia) with only R3/4 expressions, allowing specific photoconversion of the young R3/4 PRs. The anterior (younger) R3/4 are labelled by red Kaede and green GFP, while the more posterior (older) R3/4 are labelled by both green GFP and green Kaede, so the differential red-to-green ratio allows easy distinction of young vs older R3/4 axons (Fig. 3A). From the lateral view of eye disc, it is clear that the younger R3/4 axons lay at the basal layer, while the older R3/4/7 axons are located in more apical position (Fig. 3C-C’), consistent with our previous findings (Figs 1 and 2). In the lamina, the young R3/4 axons project to the anterior position (Fig. 3E).
Young R3/4 axons transiently overshoot in lamina. mCD8GFP and Kaede expression are simultaneously driven by R3/4-specific mδ0.5-GAL4 and R7-specific sev181-GAL4. Anterior is to the left for all images except (E). (A) HRP staining (cyan) shows all PR cell soma and axons. Young R3/4 cells are labeled by red Kaede (magenta) and mCD8GFP (green); mature R3/4/7 PRs are labeled by green Kaede and mCD8GFP. Box indicates the region of UV irradiation to convert Kaede. (B) R3/4 can be distinguished by cell shapes. (C-C’) Lateral view shows that HRP (cyan) appear before R3/4 axonal projections (red), which is anterior to R3/4/7 positive region (green). The extents of the three color signals are shown below. mδ0.5 > Kaede+ mCD8-GFP has low level of GFP in R3/R4 that is not apparent and is not shown in the colored bars. The young R3/4 axons (red) are basal to the older R3/4/7 (green) axons. (D) Young R3/4 axons (magenta) project past the lamina plexus while the older R3/4/7 (green) terminate at the lamina plexus in the lamina. The overshot R3/4 axons costains with HRP (blue) (D’), indicating that these are axons. (E,F) The projections can be viewed in two optical sections (red and white dashed lines in D’) in 3D reconstruction. (E,E’) The young R3/4 axons (magenta) terminate in the anterior portion of lamina, while the older axons (green) terminate in more posterior regions. (F-F”) Lateral view of lamina shows that the overshoot young R3/4 axons (arrowhead) is at the anterior portion and past the lamina plexus (arrow) marked by the older PR axon termini. The brackets indicate the lamina and medulla regions. The asterisk indicates the background of Kaede. (H) Schematic drawing of eye-brain complex showing rows of R3/4 and R3/4/7 in a late thirrd instar eye disc with ~20 rows of ommatidia. The young R3/4 axons (anterior, magenta) project pass the lamina plexus, while the older R3/4/7 axons (posterior, green) terminate at the lamina plexus. Scale bar is 5 μm for (B), 10 μm for F-F”D, 15 μm for C-C’, 20 μm for A and E-E’ and 30 μm for (F).
Interestingly, we found that whereas the older R3/4/7 axons (green) terminate in a neat line (termed the lamina plexus) in the lamina (Fig. 3E) as previously reported for all R1-6 axons15, the younger R3/4 axons (red) terminate in deeper positions in the lamina, and even in the medulla (Fig. 3D,F-F”). Some overshooting can also be observed in mδ0.5 > Kaede (Fig. 1, Supplementary Fig. 2). mδ0.5 > mCD8GFP also showed similar phenomenon (Supplementary Fig. 4B). We generated MultiColor FlpOut (MCFO) clones49 driven by the mδ0.5-GAL4. In these single cell clones, the younger R3/4 axons also showed overshooting, but the older R3/4 axons terminate at the lamina plexus (Supplementary Fig. 4C). These results suggest that the young R3/4 axons transiently overshoot and then retract back to assume their final destination in lamina plexus.
We tried to ask whether this transient overshoot of R2/5 axons is a general property of all R1-6 axons. To see this early event, we need early markers for photoreceptors. The rhodopsins express only in well differentiated PR, so is not suitable for this purpose. The 24B10 stains all PR axons, but does not show any overshoot in lamina (e.g. Yu et al.50), presumably because its expression is later than the early event. Ro-tau-lacZ marks R2-5 axons51 and showed some axonal overshoot52,53. However, since its expression includes R3/4, whether R2 and R5 axons also overshoot is not clear. MT14-GAL454 is reported to be specific for R2/5/855, although also reported to have some expression in other PRs56. We found that MT14 > mRFP axons do not show overshooting in lamina (Supplementary Fig. 4D). We generated MCFO clones driven by the MT14-GAL4. In these single cell MCFO clones, the younger PR and older PR all have axons terminate at the lamina plexus (Supplementary Fig. 4E). However, all PR cells labelled by MT14 > mRFP (magenta) are also 24B10+, therefore the MT14-GAL4 expression only marks older R2/5/8 axons. Therefore, whether the young R2/5/8 axons also overshoot cannot be determined using this reporter.
WG membrane extension into optic lamina
We examined the axonal ensheathment process at single glia cell resolution by using hs-FLP and repo-GAL4 to generate flp-out clones57,58 and monitored these clones by live imaging of ex vivo cultured eye disc (Supplementary Fig. 5). The glial membrane is visualized by mCD8-GFP. The WG and SG can be distinguished by their distinct morphologies. The SG can undergo cell division (Supplementary Fig. 5, yellow arrowheads). The WG progressively extends its membrane posteriorly toward the OS, presumably along the wrapped axons, with its nucleus move up and down in the cell (Supplementary Movie 1; Supplementary Fig. 5, blue and red arrowheads). We also used transmission electromicroscopy (TEM) to examine the axonal ensheathment by WG membrane. CD2-HRP59 was driven by the WG-specific MZ97-GAL4 and stained by Diaminobenzidine (DAB) to detect WG membrane. In a cross section of eye disc, WG membrane can be found infiltrating to PR layer and membrane surround the PR axon (Supplementary Fig. 7AI). The WG membrane can also be noted extending into the lamina (Supplementary Fig. 7AII).
