Abstract
The sperm plasma membrane is a sensitive target to oxidative stress. The most representative reactive oxygen species (ROS) scavengers in the genital tract, hypotaurine and glutathione, require, for their synthesis, cysteine whose availability is associated with the 1-carbon cycle (1-CC). Human, bovine and ascidian spermatozoa were incubated with compounds supporting the 1-CC (Vitamin B6, Methylcobalamin, 5 Methyl Tetrahydrofolate, Zinc Bisglycinate and N-acetyl-cysteine) (TRT) and compared to the effects induced solely by N-acetyl-cysteine (NAC). In control groups (CNTRL), spermatozoa were incubated with medium alone. After 90 and 180 minutes of incubation, the mitochondrial membrane potential (ΔΨM) in TRT and NAC was significantly (P < 0.01) higher than in CNTRL. At H2DCFDA evaluation, ROS production differed between species whereas, at 2-OH Ethidium, it significantly decreased in bovine TRT group. Intracellular pH (pHi) did not significantly vary in relation to treatment. In ascidian spermatozoa, the NAC supplementation decreased external pH, which in turn brought to a pHi lowering. Buffering seawater with NaHCO3 reversed the beneficial effects of N-acetyl-cysteine supplementation. In conclusion, both fully supporting the 1-CC and treatment with N-acetyl-cysteine alone improved kinetics, ΔΨM and ROS production in mammalian sperm demonstrating for the first time the direct in vitro effects of these compounds on sperm functionality.
Similar content being viewed by others
Introduction
The spermatozoon is a highly differentiated cell with a compacted nucleus, scarce cytoplasm, mitochondria and usually a flagellum. The mitochondria generate energy mainly for motility, via oxidative phosphorylation, and this depends on external sources of carbohydrate1 and fatty acids with a consequent production of large amounts of NADH and FADH2. In the mitochondria, electrons move from NADH to O2 via enzymatic complexes, such as NADH-Q oxidoreductase, Q-cytochrome C oxido-reductase, the cytochrome C oxidase, and the complex V: ATP synthase. However, approximately 1–4% of oxygen escapes and forms reactive oxygen species (ROS)2. The oxidases generating free radicals affect mitochondrial structure and activity leading to cell aging3. ATP production and leakage of ROS by the electron transport chain are dependent on the mitochondrial membrane potential (ΔΨM)4,5, by measuring which the mitochondrial energy metabolism can be monitored. The ΔΨM is, however, affected by other metabolic processes such as calcium uptake and anti-oxidant defenses6,7.
The high mitochondrial activity of spermatozoa together with the small amount of cytoplasm and a high concentration of polyunsaturated fatty acids in plasma membrane makes these cells extremely sensitive to oxidative damage, which may affect DNA, proteins and membrane integrity. In the latter case, motility8, adhesion and fusion during fertilization process and the acrosome reaction may be strongly impaired9,10. Based on these observations, the idea that antioxidant molecules might be helpful for sperm protection and storage is of current interest. Many studies have been carried out using a variety of antioxidants for improving sperm quality11. In previous studies, nucleophilic thiols were used to counteract the effects of ROS12,13 on sperm activity and prevention of DNA adducts; however, their roles on mitochondrial functionality were not evaluated.
Glutathione (GSH) is the universal cell ROS scavenger in cells; whereas, hypotaurine is the most representative ROS scavenger in the genital tract14. Both these molecules require cysteine for their synthesis15. Cysteine availability is associated with the one carbon cycle (1-CC), as homocysteine, resulting from methionine de-methylation that can be regenerated to cystathionine by the trans-sulfuration pathway leading to the formation of cysteine (Supplemental Fig. S1). Methionine is a mandatory metabolite in the methylation processes. Within the 1-CC, it is transformed by the methionine adenosyl transferase to form the universal methyl donor S-adenosylmethionine (SAM). SAM is the cofactor of the methyl transferases releasing methyl tags on proteins (histones), nucleic acids and lipids to form phospholipids16. The byproducts of these reactions, i.e., S-adenosylhomocysteine (SAH) and, then, homocysteine, must be recycled to methionine since homocysteine is an inhibitor of methylation and a cellular poison at high concentrations17. Similarly, vitamin B12 and B6 are compulsory cofactors in this methionine regeneration activity18. The betaine-homocysteine S-methyltransferase (BHMT) pathway has a weaker regeneration activity19. In addition, SAM is also necessary for the synthesis of Coenzyme Q10, a lipid-soluble, powerful antioxidant representing an essential cofactor in mitochondrial oxidative phosphorylation20.
In addition to energy availability, intracellular pH (pHi) can strongly affect sperm functionality. This is mainly regulated via defined mechanisms: the Na+/H+ antiport (NHE), Na+-HCO3−-(NaCO3−) symport, and Cl−/HCO3− exchange (AE2)21. Ionic transport is strongly linked to the hydrolysis of ATP. Hence, pHi maintenance is an energy consuming activity requiring a highly efficient energy production also needed for the transport of nutrients used as energy sources. This intra-membrane transport can be seriously impaired by lipid peroxidation produced by ROS.
The enhancement of sperm energy metabolism is, therefore, a topic of enormous interest linked to the possibility of improving sperm functionality and providing more in-depth tools and knowledge for the management of sperm conservation programs. In this line, this study aimed to evaluate the efficacy of a treatment of fully supporting the 1-CC on sperm functionality. In particular, the effects of five compounds involved in the 1-CC, i.e., Vitamins B6 and methyl B12, 5 methyl folate, zinc and N-acetyl cysteine (NAC) on different sperm parameters, such as mitochondrial activity, lipid peroxidation, ROS production and intracytoplasmic pH, were evaluated in human, bovine and the ascidian C. robusta. This treatment was compared to the impact of NAC alone, since in preliminary trials (Supplemental Fig. S2) this compound showed a predominant activity compared to that of the other individually evaluated enhancers.
Results
Sperm kinetics
In the human, we observed (Fig. 1A1) a significant improvement in sperm motility at 90 min incubation in NAC group in comparison with CNTRL (85.1 ± 9.5% vs. 56.5 ± 8.2%; P < 0.05). This beneficial effect, however, decreased at 180 min. Kinetic parameters did not show significant differences between groups neither at 90 min nor at 180 min incubation (Fig. 1A2).
