Abstract
First-principles calculations were carried out to understand how anionic isovalent-atom doping affects the electronic structures and optical properties of α-MoO3. The effects of the sulphur and selenium doping at the three unique oxygen sites (Ot, Oa, and Ot) of α-MoO3 were examined. We found that the valence p orbitals of Sulphur/Selenium dopant atoms give rise to impurity bands above the valence band maximum in the band structure of α-MoO3. The number of impurity bands in the doped material depends on the specific doping sites and the local chemical environment of the dopants in MoO3. The impurity bands give rise to the enhanced optical absorptions of the S- and Se-doped MoO3 in the visible and infrared regions. At low local doping concentration, the effects of the dopant sites on the electronic structure of the material are additive, so increasing the doping concentration will enhance the optical absorption properties of the material in the visible and infrared regions. Further increasing the doping concentration will result in a larger gap between the maximum edge of impurity bands and the conduction band minimum, and will undermine the optical absorption in the visible and infrared region. Such effects are caused by the local geometry change at the high local doping concentration with the dopants displaced from the original O sites, so the resulting impurity bands are no long the superpositions of the impurity bands of each individual on-site dopant atom. Switching from S-doping to Se-doping decreases the gap between the maximum edge of the impurity bands and conduction band minimum, and leads to the optical absorption edge red-shifting further into the visible and infrared regions.
Similar content being viewed by others
Introduction
Molybdenum trioxide (MoO3) has garnered much research attention recently due to this material offering promising applications, coupled with its non-toxic nature, low cost and outstanding catalytic properties1,2,3,4,5,6,7. Further, MoO3 is found to be one of the most important metal oxides used as the electron-injection layers and the electrode material in the fast-growing field of photovoltaics and solar-cell devices. Amongst the three different polymorphic phases of MoO3, α-MoO3 with the orthorhombic layered crystal structure is thermodynamically most stable. α-MoO3 is an n-type semiconductor with a layered crystal structure with a wide band gap of 3.2 eV8. Due to its high band gap, α-MoO3 is not optimal to be used as photocatalyst for solar-energy applications directly. In order to utilize α-MoO3 effectively as a photocatalyst, we need to modify its structure to reduce the band gap for the improved efficiency in harness the solar energy of which the major component is from the visible region. So far, significant progress has been made in the fabrication and modification of α-MoO3-based materials and devices, and the performance and reliability of the material has been greatly improved. Nano-sized MoO3, such as nanoporous, nanobelts and nanorods, has drawn substantial attention and been utilized in various applications, such as electrochromic/photochromic devices9, pseudocapacitive charge storage10, supercapacitors11,12, gas sensors13,14, lithium-ion batteries15 and effective heterogeneous catalysts16. Kumar et al.10 engineered the surface of MoO3 nanobelts for electrochemical cell applications and reported that their specific capacitance is enhanced with relatively high stability. Hamwi et al.17 studied p-type doping efficiency of MoO3 in organic hole-transport materials and White et al.18 studied the interface structure of MoO3 and organic semiconductors for organic electronic device applications. Experimentally, Qin et al. studied19 the sulfur-doped molybdenum oxide (S-MoO3) material used as anode interface layer and their study shows that doping of S atom to the MoO3 can effectively modify anode interface layer to improve the hole-transport properties of MoO3.
The electronic, optical and catalytic properties of MoO3 are related to various factors including the structures, defects, and impurities. Earlier, White et al.18 examined the effects of surface structure, oxygen vacancy, and hydrogen adsorption on the catalytic properties on MoO3 using the DFT+U method and rationalized its reaction mechanisms20. The recent work by Agarwal et al. explored the catalytic applications of the bulk MoO3 and two-dimensional MoO321. Yang et al.22 investigated MoO3-TiO2 nanotube arrays by electrochemical anodization and reported an improved efficiency for the separation of photoinduced carrier pairs and an enhanced water-splitting performance. Yu et al.23 reported MoO3-TiO2 composite nanorods films that exhibited improved electrochromic performance. Recently, Chen et al.24 synthesized MoO3 nanobelts and the proposed that the impurity band (IB) in the gap is due to the oxygen vacancies. Among the various factors that have substantial influences on the electronic, optical and catalytic properties of MoO3, the impurity that can be introduced facilely via the doping treatment can be used as a conventional way to reduce the band gap of α-MoO3 for solar energy related applications.