We then examined the temporal progression and the extent of WG membrane extension. Although Mz97-GAL4 is expressed only in WG in eye discs44, it also expressed in some glia in the optic lobe (data not shown). Therefore, this GAL4 is not suitable for tracing retinal WG membrane extension into the optic lobe. To overcome this issue, we screened the Janelia Farm collection of GAL4 lines with retinal glia expressions and identified a GMR74E02-GAL4 (referred to as WG-GAL4 in this study) that marks only the retinal WG but no other glia in the optic lobe and brain (Supplementary Fig. 6A,B). Examination of eye discs of different developmental age showed that the progressive WG membrane extension lags behind the PR axon projection through OS to lamina (Supplementary Fig. 6D–F). The WG membrane extends into lamina but stops at the anterior layer of lamina. When all glial membrane is labelled, we noticed a region in lamina that lacks glial membrane (Supplementary Fig. 6C). The termination of WG membrane extension is not because of touching the next lamina glial membrane. Since PR axons continue to extend into the lamina (R1-6) and medulla (R7/8), there is a segment of PR axon not wrapped by glial membrane. We also generated WG MCFO clones using the WG-GAL4. The WG membrane extends to the lamina neurons L1-L4, but not to the lamina L5 neurons and the lamina plexus (Supplementary Fig. 7B). The younger WG membrane extends to adjacent to the lamina neurons, while the older WG membrane extend to partially surround the lamina neurons (Supplementary Fig. 7C). Consistently, recent reports showed that WG membrane progressively extend into the lamina60,61.
WG membrane extension also follows the retinotopic order
In order to clearly demonstrate the A-P and D-V relationship of retinal WG and their membrane extension into lamina, we used two methods to clonally label WG cells. Both methods are based on the flp-out concept62. A transient heat shock during development induces the expression of hs-Flp to randomly induce the excision (flp-out) of a stop cassette in UAS-reporters in cells. The UAS-reporter is then responsive to GAL4 induction. In the first flp-out method, the UAS > CD2, y+ > mCD8GFP57,58 and Mz97-GAL4 were used. The hs-Flp was induced transiently 12 hr. before late third instar larva. If the flp-out cell and its progeny cells differentiate into WG, then the Mz97-GAL4 will drive the expression of mCD8GFP. We found that 88% of WG clones (N = 42) has only single cell, suggesting that during the 12 hr. since heat shock induction, the flp-out cell rarely undergone cell division. The advantage of this method is that live imaging is possible, but the distinction of neighboring clones is not easy. Multiple clones in a disc are numbered and the extent of their membrane, marked by mCD8GFP, can be traced. Because we never observe WG membrane extend anteriorly (Supplementary Fig. 4), the anterior extent of its membrane is interpreted as an indicator of its time and position of origin (differentiation into WG), i.e. the posterior #2 WG is older than the anterior #1 WG (Supplementary Fig. 8A). Like the PR axons, the younger WG (#1) membrane -presumably wrapping younger PR axons- is located in a more basal position in the OS than the position of older WG (#2) (Supplementary Fig. 8C,D-D’). The lateral clones #3 and #4 maintain their relative D-V positions in the OS and the medial #1 and #2 maintain their medial positions (Supplementary Fig. 8C).
The second method uses the MCFO49 and the WG-GAL4 to label multiple WG clones with different combinations of the V5, HA, FLAG tags on myristoylated (membrane) GFP, as such the extent of membrane extension can be observed. This method provides better distinction of neighboring clones. The heat shock induction was 24 hr. prior to late third instar larva, therefore most clones contain multiple cells. Similar results were obtained with Mz97-GAL4 driven MCFO analysis. WG clones preserve their relative D-V positions (Fig. 4 and Supplementary Fig. 9). In Fig. 4B, 5 WG clones were labelled: anterior WG clones (AV1, AV2) and posterior WG clones (PD1, PD2 and M). Membranes from anterior clones (younger WG) are located in the basal portion of OS, compared to those from posterior WG clones (Fig. 4C,D). The WG membrane extension follows the retinotopic rules of PRs in maintaining their D-V orders when projecting into optic lobe (Fig. 4E–H). Similar to the PR axons, the younger WG membrane extension stops at a more anterior position in the OL, while the older ones are at more posterior positions.
WG membrane extension follows the retinotopic rules as PR axons. MultiColor FlpOut (MCFO) clones were generated using WG-specific GAL4 (abbreviated WG > MCFO). Anti-V5 (magenta), anti-HA (green) and anti-Flag (blue) antibodies are used to visualize membrane of WG clones. Clones are named by their relative positions along the A/P and D/V axes of the eye disc. (A) Schematic drawing shows the types of WG clones (white). Green indicates the extent of all WG membrane from eye disc (left) to the anterior edge of lamina (right). (B,E) WG > MCFO clones in eye discs are shown with their projections into the lamina. Dorsal is up and anterior is to left. Several WG > MCFO clones can be identified by different colors due to different reporter combinations. (C) An optical cross-section of the sample in (B) at the OS. The two anterior-ventral WG clones (AV1, AV2) show WG membrane in the basal-ventral region in OS. The two posterior-dorsal clones (PD1, PD2) have membrane in the more apical-dorsal position in OS. The medial (M) clone occupies a medial position in both A-P and D-V axes in the eye disc and in the OS. (D) Schematic summary of the spatial distribution of the clones in (B,C). (E) Among the WG > MCFO clones, an anterior-dorsal (AD), an anterior-medial (AM) and a posterior-medial (PM) clone are labeled and their membrane projections traced into the OS (F) and lamina (G). The AD clone occupies a basal position in the OS and has not yet reached the lamina. The AM clone occupy a basal position in OS, and has just entered the lamina. The PM clone occupies an apical position in OS and terminated in a posterior position in lamina. Dashed line outlines the OS cross section (C,F). (H) Schematic representation of the locations of PM and AM membrane in lamina. Scale bars are 30 μm for B, 10 μm for CEG, and 5 μm for (F).