Human, bovine and ascidian sperm kinetics following exposure to metabolic enhancers. Total sperm motility and kinetics (VCL, VSL and VAP) in human (A1,A2), bovine (B1,B2) and ascidian (C1,C2) sperm divided in three groups treated with: (1) five metabolic enhancers (vitamins B6 and B12, 5 methyl THF, zinc and N-acetyl cysteine) (TRT group); (2) n-acetyl-cysteine (NAC group); and (3) medium alone (CNTRL group). Mean (± SE) values in CNTRL, TRT and NAC groups after 0, 90 and 180 min incubation. Statistically significant differences between groups for each evaluated time point are expressed as different capital (A vs. B and C vs. D; P < 0.01) and small (a vs. b; P < 0.05) letters.
In bovine, a progressive time-dependent decrease in sperm motility was observed in all the groups evaluated (Fig. 1B1). Both the TRT and NAC treatments significantly improved total motility after 180 min incubation with respect to CNTRL (35.1 ± 2.1% vs. 26.7 ± 3.2; P < 0.05 and 36.5 ± 2.0% vs. 26.7 ± 3.2%;P < 0.01). However, the beneficial effects observed in TRT and NAC groups were not sufficient to effectively counteract the natural decline of motility which was inferior to CNTRL at time 0 even after 90 min incubation (48.2 ± 2.3% and 51.2 ± 2.5% vs. 70.1 ± 1.4%; P < 0.01). Regarding kinetic indicators, only VSL values were significantly (P < 0.01) higher in TRT than in CNTRL whereas NAC was lower (P < 0.01) than TRT but it did not differ from CNTRL (Fig. 1B2).
In the ascidian, the overall sperm motility significantly decreased in TRT and NAC vs. CNTRL after 90 min incubation (29.6 ± 4.6% and 39.7 ± 3.0% vs. 81.6 ± 7.3%; P < 0.01) (Fig. 1C1). However, after 180 min incubation both treatments did not differ with respect to the CNTRL. Also the sperm kinetic parameters (Fig. 1C2), as VSL and VAP, at 90 min were lower in TRT and NAC than CNTRL groups (VSL = 22 ± 2 and 32 ± 3 vs. 43 ± 2 µm/s; P < 0.01) (VAP = 50 ± 3 and 62 ± 5 vs. 87 ± 5 µm/s; P < 0.01). After 180 min incubation, however, VSL and VAP values of TRT and NAC did not differ from CNTRL but NAC showed higher values than TRT (VSL = 52 ± 4 vs. 30 ± 1 µm/s; P < 0.05) (VAP = 88 ± 3 vs. 61 ± 1 µm/s; P < 0.05).
Mitochondrial Membrane Potential (ΔΨM)
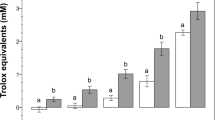
The incubation period significantly affected ΔΨM causing a progressive reduction in all three species. Moreover, the ΔΨM significantly increased in either TRT or NAC groups in all the species considered (Fig. 2). In human spermatozoa, at 90 min incubation, both treatments induced ΔΨM values significantly higher than those of CNTRL (2.9 ± 0.5 and 3.2 ± 0.4 vs. 1.6 ± 0.3; P < 0.01) and similar to those recorded in CNTRL at time 0 (3.5 ± 0.6). Even at 180 min incubation, the ΔΨM values of TRT and NAC groups were higher than CNTRL (2.4 ± 0.5 and 2.3 ± 0.4 vs. 1.1 ± 0.2; P < 0.05) and did not differ between themselves. Although lower than at 90 min, ΔΨM of TRT and NAC groups did not significantly differ with CNTRL at time 0.
ΔΨM in human, bovine and ascidian spermatozoa exposed to metabolic enhancers. Mitochondrial membrane potential (ΔΨM) in human (A), bovine (B) and ascidian (C) sperm divided in three groups treated with: (1) five metabolic enhancers (vitamins B6 and B12, 5 methyl THF, zinc and N-acetyl cysteine) (TRT group); (2) n-acetyl-cysteine (NAC group); and (3) medium alone (CNTRL group). Mean (±SE) values of ΔΨM in spermatozoa loaded with JC-1, arranged in TRT, NAC and CNTRL groups and then incubated for 0, 90 and 180 min. The fluorescence emission peak (Fo) was measured at ~595 and ~535 nm and expressed as Fo~595/Fo~535. Statistically significant differences between groups for each evaluated time point are expressed as different capital (A vs. B; P < 0.01) and small (a vs. b; P < 0.05) letters.
In bovine sperm, both treatments caused a ΔΨM increase as observed in human sperm. Indeed, at 90 min incubation, the two treatments showed ΔΨM levels significantly higher than CNTRL (11.5 ± 1.2 and 12.1 ± 0.9 vs. 6.1 ± 1.1; P < 0.05 and P < 0.01, respectively) and similar then CNTRL at time 0 (13.8 ± 1.5). At 180 min, the two treatments were higher than CNTRL (6.0 ± 0.5 and 6.7 ± 0.5 vs. 2.2 ± 0.3; P < 0.01) but significantly (P < 0.01) lower than CNTRL at time 0.
In ascidian sperm, after 90 min incubation, NAC group showed significantly higher levels of ΔΨM than CNTRL (21.3 ± 1.9 vs. 13.8 ± 1.3; P < 0.05). At 180 min, NAC maintains a significant higher value than CNTRL (15.1 ± 1.7 vs. 9.6 ± 0.7; P < 0.01). At this time, only NAC maintained levels of ΔΨM that were not significantly different from the CNTRL at time 0 (15.1 ± 1.7 vs. 20.6 ± 2.2).
Lipid peroxidation
Lipid peroxidation, as evaluated by C11- BODIPY581/591 and spectrofluorometric analysis, showed a significant (P < 0.01) time-dependent increase in all the species studied. However, no significant effect was observed in the treated groups (Fig. 3).
Lipid Peroxidation in human, bovine and ascidian spermatozoa exposed to metabolic enhancers. Lipid peroxidation in human (A), bovine (B) and ascidian (C) sperm divided in three groups treated with: (1) five metabolic enhancers (vitamins B6 and B12, 5 methyl THF, zinc and N-acetyl cysteine) (TRT group); (2) n-acetyl-cysteine (NAC group); and (3) medium alone (CNTRL group). Mean (± SE) values of lipid peroxidation in spermatozoa loaded with C11-BODIPY581/591, arranged in TRT, NAC and CNTRL groups and then incubated for 0, 90 and 180 min. Lipid peroxidation was calculated by relating the emission peak (Fo) at ~520 nm to the sum of the fluorescence emission peaks at ~520 and ~590 nm, i.e., ((Fo~520/ (Fo~520 + Fo~590)) * 100.