Sulphur and selenium are group VIA elements that are isovalent to oxygen, but have higher valence p orbital energies than O (the first ionization energies of S and Se are 10.3625 and 9.75 eV26 respectively, lower than the first ionization energy of O 13.62 eV)27. Therefore, substituting S or Se for O may lead to substantial changes to the optical properties of α-MoO3. The effects of S-doping to WO3 (both W and Mo are group VIB transition metals) have been previously examined by Wang et al.28. Their study shows the S dopants can introduce impurity bands inside the band gap, and the resulting HOMO-LUMO gap (from the maximum edge of impurity bands to conduction band minimum) is smaller than the band gap of the pure material, which facilitates the absorption of visible light. However, to the best of our knowledge, there has not been a systematic first-principles study on how anionic (S and Se) doping at different doping sites and at different concentration affects the electronic structure and optical behaviour of α-MoO3. In order to understand how such doping processes affect the properties of α-MoO3, sulphur and selenium atoms were doped into the 3 unique oxygen sites of α-MoO3 at different local doping concentrations modelled using the first-principles calculations at the density functional theory (DFT) level. Our study is an important step forward to understand the effects of the ionic doping on α-MoO3, and to learn to tweak the electronic and optical properties of α-MoO3 by controlling the simple factors such as the doping concentration.
Computational Details
The first-principles calculations were carried out using Perdew–Burke–Ernzerhof (PBE) exchange-correlation functional within generalized gradient approximation (GGA), implemented in the Vienna ab initio simulation package (VASP) code29,30. The projected augmented wave (PAW) method31 with a plane-wave basis set was used. All of the structures were fully relaxed without any symmetric constraints. The energy cutoff is set to 550 eV and the convergence criteria for energy and force are set to 10−5 eV and 0.01 eV/Å, respectively. In order to better take the effect of the on-site Coulomb repulsion of Mo 3d electrons into account, the exchange correlation energy was treated by Perdew, Burke, and Ernzerhof (PBE)32 functional, in conjunction with the PBE+U (Ueff = 8.6 eV for the Mo d electrons) approach of Dudarev et al.33,34. First, we tested different Ueff values ranging from the 3.0 to 8.6 eV for the calculation of the raw MoO3 material. The Ueff value that predicts band gap in the best agreement with the experimental values was then used in the calculations of the doped MoO3 systems. For the band structure calculations, the integration over the first Brillouin zone was performed using a 4 × 4 × 3 Monkhorst-Pack k-point grid35. In our first-principles studies, the lattice constants of the supercells were kept constant during the doping process, so that the change in electronic stucture is caused the dopants rather than by resizing the super cell.
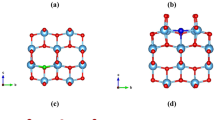
We have used a 2 × 2 × 2 MoO3 host supercell that consists of 32 Mo atoms and 96 O atoms (Fig. 1) to simulate the anionic doped MoO3-xSx and MoO3-xSex with different sulphur and selenium concentrations (indicated by different values of x). This doping process can be achieved by substituting oxygen atoms of the super cell with the dopant atoms (S/Se). Substituting one, two and three oxygen atoms of the supercell yields x = 0.03, 0.06 and 0.09 respectively. Bulk α-MoO3 has three distinct types of oxygen atoms: terminal (Ot), asymmetrical (Oa), and symmetrical (Os) oxygen atoms (Fig. 1a)20. The Oa is 2-fold by forming one long (2.20 Å) and one short (1.78 Å) bonds with two Mo atoms in the same layer. The Os is 3-fold as it forms two equal intralayer Mo-O bonds (1.95 Å) and one longer interlayer Mo-O bond (2.38 Å). The Ot forms one Mo=O bond with a single Mo atom, for which the bond distance (1.68 Å) is the shortest in the system.