WG affects PR axon projection in OS and lamina
Since the wrapping of axons is temporally tightly coupled with the extension of PR axons into the OS and lamina, we tested whether the wrapping affects the axonal retinotopic projection. In the OS, the central region consists of axons wrapped by WG membrane41, visualized by WG > mCD8-GFP (Fig. 5E,F). The periphery of OS consists of SG membrane41. FGF signaling pathway has been reported as both necessary and sufficient to promote WG differentiation46. In pan-glial knock down of the FGF receptor Heartless (Htl), the WG does not differentiate and there is no WG membrane in the center of the OS (Fig. 5D, compare with 5B), indicating the loss of WG membrane. The periphery of OS consists of SG membrane, so is not affected by Htl knockdown. When Htl is knocked down specifically in differentiated WG by the WG-GAL4, the WG membrane in OS is not obviously affected (Fig. 5H), although previous study showed that membrane extension into lamina is affected47.
Loss of Htl in WG disrupted PR retinotopic distribution in OS. (A–D) Glial membrane is visualized by repo > tdCD4GFP (green). (E–G) WG membrane is visualized by WG > mCD8GFP (green). (A–G) 24B10 (anti-Chaoptin; magenta) stains mature PR axons. Anti-HRP (cyan) stains all PR cells and axons. The combination of 24B10 and HRP can distinguish young (HRP only) and old (both HRP and 24B10) axons. (A,C) 24B10 staining stays about 8 rows behind the anterior edge of HRP signal. (B-B”’ and D-D”’) Optical cross section of OS at the position of dashed lines in (A) and (C), respectively. (B, B’) In the control group, glial membranes occupy the entire OS cross section, with the PR axons in the central region. Younger axons (HRP only) are at the basal region, while older axons (both HRP and 24B10) are at more apical region. (D, D’) When Htl is knocked down in all glia, the glial membrane in the central region of OS is lost, indicating a loss of WG membrane. The differential apical-basal distribution of old-young PR axons is lost. (E) In WG > mCD8-GFP, WG membranes colocalize with axons in the central region of OS. (F-F”’) Younger axons (HRP only) are at the basal region, while the older axons (both HRP and 24B10) are at more apical region and colocalized with WG membranes. (G) When Htl is knocked down in WG by the WG-GAL4, the WG membrane is reduced, and the differential apical-basal distribution of old-young PR axons is lost (H). (I,J) Statistics for the percentage of 24B10 axonsin the basal region of OS. (I) repo > tdCD4GFP + HtlRNAi; compared with repo > lacZ (*p < 0.05). (J) WG > mCD8GFP + HtlRNAi compared with WG > lacZ (**p < 0.001). Two-tailed of Menwhitney analysis were used. Scale bar is 20 μm for (A–G).
The young and old PR axons are distinguished by the relative timing of 24B10 and HRP staining22. HRP-only axons (cyan) represent younger axons, while 24B10-positive axons (magenta) represent older axons (Fig. 5). In wild type, about 25% (N = 6 for repo-Gal4 control and N = 17 for WG-Gal4 control) of the 24B10-positive axons are in the basal volume. The percentage increased to 39% in pan-glial Htl knockdown (N = 8) and 38% in WG-specific Htl knockdown (N = 11) (Fig. 5I,J). The 24B10 + axons in the Htl knockdown OS also appears to be more dispersed than in controls (Fig. 5I,J). In mutant animals, axons layers become extremely flattened. In addition, young (HRP staining only) and old (24B10) axons were patchily distributed. Additionally, WG membrane extends into the OS and lamina, but terminate in lamina in an irregular manner (Figs 5F’,H’ and 6C,D). Same defects of membrane extension were previously reported while overexpressed HtlDN driven by Mz97-GAL447,48. In the lamina, when dominant-negative Htl was expressed in WG, the lamina plexus is less compact than in wild type (Fig. 6C,D, compare with 6A,B). A projection from a different angle shows that the WG > HtlDN lamina has some gaps in the lamina plexus (Fig. 6C’, compare with Fig. 6A’). These results showed that the reduction of Htl in WG affects PR axon retinotopic projections in OS and lamina.
Expression of dominant-negative Htl in WG resulted in aberrant PR projection pattern in lamina. (A–D) WG membranes are visualized by WG-GAL4-driven mCD8GFP. 24B10 stains older PR axons. (A) In the control sample, R1-6 axons terminate in the lamina plexus and the R8 axons extend into the medulla. (B) An optic section lateral view along the white dashed line in (A). (C,D) WG-specific expression of a dominant-negative Htl (WG > HtlDN). The retinotopic projection of PR axons (A’, C’) are viewed from optical section indicated by red dashed line in (A, C), respectively. (A’, C’) The anterior border of lamina is to the left. (C) In WG > HtlDN, the WG membrane border in the anterior region of lamina becomes irregular. The PR axon termini at the lamina plexus is less compact than control. (D) In a lateral view, the lamina plexus is disorganized. (C’) Small gaps are found in the PR axon field in lamina, suggesting irregular PR axon projections. Scale bars are 20 μm for AA’BC’D and 10 μm for (C).
We examined the effect of WG on PR retinotopic projection by other manipulations. We first killed WG by expressing the pro-apoptotic genes head involution defective (hid) and reaper (rpr) using WG-GAL4. Since the continuous expression of death genes in WG > hid + rpr lead to larval death, we used tub-GAL80ts to temporally control the death genes expression (abbreviated as WGts > hid + rpr). After a 12 hr. induction of hid and rpr expression in late third instar larvae, there is significant increase of the apoptotic marker cleaved caspase 3 (Fig. 7A,E) and significant decrease of Cut+ cells in the eye disc (Fig. 7B,F), indicating the killing of a significant proportion of WG cells. The preference of younger PR axons to occupy the more apical region in OS is reduced (Fig. 7C and G). In the lamina, there is more and larger holes (Fig. 7D,H), suggesting a more disorganized retinotopic distribution. These results demonstrate that WG is important for the proper retinotopic projection of PR axons. When CG is similarly killed (in C135ts > mCD8GFP + hid + rpr), the number of WG is slightly reduced, the PR retinotopic projection pattern in OS is not affected, but there are more holes in the lamina (Supplementary Fig. 10G–J). When hid were driven by the SG-specific C527-GAL4, the number of WG was not affected. Although WG is derived from SG44, the 12 hr of hid induction was too short to significantly affect the transition from SG to WG. In C527ts > hid, the PR retinotopic projection pattern in OS and in lamina were not significantly affected (Supplementary Fig. 10O-R). These results showed that the WG has a major role in PR retinotopic projection in OS and in lamina.