ROS production
The production of ROS, detected by H2DCFDA, increased with incubation time in the CNTRL of all the considered species (Fig. 4). Instead, the effects of the treatments varied according to the species. In particular, in human, ROS production significantly (P < 0.01) increased in NAC at both 90 and 180 min while TRT did not differ from CNTRL. In bovine spermatozoa, both TRT and NAC showed ROS levels significantly (P < 0.05) lower than in the CNTRL group at 180 min incubation.
H2DCFDA-detected ROS in human, bovine and ascidian spermatozoa exposed to metabolic enhancers. Reactive Oxygen Species (ROS) detected by H2DCFDA in human (A), bovine (B) and ascidian (C) sperm divided in three groups treated with: (1) five metabolic enhancers (vitamins B6 and B12, 5 methyl THF, zinc and N-acetyl cysteine) (TRT group); (2) n-acetyl-cysteine (NAC group); and (3) medium alone (CNTRL group). Mean (±SE) values of ROS were evaluated in H2DCFDA-loaded spermatozoa, arranged in TRT, NAC and CNTRL groups and then incubated for 0, 90 and 180 min. ROS production was calculated by evaluating fluorescence intensity (Fo) at the emission peak (~520 nm). Statistically significant differences between groups for each evaluated time point are expressed as different capital (A vs. B; P < 0.01) and small (a vs. b; P < 0.05) letters.
Ascidian sperm did not show significant differences between groups at either 90 or 180 min incubation.
The production of ROS, detected by DHE (O2−-sensitive probe), varied among time points (Fig. 5). In human sperm, analyzing data without considering the effect of the incubation time, 2-OH E levels were significantly (P < 0.01) lower in TRT than in CNTRL and NAC groups. In bovine sperm, after 90 min incubation, the 2-OH E levels of TRT and NAC groups were significantly lower than in CNTRL group. After 180 min incubation, TRT group showed lower (P < 0.01) 2-OH E levels than NAC but it did not significant differ from CNTRL.
DHE-detected ROS in human, bovine and ascidian spermatozoa exposed to metabolic enhancers. Reactive Oxygen Species (ROS) detected by dihydroethidium (DHE) in human (A), bovine (B) and ascidian (C) sperm divided in three groups treated with: (1) five metabolic enhancers (vitamins B6 and B12, 5 methyl THF, zinc and N-acetyl cysteine) (TRT group); (2) n-acetyl-cysteine (NAC group); and (3) medium alone (CNTRL group). Mean (±SE) values of ROS production in spermatozoa loaded with DHE, arranged in TRT, NAC and CNTRL groups and then incubated for 0, 90 and 180 min. ROS production was calculated by fluorescence intensity (Fo) at the emission peak (~590 nm). Statistically significant differences between groups for each evaluated time point are expressed as different capital (A vs. B; P < 0.01) and small (a vs. b; P < 0.05) letters.
In ascidian sperm, the pattern of 2-OH E levels resembled those already described in human sperm; however, due to a higher variability among experiments, the comparison between groups did not show significant differences.
Intracellular pH (pHi)
The pHi in the CNTRL group in human and ascidian sperm significantly (P < 0.01) increased as a function of the time of incubation, whereas in the bovine sperm it maintained a stable pattern (Fig. 6). In human and bovine species, both treatments did not significantly affect pHi although a small decrease of pHi was found in both TRT and NAC groups with respect to the CNTRL group at either 90 or 180 min incubation. In ascidian sperm, a dramatic decrease of pHi was observed in TRT and NAC groups at either 90 or 180 min incubation. Following the pH measurement of culture media used to incubate each species, we found a decrease of the pH value of 0.2–0.4 units when human and bovine culture media (i.e., SP and TRIS-fructose, respectively) were supplemented with either the five treatment compounds or the only NAC; however, this decrease was equivalent to 2.0–2.5 pH units in filtered seawater used to incubate ascidian spermatozoa. When FNSW was buffered to pH = 8.2 with NaHCO3 in the ascidian TRT or NAC groups, different results were obtained in relation to ΔΨM, pHi and ROS production (Fig. 7). In particular, the ΔΨM increase found in NAC at 180 min incubation was annulled (P < 0.05) when FNSW was buffered (Fig. 7A); the production of ROS, detected by either H2DCFDA or DHE (Fig. 7B,C), did not show significant differences; the pHi significantly increased in NAC group following FNSW buffering (Fig. 7D), however, in TRT-buffered group this increase did not significantly reset pHi to CNTRL values at neither 90 nor 180 min incubation.
Intracellular pH in human, bovine and ascidian spermatozoa exposed to metabolic enhancers. Intracellular pH (pHi) in human (A), bovine (B) and ascidian (C) sperm divided in three groups treated with: (1) five metabolic enhancers (vitamins B6 and B12, 5 methyl THF, zinc and N-acetyl cysteine) (TRT group); (2) n-acetyl-cysteine (NAC group); and (3) medium alone (CNTRL group). Mean (±SE) values of pHi in spermatozoa loaded with BCECF, arranged in TRT, NAC and CNTRL groups and then incubated for 0, 90 and 180 min. The fluorescence emission peaks (Fo) were measured at 535 nm after excitation at 440 and 490 nm. Statistically significant differences between groups for each evaluated time point are expressed as different capital (A vs. B; P < 0.01) letters.
Effect of extracellular pH in ascidian spermatozoa exposed to metabolic enhancers. Mitochondrial membrane potential (ΔΨM) (A), Reactive Oxygen Species (ROS) detected by either H2DCFDA (B) or dihydroethidium (C) and intracellular pH (D) in ascidian sperm divided in five groups treated with: (a) five metabolic enhancers (vitamins B6 and B12, 5 methyl THF, zinc and N-acetyl cysteine) simply supplemented to FNSW (TRT group) and NFSW that was, after this supplementation, buffered at pH = 8.2 (TRT buf group); (b) n-acetyl-cysteine simply supplemented to FNSW (NAC group) and NFSW that was, after this supplementation, buffered at pH = 8.2 (NAC buf group); and (c) FNSW alone (CNTRL group). Mean (±SE) values obtained for each analytical determination were reported at 0, 90 and 180 min incubation. (n = 6 replications). Statistically significant differences between groups for each evaluated time point are expressed as different capital (A vs. B and C vs. D; P < 0.01) and small (a vs. b; P < 0.05) letters.