Replacing one of the three oxygen site variants (Ot, Os and Oa) of the supercell with one S atom leads to three unique mono-atomic S-doped structures, namely MoO2.97(St)0.03, MoO2.97(Sa)0.03, and MoO2.97(Ss)0.03, with a Mo32O95S formula for the supercell. The bi-S doped structure was constructed by replacing an Ot and an Oa about the same Mo in the supercell, and the resulting structure is denoted as MoO2.94(StSa)0.03 with a Mo32O94S2 formula for the supercell. The tri-S doped structure was constructed by replacing an Ot, and an Oa, and an Os about the same Mo in the supercell, denoted as MoO2.91(StSaSs)0.03 with a Mo32O93S3 formula. Similar construction procedures were applied to generate the Se-doped structures (Fig. S1).
In addition, to examine the doping effects of uniform doping (i.e. all the dopant atoms substituting for the same type of O site) at higher concentration, in contrast to the bi- and tri- S doped at the same Mo-site, we also explored the bi-S and tri-S doped structures where single-type O sites about multiple Mo atoms in the supercell are replaced by the dopants. The bi-S doped structure was constructed by replacing two Ot atoms that are coordinated to two different Mo’s in the supercell, denoted as MoO2.94(St)0.06 with a Mo32O94S2 formula. The tri-S doped structure was constructed by replacing three Ot atoms (about three Mo atoms) in the supercell, denoted as MoO2.91(St)0.09 with a Mo32O93S3 formula. These corresponding relaxed structures are shown in Fig. S2(a,b) in the supplementary information (SI), and similar construction for Se-doped structures with higher dopant concentrations are shown in Fig. S2(c,d) in the SI.
The optical absorption spectra of pure and S/Se doped systems were calculated based on the dielectric function. The imaginary part ε2(ω) of the dielectric function can be calculated from the momentum matrix elements between the occupied and unoccupied wave functions with appropriate selection rules36. The real part ε1(ω) of the dielectric function was obtained by the Kramer–Kronigs relations. The optical absorption coefficient α(ω) can be computed using following formula
Availability of materials and data
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Results and Discussion
For the DFT + U calculations of α-MoO3, a scattering range of Ueff values from 1 to 8.6 eV have been used in the literature21. The value of Ueff is usually empirically determined, and it is near impossible to find a universal Ueff that is effective for all the different cases. In the current study, the Ueff value that predicts lattice parameters, Mo-O bond distances and band gap (Eg) in the best agreement with the experimental data for the bulk α-MoO3 was chosen to be applied to the MoO3-xSx and MoO3-xSex calculations. The calculated lattice parameters and band gap values at the PBE+U level with different Ueff values are given in Table S1 in the SI. From the Table S1, we clearly observed that at the PBE level essentially all of the tested Ueff values predict lattice parameters and Mo-O bond distances in good agreements with the experimental values37, i.e., within ~1% of the experimental values. Different Ueff values, however, result in different Eg prediction values, ranging from 1.55 to 2.58 eV (Table S1 and Fig. S3 in the SI). The closest Eg value to the experimental value (3.2 eV)38,39 is given by Ueff = 8.6 eV. The underestimation of Eg (by this study and previous studies)40 is mostly due to the inadequacy of the theory that DFT fails to accurately describe the relative energies of occupied and unoccupied electron energy levels. The present paper is focused to see how the anionic atom doping affects the observed band gaps and the presence of the impurity bands (gap states), for which the Eg shifts and the relative positions of the impurity bands in response to the different concentrations of the impurity anion are particularly interested. For the systems studied in the current study, the doping concentration is not high enough to affect the bulk electronic structure, and hence the doping effect on Eg is negligible (see below discussions). We also tested using different Ueff, the calculated energy difference between the impurity band and valence band maximum for a doped MoO3 system (Table S2 in the SI) and found the impurity band–valence band maximum gap is essentially invariant to the choice of Ueff. Hence, we have proceeded our calculations with Ueff = 8.6 eV.