Killing of wrapping glia caused defect in retinotopic projection. The death genes hid and rpr were expressed by WG-GAL4 with tub-GAL80ts (WGts > hid + rpr). (A–D) WGts > lacZ served as control. (E–F) After 12 hrs temperature shift to 30 °C to induce death genes expression, the eye-brain complex is examined immediately. The basal WG layer showed significant increase of the apoptotic marker cleaved caspase 3 signals (E, green) and decrease of Cut+ WG cell number (F, red; quantitative analysis in I). Small Cut+ puncta, in contrast to the nuclear staining and probably representing debris of apoptotic WG cells can be detected in the middle region of the WG > hid + rpr eye disc (F). (C,G) In the optic stalk, the younger axons (24B10 alone, magenta) showed differential apical localization of in the control group (C), but in WG > hid + rpr are more disorganized in the optic stalk with more basal localization (G; quantitative analysis in J). (D,H) In the optic lamina, the retinotopic projections in lamina plexus are more disorganized in WG > hid + rpr (H) as compared with the control group (D). (K) Quantification data of the number of holes and the number of large holes (arbitrarily defined as holes with perimeter Lt > 20 μm). Scale bars are 20 μm for all.
We also blocked WG membrane extension to test for the effect on PR retinotopic projection. The Borderless (Bdl) receptor is expressed in WG to receive the signal Turtle (Tutl) expressed in the PR axon47,48. We knocked down Bdl in WG (WG > mCD8GFP + Bdl-RNAi). As previously reported47, Bdl knockdown reduced WG membrane extension in the lamina (Supplementary Fig. 11D-D’). The retinotopic projection pattern in OS is not affected (Supplementary Fig. 11B,D), probably because the WG membrane in OS was not affected. The retinotopic projection in lamina was disturbed (Supplementary Fig. 11C,F). All of these results indicate that the WG, especially its membrane, plays a role in the retinotopic projection of PR axons into the optic lamina.
Discussions
Retinotopic map preserves the spatial information from retina to OS and lamina
In this study, we focus on the retinotopic map from retina to lamina. Our single-cell analyses of PR showed that the relative A-P and D-V positions are preserved from the retina to the OS and to the lamina, confirming previous findings22. The D-V axon guidance information is provided by the gradient of the DWnt4 expressed in ventral lamina and the receptor Dfrizzled2 along with its downstream signaling component disheveled in the retinal axons, respectively. The dorsal-specific transcription factor Iroquois acts in dorsal PRs to attenuate the response to DWnt430. However, lamina DWnt4 gradient is unlikely to act in the OS. Therefore, the maintenance of the retinotopic map in the OS may rely on as yet unidentified guidance system or on the interaction among neighboring axons.
Transient overshoot of young R3/4 axons in lamina
R1-6 PR axons terminate in the lamina plexus between the layers of epithelial glia and marginal glia15,36. Such termination of R1-6 axons in lamina is dependent on the interaction between the PR axons and lamina glia36,37,38. Unexpectedly, we found that the young R3/4 axons overshoot and pass the lamina plexus to medulla (Fig. 3). This is not observed in older R3/4 axons, suggesting that the overshoot is a transient event (Fig. 8B). This transient axonal overshoot has not been reported before. One possible reason is that previous studies have used 24B10 as a marker for all PR axons22. 24B10 is expressed later than the mδ0.5-GAL4 and thus only in older PR axons, therefore the earlier overshoot event was not observed. We tried to test whether all R1-6 axons transiently overshoot but have not obtained conclusive result. Specific markers for R1/2/5/6 that are expressed early are needed. The R2/5/8-specific MT14-GAL4 unfortunately marks only more mature R2/5/8 (Supplementary Fig. 4).
Summary of spatial and temporal correlation between PR and WG retinotopic projections. (A) Schematic illustration of the progressive WG membrane extension and the temporal and spatial relationship with the PR axon extension into OS and lamina. (B) Schematic illustration of the R3/4 axons projection over time. The youngest R3/4 axons is depicted to the left and is extending their axons into lamina. The axons overshoot past the lamina plexus. More mature R3/4 axons terminate at the lamina plexus.
R1-6 overshoot phenotype has been observed in other situations. Several transcription factors including Brakeless (Bks), Runt (Run), Sequoia (Seq), Hindsight (Hnt) (and its target genes tiggrin (tig), jitterbug (jbug)/filamin, off-track (otk)), transcriptional coactivator and phosphatase Eyes absent (Eya), receptor tyrosine phosphatase PTP69D, the Jak/STAT pathway and the kinase Pelle are all required for proper lamina termination of R1-6 axons23,55,63,64,65,66,67. Whether these genes act on the retraction of the transiently overshot axons or changed the targeting specificity from lamina to medulla is not clear.
Glia in the target field also plays a role in the R1-6 termination in lamina. In glia cell missing (gcm) and gcm2 double mutant, lamina gliogenesis is affected38. In addition, ubiquitin protease Nonstop (Not) is required for the migration of the epithelial, marginal and medulla glia to their proper positions in lamina36. Lamina glia migration can be affected by the JAB1/CSN5 subunit of the COP9 signalosome complex acting in PRs37. Mutations or malfunction of these molecules all affected lamina glia and resulted in the R1-6 overshoot phenotypes, suggesting a role of lamina glia in R1-6 axon targeting. Whether the transient overshoot and retraction of R3/4 axons is also regulated by lamina glia is not clear.
WG is important for the retinotopic map formation
Lamina glia are critical for the proper R1-6 axon termination in lamina to form the lamina plexus36,37,38. Whether the retinal basal glia affects retinotopy is not known. Here we show that WGs in eye discs non-autonomously affect the retinotopic projections of PR axons in OS and in lamina. When Htl is reduced, the apical-basal distribution of old-young PR axon projection in the OS is disturbed, and the proper termination of R1-6 axons in the lamina and the formation of lamina plexus is also disturbed. Since the retinal WG membrane does not extend to the lamina plexus, how do WG affect the R1-6 termination at lamina plexus presents an interesting question. Recent study has shown novel ability of WG to synchronize the differentiation of lamina neuron through the secretion of insulin-like peptides60. It is possible that WG affects the R1-6 termination at lamina plexus by secreted molecules.