Discussion
The progressive decline in human male fertility over the last years22 highlights the need for strategies to prevent further drops in sperm quality, which is especially manifest when treating males for assisted reproductive technologies. The 1-CC is an important target for maintaining sperm quality as it is involved in methylation and generation of three major antioxidants: hypotaurine, glutathione and Coenzyme Q1023,24. The 1-CC, which links folate and methionine cycles, underlies this effect in conjunction with the trans-sulfuration and BHMT pathways. The 1-CC and the associated folic acid pathway allow the regeneration of homocysteine to methionine and the synthesis of S adenosyl methionine (SAM). SAM is the universal methyl donor, the precursor of polyamines and a compulsory stabilizer of DNA24. The trans-sulfuration pathway, via the genesis of cysteine, enhances the synthesis of glutathione. In the present study, we tested the supplementation of sperm culture medium with some of the major effectors of 1-CC, i.e., Vitamins B6 and methyl B12, 5MTHF, NAC and Zinc. Furthermore, we focused on NAC that plays a special role in the production of glutathione and hypotaurine25.
These effects were evaluated together with standard computer-assisted sperm kinetics, by using a simple, accurate, rapid, and sensitive methodology, which combines fluorescent probes with spectrofluorimetric analysis for the assessment of ΔΨM, lipid peroxidation, ROS production and pHi in mammalian (human and bovine), as well as in the marine invertebrate (C. robusta) spermatozoa. In human sperm, this methodology is a novelty whereas in bovine and ascidian sperm this methodological approach have been partially tested in recent studies26,27,28. Beneficial effects of NAC on semen parameters including sperm motility have been already described in several mammalian species, such as bovine29, human30 as well as in the rooster31.
The analysis of ΔΨM revealed that both the full mixture of enhancers or NAC alone have beneficial effects up to 180 min incubation in the two mammalian species. In ascidian sperm, only NAC showed a significant improvement of ΔΨM either at 90 min or 180 min incubation showing a clear discrepancy with respect to the combined treatment.
N-Acetylcysteine (NAC) improves sperm function32 and when combined with Acetyl-L-Carnitine and α-Lipoic Acid has a significant beneficial effect on in vitro fertilized mouse pronucleate oocyte development, especially under oxidative stress33. In addition, as demonstrated in H9c2 cells, it has an antiapoptotic activity against glucose/glucose oxidase-induced oxidative stress by the inhibition of mitochondrial damage and the maintenance of ΔΨM values34. The ΔΨM is generated by the electron-transport chain and regulated by an oxidation–reduction equilibrium of ROS, pyridine nucleotides (NADH/NAD+ and NADPH/NADP), and reduced glutathione (GSH)35. ΔΨM, together with the production of ROS, is crucial for the forward motility and fertility of spermatozoa36,37,38. Hence, the beneficial effects of NAC on ΔΨM was predictable as well as the combined treatment containing NAC. There was no additive effect in human or bovine sperm from the other four components, as demonstrated in preliminary trials of single compound exposure (Supplemental Fig. S2), where, unlike NAC, no significant effects were observed on ΔΨM values by each single supplementation. In ascidians, the lower ΔΨM values observed in the TRT group may be due to the negative effects of the four other components. The role of vitamins in marine organisms is, in fact, critical. In the marine systems, taxonomic changes observed in marine phytoplankton communities could be the result of their specific vitamin requirements and/or vitamin availability. Dissolved vitamin concentrations show that large areas of the world oceans are devoid of B vitamins, suggesting that vitamin limitation could be important for the efficiency of carbon and nitrogen fixation in these regions39. Consequently, the effects of adding vitamins to marine animals may depend on conditions particular to these marine organisms in nature.
Sperm lipid peroxidation is an effective indicator of sperm quality, oxidative stress and storage resistance40. Moderate ROS levels exert positive effects during sperm capacitation and fertilization41; however, high ROS levels cause sperm damage, affect all biomolecules and, ultimately, lead to a cellular stress or even death42. The lipophilic fluorescent probe C11-bodipy shows oxidative damage in cells by changing its fluorescence when it interacts with peroxyradicals27. In our study, we did not find differences between TRT, NAC and CNTRL groups in relation to peroxidation, which may be due to the short incubation time used; however, the progressive increase in lipid peroxidation with time of incubation suggests the experimentation was correct. This was further demonstrated by the use of positive controls, by adding 750 µM ascorbic acid and 150 µM FeSO4 to the sperm extender27.
ROS production was determined by evaluating either dichlorofluorescein (H2DCFDA) or 2-OH ethidium fluorescence by spectrofluorometric analysis. Dichlorofluorescein (H2DCFH) enters cells in the diacetate form (H2DCFH-DA). It is hydrolyzed and trapped as DCFH, a nonfluorescent compound. Subsequent oxidation of DCFH by H2O2, catalyzed by peroxidases, yields the highly fluorescent DCF43. This chemical reporter is widely applied as a “hydrogen peroxide (H2O2)-specific probe” in intact cells44. However, the range of ROS detected by this probe is much broader since DCF formation is also induced by other radical species including nitrogen derived free radicals45,46 rather than O2−47. However, the superoxide radical can be enzymatically dismutated to form H2O2 and further stimulate amplification of the DCF signal48. In our study, this ROS indicator shows clear species-specific modifications. In all the studied species, it increased along with incubation time. In human sperm, ROS levels appear higher in the NAC than in the CNTRL with an intermediate value in the TRT group. The high concentration of NAC (2 mM) may have exerted a paradox pro-oxidant effect via reduction of transition metal ions and enhancement of Fenton chemistry, as shown for high dose vitamin C49. This paradox effect was likely partially compensated by the antioxidant effect of other substances in TRT group. This was not seen in bovine sperm where the CNTRL group exhibits higher levels of ROS than treated groups at both 90 and 180 min of incubation. Indeed, bovine sperm unlike the other species have been subjected to freezing/thawing procedure, a particularly stressful condition that has led them to benefit from the antioxidant treatment50. In ascidian sperm, the situation is also different without significant differences between groups.