Methods
Drosophila stocks
Fly stocks are maintained at room temperature (RT). For all experiments, the flies are raised at 25 °C unless otherwise indicated. Special conditions were indicated individually. Fly stocks from Bloomington Stock Center are: repo-GAL4 (BSDC#7415)68; mδ0.5-GAL469 (BSDC#41782 and #41795, on chromosome II and III, respectively); GMR74E02-GAL4 (BSDC #48320); hsFLP; UAS > STOP > myr-smGdP-HA UAS- > STOP > myr-smGdP-V5 UAS > STOP > myr-smGdP-FLAG (BSDC #64085)49; Mz97-GAL444; hsFLP122; USA > STOP > mCD8GFP (III)57,58; y1 w*;.UAS-htl-DN; UAS-htl.DN (BDSC#5366)70; MT14-GAL454 (BDSC#37293); C135-GAL444 (BDSC#6978); C527-GAL444; UAS-hid; tub-GAL80ts(II) (BDSC#7019). UAS-Bdl-RNAi (V4806) is from VDRC and also gift from Dr. Yong Rao. sev181-GAL4 (kindly provided by Chi-Hon Lee)71; Htl-RNAi (kindly provided by Christian Klämbt)46; UAST-myr-GFP-V5-P2A-H2B-mCherry-HA is an unpublished gift from Yung-Heng Chang and Joshua Dubnau (Department of Anesthesiology, Stony Brook University); UAS-Kaede.K22 (kindly provided by Ann-Shyn Chiang)33; UAS-hid, UAS-rpr (gift from Suewei Lin); UAS-CD2-HRP (kindly provided by Tzu-Yang Lin); Ro-tau-lacZ (kindly provided by Philip A. Barker).
Clonal induction
Mz97-GAL4 flp-out clones were generated using hs-FLP122; Mz97-GAL4; UAS > CD2 > UAS-mCD8GFP. MCFO clones were induces by hs-FLPG5 and driven by GMR74E02-GAL4. In both experiments, larvae of mixed ages were heat-shocked for 10 minutes in 38 °C water bath. Larvae were raised for another 12 hr. (for Mz97-GAL4 flp-out clones) or 48 hr. (for MCFO clones) after the heat-shock and larvae at late third larval stage were dissected to examine the clones. The glial flp-out clones were generated by heat shock of repo > hs-Flp122;+/+; UAS-mCD8-GFP for 15 min, and the eye disc was dissected 24 hr. after heat shock for ex vivo culture.
Conditional inactivation of GAL80ts
The flies after mating were raised at 17 °C (permissive temperature). When 12 hr before late 3rd larval stage, the larvae were shifted to a nonpermissive temperature (30 °C) to induce death genes expression for the indicated time.
Immunohistochemistry and confocal microscopy
The eye-brain complex was dissected from late third instar larvae and fixed in 4% EM-grade paraformaldehyde (PFA, Electron Microscopy Sciences, 30525-89-4) in 1X PBS (phosphate buffered saline) for 20-25 minutes and washed three times in 1xPBS. Primary antibodies were: mouse anti-2B10(CUT) (DSHB, 1:50); mouse anti-24B1027 (DSHB, 1:200), anti-HA (4C12 mouse Ab, Abcam ab1424; 1:200), anti-FLAG (anti-dykddddk rabbit Ab, Sigma Aldrich, F2555; 1:200), anti-V5 (rat Ab, Novus Biologicals #NBP1-06712; 1:200) and rabbit anti-Cleaved Caspase-3 (Asp175, 1:200, Cell Signaling). Secondary antibodies used were Alexa-488, Cy3, Alexa-647 and HRP-Alexa-647(Jackson Immunoresearch, mouse and rat minimal cross-talk versions). All antibodies were diluted by PBST with 10% (v/v) normal goat serum (Jackson Immunoresearch). All images of fixed samples were acquired by Zeiss LSM 780 with the Plan-Apochromat 40x/1.4 oil objective. For live imaging and UV photoconversion of Kaede, LSM 710 inverted microscope was used with C-Apochromat 40x/1.2 W korr objectives. Optical sections were 0.2 (for 24B10/HRP staining), 0.8 and 1.2 μM thickness for fixed and live samples, respectively.
Image processing and analysis
3D images were reconstructed and analyzed by the commercial software IMARIS 8.4.1 or 9.0.0 (Bitplane, Germany). XY figures are all presented as whole Z-stacks processed by IMARIS unless otherwise indicated. Cross and longitudinal sections were shown in partial Z-stack projections (2.5–10 μM). For live imaging, images were recorded every 10 minutes for 10–16 hrs. To analyze axonal movement, images were cropped into smaller region that only covers OS. IMARIS threshold cutoffs of 13.5 and 7.5 were used for green channel and red channel respectively to get rid of global background signals and make the outline of the OS clearer. The “Measurement points” function was used to define the length from top to bottom and from top to center of axons in OS around 40 time points. To analyze 24B10 distribution in optic stalk in Fig. 5, OS sections were cropped by IMARIS. Next, volumes of 24B10 and HRP staining were obtained after 3D surface rendering. The region covering the OS was then divided equivalently along Z axis. Percentage of 24B10 volume in apical region and basal regions were recorded. To analyze the size of hole in Fig. 7, the perimeter of the holes was measured.
Ex vivo tissue culture and confocal microscopy
Eye-brain complexes were cultured ex vivo as described previously45,72.