Dihydroethidium (DHE) is an intracellular indicator of the ROS superoxide ion quota51. This probe is able to detect a non-mitochondrial ROS source that cannot be disrupted by mitochondrial inhibitors such as rotenone and CCCP52. However, this hypothesis was revised when a derivative of DHE, MitoSOX Red, was developed. This probe contains a positively charged triphosphonium cation that allows it to concentrate in the mitochondrial matrix and efficiently detects mitochondrial-derived O2− in human sperm53. More recently, Aitken et al.54 demonstrated in human sperm exposed to oxidative stress that DHE was highly correlated with MitoSOX Red (r = 0.993) for the evaluation of O2− generation. In our study, the specificity of superoxide detection is confirmed by the analysis at spectrofluorometer of 2OH-Etidium in positive controls obtained by treating sperm samples for 1 hour with 30 µM pyrogallol, a potent superoxide ion generator55 (Supplemental Fig. S3). In all the studied species, we found a similar effect from the evaluated treatment. In particular, the combination of the five vitamin-antioxidant components linked to the 1-CC cycle significantly reduced the production of superoxide ions whereas NAC alone did not. This finding agrees with studies carried out in other species. In the horse, incubation of spermatozoa under both hyposmotic and hyperosmotic conditions increases superoxide anion generation; this production can be inhibited by tiron, a superoxide scavenger as well as MAP kinase p38 inhibitor, SB20358056.
No significant effects were observed on the intracellular pHi of human and bovine sperm in relation to the treatments used. The high buffering capacity of the extenders used in human and bovine species may have counteracted the NAC-induced acidification found in ascidian sperm; this was based on the reduced buffering potential of natural sea water and was indeed reverted by buffering with NaHCO3. However, the NAC-induced acidification of the medium resulted to be the determining factor for the positive effects of treatment in this species. Actually, the fact that the NAC exhibits an isoelectric point equal to 5, makes the treatment more effective in acidic environments, canceling its effects under the typical alkaline conditions of ascidian sperm. In the human, previous studies57 found that 0.5 mM NAC prevented sperm immobilization induced by Stat3 inhibitory compound V (Stattic V) as well as the production of superoxide anion, mitochondrial membrane depolarization and the oxidation of protein free thiols caused by Stattic V; however, with regards to pHi, 0.5 mM NAC did not significantly affect pHi but efficiently counteracted Stattic V-induced acidification.
Overall, compared to NAC only, the association of the other 1-CC enhancers (TRT) produced some further advantages. TRT groups showed lower production of ROS detected by DHE compared to CNTRL and NAC. TRT also partially restored the excess of ROS production measured by H2DCFDA in NAC group, a paradox pro-oxidant effect due to its high concentration. Indeed, the activation of the 1-CC serves as a homeostatic signal for the up-regulation of GSH synthesis58,59. This added antioxidant benefit may account for the TRT improvements over NAC alone on ROS production and may be very relevant in in vivo conditions. Indeed, NAC oral administration in humans generates circulating concentrations in the range of 5 µM, which is far below the concentration tested in this study and that are supposed to benefit only by inducing GSH synthesis without any real direct antioxidant effect60. Therefore, the positive in vivo effect of NAC is linked to the induction of GSH synthesis and it is predicted to benefit from an extended 1-CC support.
Although both the extenders used for human (SP) and bovine (Tris-fructose) semen contain components such as human serum albumin and insulin (SP) and bovine serum albumin (Tris-fructose) which could perform a per se antioxidant role and interact with the evaluated treatments, the use of these media is mandatory to assure optimal in vitro culture conditions of spermatozoa.
In conclusion, the proposed multiple vitamin/antioxidant treatment efficiently enhanced human and bovine sperm metabolic activity based on sperm kinetics and ΔΨM indications. Since there were no significant differences between the TRT and the NAC treatment alone, we may attribute these beneficial effects, except for the inhibition of ROS production, to NAC. The association of other 1-Carbon Cycle enhancers further improved the effect on sperm kinetics and ROS production indicating that they may be relevant in the clinical setting when the attainable concentration of NAC is a lot lower. The fast metabolism associated with the reduced cytoplasmic component typical of spermatozoon makes this specialized cell an unexpected user of the 1-Carbon Cycle. Ascidian C. robusta sperm showed lower advantages following the vitamin/antioxidant treatment that were, however, strictly dependent on the extra- and intra-cellular acidification induced.
Materials and Methods
Chemicals
Nigericin, Triton X-100, sodium citrate, HEPES, Penicillin-Streptomycin, pyridoxine (Vitamin B6), zinc bisglycinate, n-acetyl-cysteine, water and all the culture medium compounds were purchased by Sigma Chemical Company (Milan, Italy) and cell culture tested.
(6S)-5-methyltetrahydrofolate/glucosamine salt (5MTHF) and methylcobalamin (reduced vitamin B12) were kindly provided by Parthenogen, Switzerland. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1), 4,4-difluoro-5-(4-phenyl-1,3,butadienyl)4-bora-3a,4a-diaza-s-indacene-33-undecanoic acid (C11-BODIPY581/591), 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxy-fluorescein acetoxymethyl ester (BCECF-AM), 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) and dihydroethidium (DHE) were obtained from Life Technologies (Milan, Italy).
Human sperm preparation
Sperm samples were provided by patients, with written consent, attending to the Centre for Assisted Fertilization (CFA), Naples, Italy, between October 2016 and April 2017. The sperm used for experimentation was excess material not used for clinical purposes and was normally discarded. The experimentation on human spermatozoa was approved by the ethical committee of CFA. Each semen sample was evaluated for sperm concentration and motility by using a Makler chamber (Sefi Medical Instruments, Haifa, Israel). Only ejaculates from normospermic individuals according to WHO criteria61 were used for experiments.
Spermatozoa were isolated by centrifugation over 45% and 80% Percoll gradients for 30 min at 200 g. They were washed, resuspended in Sperm Preparation (SP) medium (Origio, DK) and evaluated for their concentration and motility.
Bovine sperm preparation
Frozen bovine semen from three ejaculates of three Holstein Friesian bulls of proven fertility were used in all experiments and obtained from Inseme (Modena, Italy). For each experiment, straws of each bull were thawed in air (10 sec), then, in a water bath at 37 °C for 30 sec and mixed in a pool. Spermatozoa were isolated by centrifugation over 45% and 80% percoll gradient for 30 min at 200 g. They were washed in tris-fructose medium - i.e., 247.7 mM Tris (hydroxymethyl) aminomethane, 57.8 mM citric acid, 69.4 mM fructose, supplemented with 3 mg bovine serum albumin/mL and 1x Penicillin-Streptomycin solution in 1 L of final solution (pH = 7.5, 291 mOsm)62 - by centrifugation at 200 g for 10 min. Spermatozoa were resuspended in tris-fructose medium and evaluated for their concentration and motility.