Transmission electron microscopy (TEM)
The larvae were cut into halves by dissecting scissor to reduce the sheared damage on the tissues. The head cuticle was gently removed by scissor to expose the eye-brain complex. The dissected eye-brain complex was quickly fixed in fixative containing 4% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate (Sigma), pH7.4 in 4 °C for overnight. The tissues were washed three times (15 minutes each) with 0.1 M sodium cacodylate, 2% OsO4 (Electron Microscopy Sciences) in 0.1 M sodium cacodylate in RT for 1 h, three times in Milli-Q water and switched to 2% uranyl acid (Polysciences) for 30 mins in RT, then washed by Milli-Q water, and dehydrated by a series of different concentration of ethanol (50% once, 70% once, 80% once, 90% once, 95% once and 100% three times, propylene oxide twice; 15 minutes for each series dehydration). The dehydrated tissues then underwent a series of resin replacement in increasing ratio of Epon (EMbed-812 Kit)/propylene oxide (SIGMA-ALDRICH #471968) (1:3, 2:1, 1:1, 1:2, 1:5, then pure Epon for three times). In the final step, individual eye-brain complex was embedded in resin. Resin blocks were baked in 60 °C oven for 48 hrs. 80 nm-thick sections were acquired with a diamond knife (Ultracut, Reichert-Jung, Vienna, Austria) and examined by TEM (Tecnai G2 Spirit TWIN, FEI Company, Hillsboro, OR) equipped with a Gatan CCD Camera (794.10.BP2 MultiScanTM). For visualization of HRP-expressing WG, Diaminobenzidine (DAB) staining are as described73 but without Ni-intensified.
Photoconversion of Kaede
The photoconvertible fluorescent protein, Kaede31,32,33 was used in this study. UV irradiation induces a peptide cleavage resulting in the rapid and irreversible conversion from green fluorescence to red fluorescence32. The red signal slowly decays, while the green signal slowly recovers, probably due to newly synthesized Kaede protein. The green-to-red photoconversion was induced by 405 nm laser beam with 1.5–7% power of 25 mW laser (LASOS lasertechnik GmbH, LGN3001) for 300 hits (over 4.53 s; 0.01513 s/hit; without interval). The irradiating conditions were optimized (Supplementary Fig. 1). Low laser power and longer exposure work well in single PR irradiation. In order to reduce photoconversion in non-target cells, we only irradiated a single PR pair in a region smaller than 3 × 3 pixel2. The bleaching mode in Zen software was used to draw region of interests (ROI). Once the red-to-green ratio was raised, the activated region could be distinguished until the end of imaging period (9–16 hr). Due to the background photoconversion during dissecting and mounting, the red signals are clearer when overlaid with green signals.
References
Triplett, J. W. & Feldheim, D. A. Eph and ephrin signaling in the formation of topographic maps. Semin Cell Dev Biol 23, 7–15, https://doi.org/10.1016/j.semcdb.2011.10.026 (2012).
Reese, B. E. Development of the retina and optic pathway. Vision Res 51, 613–632, https://doi.org/10.1016/j.visres.2010.07.010 (2011).
Chedotal, A. & Richards, L. J. Wiring the brain: the biology of neuronal guidance. Cold Spring Harb Perspect Biol 2, a001917, https://doi.org/10.1101/cshperspect.a001917 (2010).
Huberman, A. D., Feller, M. B. & Chapman, B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 31, 479–509, https://doi.org/10.1146/annurev.neuro.31.060407.125533 (2008).
Ready, D. F., Hanson, T. E. & Benzer, S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol 53, 217–240 (1976).
Melamed, J. & Trujillo-Cenoz, O. The fine structure of the central cells in the ommatidia of dipterans. J Ultrastruct Res 21, 313–334 (1967).
Trujillo-Cenoz, O. & Melamed, J. Compound eye of dipterans: anatomical basis for integration–an electron microscope study. J Ultrastruct Res 16, 395–398 (1966).
Braitenberg, V. Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp Brain Res 3, 271–298 (1967).
Horridge, G. A. & Meinertzhagen, I. A. The accuracy of the patterns of connexions of the first- and second-order neurons of the visual system of Calliphora. Proc R Soc Lond B Biol Sci 175, 69–82 (1970).
Meinertzhagen, I. A. The development of neuronal connection patterns in the visual systems of insects. Ciba Found Symp 0, 265–288 (1975).
Kunes, S. & Steller, H. Topography in the Drosophila visual system. Curr Opin Neurobiol 3, 53–59 (1993).
Clandinin, T. R. & Feldheim, D. A. Making a visual map: mechanisms and molecules. Curr Opin Neurobiol 19, 174–180, https://doi.org/10.1016/j.conb.2009.04.011 (2009).
Tomlinson, A. & Ready, D. F. Cell fate in the Drosophila ommatidium. Dev Biol 123, 264–275 (1987).
Dittrich, K.-F. F. P. M. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell and Tissue Research 258, 441–475 (1989).
Winberg, M. L., Perez, S. E. & Steller, H. Generation and early differentiation of glial cells in the first optic ganglion of Drosophila melanogaster. Development 115, 903–911 (1992).
Melnattur, K. V. & Lee, C. H. Visual circuit assembly in Drosophila. Dev Neurobiol 71, 1286–1296, https://doi.org/10.1002/dneu.20894 (2011).
Clandinin, T. R. & Zipursky, S. L. Making connections in the fly visual system. Neuron 35, 827–841 (2002).
Hadjieconomou, D., Timofeev, K. & Salecker, I. A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol 21, 76–84, https://doi.org/10.1016/j.conb.2010.07.012 (2011).
Sanes, J. R. & Zipursky, S. L. Design principles of insect and vertebrate visual systems. Neuron 66, 15–36, https://doi.org/10.1016/j.neuron.2010.01.018 (2010).
Cajal SR, S. D. Contribucion. al conocimiento de los centros nerviosos del los insectos. Trab. Lab. Invest. Biol. 13, 1–167 (1915).
Meinertzhagen I. A. H.T. The development of the optic lobe. Cold Spring Harbor Laboratory Press, 1363–1491 (1993).
Kunes, S., Wilson, C. & Steller, H. Independent guidance of retinal axons in the developing visual system of Drosophila. J Neurosci 13, 752–767 (1993).
Newsome, T. P., Asling, B. & Dickson, B. J. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860 (2000).
Jan, L. Y. & Jan, Y. N. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci USA 79, 2700–2704 (1982).
Fabini, G., Freilinger, A., Altmann, F. & Wilson, I. B. Identification of core alpha 1,3-fucosylated glycans and cloning of the requisite fucosyltransferase cDNA from Drosophila melanogaster. Potential basis of the neural anti-horseadish peroxidase epitope. J Biol Chem 276, 28058–28067, https://doi.org/10.1074/jbc.M100573200 (2001).
Paschinger, K., Rendic, D. & Wilson, I. B. Revealing the anti-HRP epitope in Drosophila and Caenorhabditis. Glycoconj J 26, 385–395, https://doi.org/10.1007/s10719-008-9155-3 (2009).
Fujita, S. C., Zipursky, S. L., Benzer, S., Ferrus, A. & Shotwell, S. L. Monoclonal antibodies against the Drosophila nervous system. Proc Natl Acad Sci USA 79, 7929–7933 (1982).
Zipursky, S. L., Venkatesh, T. R., Teplow, D. B. & Benzer, S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell 36, 15–26 (1984).
Reinke, R., Krantz, D. E., Yen, D. & Zipursky, S. L. Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell 52, 291–301 (1988).
Sato, M., Umetsu, D., Murakami, S., Yasugi, T. & Tabata, T. DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci 9, 67–75, https://doi.org/10.1038/nn1604 (2006).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA 99, 12651–12656, https://doi.org/10.1073/pnas.202320599 (2002).
Mizuno, H. et al. Photo-induced peptide cleavage in the green-to-red conversion of a fluorescent protein. Mol Cell 12, 1051–1058 (2003).
Chen, C. C. et al. Visualizing long-term memory formation in two neurons of the Drosophila brain. Science 335, 678–685, https://doi.org/10.1126/science.1212735 (2012).
Vecino, E., Rodriguez, F. D., Ruzafa, N., Pereiro, X. & Sharma, S. C. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res, https://doi.org/10.1016/j.preteyeres.2015.06.003 (2015).
Vecino, E., Rodriguez, F. D., Ruzafa, N., Pereiro, X. & Sharma, S. C. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res 51, 1–40, https://doi.org/10.1016/j.preteyeres.2015.06.003 (2016).
Poeck, B., Fischer, S., Gunning, D., Zipursky, S. L. & Salecker, I. Glial cells mediate target layer selection of retinal axons in the developing visual system of Drosophila. Neuron 29, 99–113 (2001).
Suh, G. S. et al. Drosophila JAB1/CSN5 acts in photoreceptor cells to induce glial cells. Neuron 33, 35–46 (2002).
Chotard, C., Leung, W. & Salecker, I. glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron 48, 237–251, https://doi.org/10.1016/j.neuron.2005.09.019 (2005).
Choi, K. W. & Benzer, S. Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron 12, 423–431 (1994).
Rangarajan, R., Gong, Q. & Gaul, U. Migration and function of glia in the developing Drosophila eye. Development 126, 3285–3292 (1999).
Hummel, T., Attix, S., Gunning, D. & Zipursky, S. L. Temporal control of glial cell migration in the Drosophila eye requires gilgamesh, hedgehog, and eye specification genes. Neuron 33, 193–203 (2002).
Tavares, L. et al. Drosophila PS2 and PS3 integrins play distinct roles in retinal photoreceptors-glia interactions. Glia 63, 1155–1165, https://doi.org/10.1002/glia.22806 (2015).
Rangarajan, R., Courvoisier, H. & Gaul, U. Dpp and Hedgehog mediate neuron-glia interactions in Drosophila eye development by promoting the proliferation and motility of subretinal glia. Mech Dev 108, 93–103 (2001).
Silies, M. et al. Glial cell migration in the eye disc. J Neurosci 27, 13130–13139, https://doi.org/10.1523/JNEUROSCI.3583-07.2007 (2007).
Tsao, C. K., Ku, H. Y., Lee, Y. M., Huang, Y. F. & Sun, Y. H. Long Term Ex Vivo Culture and Live Imaging of Drosophila Larval Imaginal Discs. PLoS One 11, e0163744, https://doi.org/10.1371/journal.pone.0163744 (2016).
Franzdottir, S. R. et al. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature 460, 758–761, https://doi.org/10.1038/nature08167 (2009).
Cameron, S., Chen, Y. & Rao, Y. Borderless regulates glial extension and axon ensheathment. Dev Biol 414, 170–180, https://doi.org/10.1016/j.ydbio.2016.04.020 (2016).
Chen, Y., Cameron, S., Chang, W. T. & Rao, Y. Turtle interacts with borderless in regulating glial extension and axon ensheathment. Mol Brain 10, 17, https://doi.org/10.1186/s13041-017-0299-6 (2017).
Nern, A., Pfeiffer, B. D. & Rubin, G. M. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci USA 112, E2967–2976, https://doi.org/10.1073/pnas.1506763112 (2015).
Yu, L., Zhou, Y., Cheng, S. & Rao, Y. Plexin a-semaphorin-1a reverse signaling regulates photoreceptor axon guidance in Drosophila. J Neurosci 30, 12151–12156, https://doi.org/10.1523/JNEUROSCI.1494-10.2010 (2010).
Garrity, P. A. et al. Retinal axon target selection in Drosophila is regulated by a receptor protein tyrosine phosphatase. Neuron 22, 707–717 (1999).
Ruan, W., Long, H., Vuong, D. H. & Rao, Y. Bifocal is a downstream target of the Ste20-like serine/threonine kinase misshapen in regulating photoreceptor growth cone targeting in Drosophila. Neuron 36, 831–842 (2002).
Apitz, H. & Salecker, I. Retinal determination genes coordinate neuroepithelial specification and neurogenesis modes in the Drosophila optic lobe. Development 143, 2431–2442, https://doi.org/10.1242/dev.135004 (2016).
Tissot, M., Gendre, N., Hawken, A., Stortkuhl, K. F. & Stocker, R. F. Larval chemosensory projections and invasion of adult afferents in the antennal lobe of Drosophila. J Neurobiol 32, 281–297 (1997).