Ascidian sperm preparation
Adults of C. robusta were collected from the Gulf of Naples (Italy) and maintained in aquaria with running seawater at 18 °C for at least two days until the experiments. This ascidian is not protected by any environmental agency in Italy and Europe.
After anesthetization of animals on ice, spermatozoa were collected from sperm ducts. Sperm concentration and motility were evaluated by using a Makler chamber and, then, diluted to the desired concentration in filtered (Millipore 0.22 mm; MilliQ, Medford, MA) natural seawater (FNSW) (38 g/L salinity, pH 8.2 ± 0.1).
Experimental design
Human, bovine and ascidian spermatozoa were suspended in their specific sperm extenders, i.e., SP and tris-fructose media and NFSW, respectively, and loaded with fluorescent indicators for the assessment of ΔΨM, pHi, lipid peroxidation and ROS production. Specific extenders were supplemented with: (1) five sperm metabolic enhancers, i.e., 60 µM pyridoxine, 40 µM 5MTHF, 7 µM methylcobalamin, 130 µM zinc bisglycinate and 2 mM n-acetyl-cysteine (treated group, TRT); (2) 2 mM n-acetyl-cysteine (n-acetyl-cysteine-treated group, NAC); (3) medium alone (control group, CNTRL). Fluorochrome-loaded spermatozoa were distributed in these three groups and incubated for 0, 90 and 180 minutes at 37 °C (human and bovine) and 18 °C (ascidian). All the sperm metabolic enhancers were water soluble; hence, 100X stock solutions of each compound were previously prepared and stored at −80 °C.
Sperm kinetics
The assessment of the human and bovine sperm kinetics was carried out with a SCA 5.0 system (Microptic, Barcelona, Spain), whereas ascidian sperm kinetics were analyzed by CASA application of the open source ImageJ software. The extended spermatozoa were loaded into a Makler cell chamber and recorded using video cameras (CCD Hitachi, Tokyo, Japan and AxioCam 506, Ziess, Oberkochen, Germany). Sperm motility patterns were analyzed at 25 or 40 video frames per sec with the SCA and CASA systems, respectively. Sperm concentration was adjusted with specific extenders to a final concentration of 20 × 106 spermatozoa/mL. The kinematic values recorded included: the overall percentage of motile spermatozoa; the curvilinear velocity (VCL, µm/s); the straight-line velocity (VSL, µm/s); the average path velocity (VAP, µm/s)63.
Evaluation of Mitochondrial Membrane Potential (ΔΨM)
Sperm aliquots were diluted in their specific extender in order to obtain a concentration of approximately 1 × 107 spermatozoa/mL and incubated with 5 µM JC-1 at room temperature (RT) for 30 min13. After incubation, sperm suspensions were centrifuged at 200 g for 10 min (human and bovine) at RT or at 800 g for 10 min at 4 °C (ascidian). Later, they were resuspended in their specific extender, divided in three aliquots at the same sperm concentration and arranged accordingly to CNTRL, TRT and NAC groups, as described above. In addition, one tube with the addition of CCCP to a final concentration of 2 µM served as a negative control27.
The fluorescence spectra were recorded in duplicate: 400 µL of each sample was placed in a quartz microtube (10 × 4 mm) high precision cell (Hellma Analytics, Müllheim, Germany) and read with a spectrofluorometer (Shimadzu RF-5301, Tokyo, Japan). The excitation wavelength was set at 490 nm, and the emission spectrum was recorded in the range of 500–650 nm (Supplemental Fig. S3). The ΔΨM was measured by evaluating the ratio of the fluorescence values at ~595 nm and ~535 nm.
Evaluation of Intracellular pH (pHi)
Sperm suspensions (1 × 107 spermatozoa/mL) of each species were incubated with 5 μM BCECF-AM, for 30 min at 37 °C (human and bovine) or 18 °C (ascidian) and, then, washed by centrifugation, as referred above. Later, spermatozoa were incubated for an additional 30 min to allow BCECF de-esterification. The spermatozoa were then washed, suspended in their specific extender and divided at the same sperm concentration in three groups. Aliquots of each sperm suspension were sampled, mixed and equally divided into three tubes containing the calibration solution (143 mM KCl, 5 mM HEPES, 290 mOsm) at different pH values supplemented with nigericin64,65.
At the end of each incubation time and at time 0 in the control group, samples were read with a spectrofluorometer, as described above. Data were obtained by dividing the emission intensity at 535 nm after excitation at 490 nm by the emission intensity at 535 nm after excitation at 440 nm. A linear-regression analysis of the calibration produced a formula that was used to obtain pHi values for each experiment.
Evaluation of ROS production
ROS production was determined by spectrofluorometric analysis evaluating either H2DCFDA or DHE fluorescence spectra. H2DCF has very low reactivity toward superoxide radicals whereas hydrogen peroxide indirectly facilitate its oxidation48.
DHE is a cell-permeable compound that, in the presence of superoxide anion, is oxidized to unspecific products, as ethidium, and a single superoxide-specific product, 2-OH-ethidium (2-OH E)51,66. For the H2DCFDA examination, sperm suspensions (2 × 107 spermatozoa/mL) were incubated for 30 min at 37 °C (human and bovine) or 18 °C (ascidian) with 10 µM H2DCFDA. Then, spermatozoa were washed and incubated as described above for additional 30 min. Later, spermatozoa were washed and divided in tubes containing the specific extenders arranged for the three experimental groups and the three times of evaluation. Positive control was obtained by incubating 1 h samples of sperm suspension with 25 µM H2O2. Samples were read with a spectrofluorometer, as described above. The excitation wavelength was set at 490 nm, and the emission spectrum was recorded at 500–560 nm (Supplemental Fig. S3). ROS production was evaluated as arbitrary units of fluorescent signal.
For the DHE protocol, sperm suspensions (30 × 107 spermatozoa/mL) of the three experimental groups, at the end of each incubation time and at time 0 in the control group, were incubated for 30 min at 37 °C (human and bovine) or 18 °C (ascidian) with 20 µM DHE. Positive control was obtained by incubating samples of sperm suspension with 30 µM pyrogallol together with DHE.
At the end of the incubation time, samples were read with a spectrofluorometer, as described above. The excitation wavelength was set at 350 nm, and the emission spectrum was recorded in the range of 550–670 nm (Supplemental Fig. S3). ROS production was evaluated as arbitrary units of fluorescent signal.