Mindorff, E. N. et al. A gain-of-function screen for genes that influence axon guidance identifies the NF-kappaB protein dorsal and reveals a requirement for the kinase Pelle in Drosophila photoreceptor axon targeting. Genetics 176, 2247–2263, https://doi.org/10.1534/genetics.107.072819 (2007).
Edwards, T. N. & Meinertzhagen, I. A. Photoreceptor neurons find new synaptic targets when misdirected by overexpressing runt in Drosophila. J Neurosci 29, 828–841, https://doi.org/10.1523/JNEUROSCI.1022-08.2009 (2009).
Wang, J., Zugates, C. T., Liang, I. H., Lee, C. H. & Lee, T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron 33, 559–571 (2002).
Li, W. et al. Morphological characterization of single fan-shaped body neurons in Drosophila melanogaster. Cell Tissue Res 336, 509–519, https://doi.org/10.1007/s00441-009-0781-2 (2009).
Larsen, C. W., Hirst, E., Alexandre, C. & Vincent, J. P. Segment boundary formation in Drosophila embryos. Development 130, 5625–5635, https://doi.org/10.1242/dev.00867 (2003).
Fernandes, V. M., Chen, Z., Rossi, A. M., Zipfel, J. & Desplan, C. Glia relay differentiation cues to coordinate neuronal development in Drosophila. Science 357, 886–891, https://doi.org/10.1126/science.aan3174 (2017).
Rossi, A. M. & Fernandes, V. M. W. G. Morphogenesis and Signaling Control the Timing and Pattern of Neuronal Differentiation in the Drosophila Lamina. J Exp Neurosci 12, 1179069518759294, https://doi.org/10.1177/1179069518759294 (2018).
Basler, K. & Struhl, G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature 368, 208–214, https://doi.org/10.1038/368208a0 (1994).
Rao, Y., Pang, P., Ruan, W., Gunning, D. & Zipursky, S. L. brakeless is required for photoreceptor growth-cone targeting in Drosophila. Proc Natl Acad Sci USA 97, 5966–5971, https://doi.org/10.1073/pnas.110135297 (2000).
Kaminker, J. S., Canon, J., Salecker, I. & Banerjee, U. Control of photoreceptor axon target choice by transcriptional repression of Runt. Nat Neurosci 5, 746–750, https://doi.org/10.1038/nn889 (2002).
Oliva, C. & Sierralta, J. Regulation of axonal development by the nuclear protein hindsight (pebbled) in the Drosophila visual system. Dev Biol 344, 911–921, https://doi.org/10.1016/j.ydbio.2010.06.007 (2010).
Hoi, C. S., Xiong, W. & Rebay, I. Retinal Axon Guidance Requires Integration of Eya and the Jak/Stat Pathway into Phosphotyrosine-Based Signaling Circuitries in Drosophila. Genetics 203, 1283–1295, https://doi.org/10.1534/genetics.115.185918 (2016).
Kulkarni, A., Ertekin, D., Lee, C. H. & Hummel, T. Birth order dependent growth cone segregation determines synaptic layer identity in the Drosophila visual system. Elife 5, e13715, https://doi.org/10.7554/eLife.13715 (2016).
Sepp, K. J., Schulte, J. & Auld, V. J. Peripheral glia direct axon guidance across the CNS/PNS transition zone. Dev Biol 238, 47–63, https://doi.org/10.1006/dbio.2001.0411 (2001).
Cooper, M. T. & Bray, S. J. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature 397, 526–530, https://doi.org/10.1038/17395 (1999).
Carmena, A., Gisselbrecht, S., Harrison, J., Jimenez, F. & Michelson, A. M. Combinatorial signaling codes for the progressive determination of cell fates in the Drosophila embryonic mesoderm. Genes Dev 12, 3910–3922 (1998).
Lee, C. H., Herman, T., Clandinin, T. R., Lee, R. & Zipursky, S. L. N-cadherin regulates target specificity in the Drosophila visual system. Neuron 30, 437–450 (2001).
Tsao, C. K., Ku, H. Y. & Sun, Y. H. Long-term Live Imaging of Drosophila Eye Disc. J Vis Exp, https://doi.org/10.3791/55748 (2017).
Matzat, T. et al. Axonal wrapping in the Drosophila PNS is controlled by glia-derived neuregulin homolog Vein. Development 142, 1336–1345, https://doi.org/10.1242/dev.116616 (2015).
Acknowledgements
We are grateful to Ya-Hui Chou, Tzu-Yang Lin, Chi-Hon Lee for valuable discussions. We are grateful to Chi-Hon Lee, Yung-Heng Chang, Joshua Dubnau, Ann-Shyn Chiang and Christian Klämbt for generously providing reagents. We are grateful to Chun-Lan Hsu and Yu-Chi Yang for preparing fly food and maintaining fly stocks, and to Su-Ping Lee, Su-Ping Tasi and the IMB Imaging Core for help in confocal microscopy and TEM. This study was supported by grants to Y.H.S. (NSC 100-2321-B-001 -012, NSC 101-2321-B-001 -004, NSC 102-2321-B-001 -002, MOST 103-2311-B-001 -035 -MY3) and to Y.C.C (NSC 103-2917-I-010 -001) from the National Science Council and the Ministry of Science and Technology of the Republic of China.
Author information
Authors and Affiliations
Contributions
Y.C.C. and Y.H.S. designed the experiment. Y.C.C. performed the experiments, processed and analyzed the data. C.K.T. prepared ex vivo imaging in Supplementary Figure 5. Y.C.C. and Y.H.S. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, YC., Tsao, CK. & Sun, Y. Temporal and spatial order of photoreceptor and glia projections into optic lobe in Drosophila. Sci Rep 8, 12669 (2018). https://doi.org/10.1038/s41598-018-30415-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30415-8
This article is cited by
-
The exit of axons and glial membrane from the developing Drosophila retina requires integrins
Molecular Brain (2022)
-
An RNAi screen for secreted factors and cell-surface players in coordinating neuron and glia development in Drosophila
Molecular Brain (2020)
-
Early lineage segregation of the retinal basal glia in the Drosophila eye disc
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.