Evaluation of Lipid Peroxidation
Lipid peroxidation was evaluated by C11- BODIPY581/591 fluorescence and spectrofluorometric analysis67. Sperm aliquots (2 × 107 spermatozoa/mL) were incubated with 5 µM C11-BODIPY581/591 for 30 min at 37 °C (human and bovine) and 18 °C (ascidian). The spermatozoa were then centrifuged and resuspended in each specific extender. Aliquots of each sample were mixed and used as a positive control which involved incubating for 1 h with 150 µM FeSO4 and 750 µM ascorbic acid before spectrofluorometric evaluation27.
After incubation, aliquots of each sample were read with a spectrofluorometer, as described above. The excitation wavelength was set at 490 nm, and the emission spectrum was recorded in the range of 500–650 nm. Lipid peroxidation was evaluated by relating the fluorescence peak values at ~520 nm to the sum of the fluorescence peak values at ~520 and ~595 nm (Supplemental Fig. S3).
Confocal laser scanning microscopy analysis
Each fluorochrome used in this study was first evaluated by confocal laser scanning microscopy (Zeiss LSM 510) to assess its localization within a specific cell compartment (Supplemental Fig. S4).
Statistical analysis
Data were obtained in six to eight replicates and entered in a spreadsheet as follows: the replication number, the time of incubation, the three experimental groups (TRT, NAC and CNTRL), and the sperm parameters evaluated, i.e., total sperm motility and kinetics, ΔΨM, pHi, ROS production by either H2DCFDA or DHE evaluations and lipid peroxidation. Before the analyses, percentage values were transformed in arcsin whereas, for pHi, H+ concentrations were log transformed. Repeated measures ANOVA (Systat 11.0) was used for evaluating either the time of incubation or treatment effects for each sperm parameter evaluated. Shapiro-Wilks test was used to evaluate the normal distribution of the data. Levene test was used to verify homogeneity of variances. Log transformation was applied when the normality hypothesis was rejected. Pair-wise comparisons of the means were performed with Bonferroni test.
References
Peterson, R. & Freund, M. ATP synthesis and oxidative metabolism in human spermatozoa. Biol Reprod 3, 47–54 (1970).
Richter, C. Do mitochondrial DNA fragments promote cancer and aging? FEBS Lett 241, 1–5 (1988).
Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005).
Kawamata, H. & Manfredi, G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech Ageing Dev 131, 517–526 (2010).
Starkov, A. A. & Fiskum, G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD (P) H redox state. J Neurochem 86, 1101–1107 (2003).
Rottenberg, H. & Scarpa, A. Calcium uptake and membrane potential in mitochondria. Biochemistry-US 13, 4811–4817 (1974).
Pena, F., Johannisson, A., Wallgren, M. & Martinez, H. R. Antioxidant supplementation in vitro improves boar sperm motility and mitochondrial membrane potential after cryopreservation of different fractions of the ejaculate. Anim Reprod Sci 78, 85–98 (2003).
O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J Androl 17, 8 (2015).
Flesch, F. M. & Gadella, B. M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. BBA-Rev Biomemb 1469, 197–235 (2000).
Tosti, E. & Ménézo, Y. Gamete activation: basic knowledge and clinical applications. Hum Reprod Update 22, 420–439 (2016).
Agarwal, A., Durairajanayagam, D. & Du Plessis, S. S. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrin 12, 112 (2014).
Oeda, T., Henkel, R., Ohmori, H. & Schill, W. B. Scavenging effect of N‐acetyl‐L‐cysteine against reactive oxygen species in human semen: a possible therapeutic modality for male factor infertility? Andrologia 29, 125–131 (1997).
Aitken, R. J. et al. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod 87 (2012).
Guerin, P., El Mouatassim, S. & Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update 7, 175–189 (2001).
Stipanuk, M. H., Dominy, J. E., Lee, J.-I. & Coloso, R. M. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 136, 1652S–1659S (2006).
Bauerle, M. R., Schwalm, E. L. & Booker, S. J. Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation. J Biol Chem 290, (3995–4002 (2015).
Menezo, Y. et al. Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sci 44, 1601–1609 (1989).
Selhub, J. F. vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging 6, 39–42 (2001).
Park, E. I. & Garrow, T. A. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J Biol Chem 274, 7816–7824 (1999).
Tran, U. C. & Clarke, C. F. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 7, S62–S71 (2007).
Jentsch, T., Janicke, I., Sorgenfrei, D., Keller, S. & Wiederholt, M. The regulation of intracellular pH in monkey kidney epithelial cells (BSC-1). Roles of Na+/H+ antiport, Na+-HCO3 (−)-(NaCO3−) symport, and Cl-/HCO3-exchange. J Biol Chem 261, 12120–12127 (1986).
Virtanen, H. E., Jørgensen, N. & Toppari, J. Semen quality in the 21st century. Nat Rev Urol 14, 120–130 (2017).
Ebisch, I., Thomas, C., Peters, W., Braat, D. & Steegers-Theunissen, R. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update 13, 163–174 (2006).
Cornet, D. et al. Association between the MTHFR-C677T isoform and structure of sperm DNA. J Assist Reprod Gen 1–6 (2017).
Waterfield, C. & Timbrell, J. The biosynthesis of taurine fromN-acetyl-l-cysteine and other precursorsin vivo and in rat hepatocytes. Amino acids 10, 173–185 (1996).
Gallo, A., Boni, R., Buttino, I. & Tosti, E. Spermiotoxicity of nickel nanoparticles in the marine invertebrate Ciona intestinalis (ascidians). Nanotoxicology 10, 1096–1104 (2016).
Boni, R., Gallo, A. & Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 5, 133–145 (2017).
Gallo, A., Boni, R. & Tosti, E. Sperm viability assessment in marine invertebrates by fluorescent staining and spectrofluorimetry: A promising tool for assessing marine pollution impact. Ecotox Environ Safe 147, 407–412 (2018).
Bilodeau, J.-F., Blanchette, S., Gagnon, C. & Sirard, M.-A. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 56, 275–286 (2001).
Ciftci, H., Verit, A., Savas, M., Yeni, E. & Erel, O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology 74, 73–76 (2009).
Partyka, A., Niżański, W., Bajzert, J., Łukaszewicz, E. & Ochota, M. The effect of cysteine and superoxide dismutase on the quality of post-thawed chicken sperm. Cryobiology 67, 132–136 (2013).
Barekat, F. et al. A Preliminary Study: N-acetyl-L-cysteine Improves Semen Quality following Varicocelectomy. Int J Fertil Steril 10, 120 (2016).
Truong, T. & Gardner, D. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum Reprod 32, 2404–2413 (2017).
Kumar, S. & Sitasawad, S. L. N-acetylcysteine prevents glucose/glucose oxidase-induced oxidative stress, mitochondrial damage and apoptosis in H9c2 cells. Life Sci 84, 328–336 (2009).
Perl, A., Gergely, P., Puskas, F. & Banki, K. Jr. Metabolic switches of T-cell activation and apoptosis. Antioxid Redox Sign 4, 427–443 (2002).
Fisher, H. M. & Aitken, R. J. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool Part A 277, 390–400 (1997).
Marchetti, C., Obert, G., Deffosez, A., Formstecher, P. & Marchetti, P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod 17, 1257–1265 (2002).
Perl, A. et al. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. PNAS 103, 14813–14818 (2006).
Sañudo-Wilhelmy, S. A., Gómez-Consarnau, L., Suffridge, C. & Webb, E. A. The role of B vitamins in marine biogeochemistry. Annu Rev Mar Sci 6, 339–367 (2014).
Kothari, S., Thompson, A., Agarwal, A. & du Plessis, S. S. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol 48, 10 (2010).
De Lamirande, E. & Gagnon, C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod 10, 15–21 (1995).
Tremellen, K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update 14, 243–258 (2008).
Cathcart, R., Schwiers, E. & Ames, B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134, 111–116 (1983).
Keston, A. S. & Brandt, R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem 11, 1–5 (1965).
Kalyanaraman, B. et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Bio Med 52, 1–6 (2012).
Winterbourn, C. C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. BBA-Gen Subjects 1840, 730–738 (2014).
Guthrie, H. & Welch, G. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J Anim Sci 84, 2089–2100 (2006).
Grisham, M. B. Methods to detect hydrogen peroxide in living cells: Possibilities and pitfalls. Comp Biochem Phys A 165, 429–438 (2013).
Halliwell, B. Commentary: vitamin C: antioxidant or pro-oxidant in vivo? Free Radical Res 25, 439–454 (1996).
Eidan, S. M. Effect on post-cryopreserved semen characteristics of Holstein bulls of adding combinations of vitamin C and either catalase or reduced glutathione to Tris extender. Anim Reprod Sci 167, 1–7 (2016).
Nazarewicz, R. R., Bikineyeva, A. & Dikalov, S. I. Rapid and specific measurements of superoxide using fluorescence spectroscopy. J Biomol Screen 18, 498–503 (2013).
De Iuliis, G. N., Wingate, J. K., Koppers, A. J., McLaughlin, E. A. & Aitken, R. J. Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. J Clin Endocr Metab 91, 1968–1975 (2006).
Koppers, A. J., De Iuliis, G. N., Finnie, J. M., McLaughlin, E. A. & Aitken, R. J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocr Metab 93, 3199–3207 (2008).
Aitken, R. et al. On methods for the detection of reactive oxygen species generation by human spermatozoa: analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology 1, 192–205 (2013).
Hsieh, Y. Y. et al. Superoxide dismutase activities of spermatozoa and seminal plasma are not correlated with male infertility. J Clin Lab Anal 16, 127–131 (2002).
Burnaugh, L., Ball, B., Sabeur, K., Thomas, A. & Meyers, S. Osmotic stress stimulates generation of superoxide anion by spermatozoa in horses. Anim Reprod Sci 117, 249–260 (2010).
Lachance, C., Goupil, S., Tremblay, R. & Leclerc, P. The immobilization of human spermatozoa by STAT3 inhibitory compound V results from an excessive intracellular amount of reactive oxygen species. Andrology 4, 133–142 (2016).
Dattilo, M., Ettore, C. & Ménézo, Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: mechanisms, clinical results, insights on gene-environment interactions and the role of diet. J Assist Reprod Gen 33, 1633–1648 (2016).
Joseph, J. & Loscalzo, J. Methoxistasis: integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients 5, 3235–3256 (2013).
Rushworth, G. F. & Megson, I. L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Therapeut 141, 150–159 (2014).
Organisation, W. H. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. (Cambridge university press, 1999).
Crespilho, A. et al. Sperm fertility and viability following 48h of refrigeration: Evaluation of different extenders for the preservation of bull semen in liquid state. Anim Reprod Sci 146, 126–133 (2014).
Mortimer, S. T. CASA—practical aspects. J Androl 21, 515–524 (2000).
Thomas, J. A., Buchsbaum, R. N., Zimniak, A. & Racker, E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry-US 18, 2210–2218 (1979).
Zeng, Y., Oberdorf, J. A. & Florman, H. M. pH regulation in mouse sperm: Identification of Na+-, Cl−-, and [formula] dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol 173, 510–520 (1996).
Zielonka, J., Vasquez-Vivar, J. & Kalyanaraman, B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3, 8 (2008).
Brouwers, J. F. & Gadella, B. M. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radical Bio Med 35, 1382–1391 (2003).
Acknowledgements
We thank Dr. Alessia Cuccaro and Dr. Maria Consiglia Esposito for participation in the spectrofluorometric analyses and prof. Brigida Bochiccio for precious chemical advices. We thank Mauro Fiaschi and Inseme (San Giuliano Saliceta, Modena, Italy) for kindly providing the bovine semen. Thanks are also due to Mr. Gianluca Zazo and Mr. Alberto Macina for providing and maintaining ascidians, and Mr. Vincenzo Monfrecola for technical assistance in experiments on ascidians. This work was supported by University of Basilicata (R.I.L.). Alessandra Gallo has been supported by Stazione Zoologica Anton Dohrn Post-doc Fellowship.
Author information
Authors and Affiliations
Contributions
Alessandra Gallo and Elisabetta Tosti carried out the methodological set up and the experiments on ascidian spermatozoa. Yves Menezo and Brian Dale conceptualized the study and revised the manuscript. Gianfranco Coppola carried out experiments on human spermatozoa. Maurizio Dattilo provided critical inputs to the analysis strategy. Raffaele Boni conceptualized and designed the study, performed experiments on bovine and human spermatozoa and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallo, A., Menezo, Y., Dale, B. et al. Metabolic enhancers supporting 1-carbon cycle affect sperm functionality: an in vitro comparative study. Sci Rep 8, 11769 (2018). https://doi.org/10.1038/s41598-018-30066-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30066-9
This article is cited by
-
Epidermal growth factor alleviates the negative impact of urea on frozen-thawed bovine sperm, but the subsequent developmental competence is compromised
Scientific Reports (2021)
-
Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men
Reproductive Biology and Endocrinology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.