Before we discuss the S/Se- doping effects, initial calculations have been carried out on the bulk MoO3 (2 × 2 × 2) without any symmetry constrains (with the optimized structure shown in Fig. 1a); the calculated bond distances of MoO3 are 1.68 Å for Mo-Ot, 1.77 Å and 2.21 Å for Mo-Oa, and 1.95 Å for Mo-Os, respectively. These bond distances are in good agreement with the experimentally measured unit cell parameters (Table S1 in the SI). The predicted density of states (DOS) for the pure α-MoO3(2 × 2 × 2) indicates that the valence band edge of the material is dominated by O 2p states and conduction band edge is dominated by Mo 3d states (Fig. 2). The calculated band structure of α-MoO3 shows that the valence band maximum (VBM) and conduction band minimum (CBM) are at Γ, suggesting α-MoO3 is a direct band gap semiconductor. The band edges at X are comparable in energy to the edges at Γ. At U, the valence band edge is ~0.2 eV lower than the VBM and the conduction band edge is ~0.1 eV higher than the CBM. This is different from the prior prediction by Scanlon et al. using the PBE functional41. Their study suggested that the VBM is situated at U and the CBM is at Γ, which lead to a minimal indirect gap of 1.95 eV and a minimal direct gap of 2.76 eV.
Doping sites favorability
The optimized geometries of the mono-S doped MoO2.97(St)0.03, and MoO2.97(Sa)0.03 and MoO2.97(Ss)0.03 (Fig. 1(b,c)) structures are essentially similar to the pure α-MoO3. The Mo-S bonds, however, are longer than the corresponding Mo-O bonds because S has a large ionic radius than O. In the mono-S doped MoO2.97(St)0.03 system, the dopant St forms one Mo-St bond with the surrounding environment, and the Mo-St bond distance is 2.073 Å. In MoO2.97(Sa)0.03, Sa forms two unequal Mo-S bonds with two Mo atoms, and the corresponding distances are 2.333 and 2.166 Å (Table S3 in the SI). In MoO2.97(Ss)0.03 Ss forms three equal Mo-S bonds with three Mo atoms with a distance of 2.297 Å. It is noted that all the Mo-S bonds are longer than their Mo-O counterparts, which is mostly due to S having the greater ionic radius than O. The bond length difference between the two Mo-Sa bonds (0.17 Å) of MoO2.97(Sa)0.03 is found to be much smaller than the difference between the two Mo-Oa bonds (0.42 Å) of the pure material, indicating a substantial change in the local geometry upon the Sa-doping treatment. For the mono-Se doped systems, Mo-Set, Mo-Sea and Mo-Ses bond distances are 2.199, 2.445(2.270) and 2.405 Å, respectively (Table S4 in the SI). The stabilities and electronic structures of mono-S doped MoO3(2 × 2 × 2) structures with one S atom substituted for each type of oxygen site (Ot, Oa, and Os) were examined. Among the three mono-S substituted structures, MoO2.97(St)0.03 (Fig. 1) is found to be more stable than the MoO2.97(Sa)0.03 and MoO2.97(Ss)0.03 (the corresponding relative energies are 0.0, 1.14 and 0.99 eV respectively). Similarly, and the results show that MoO2.97(Set)0.03 (Fig. S1 in SI)) is more stable than MoO2.97(Sea)0.03 and MoO2.97(Ses)0.03 with the corresponding relative energies being 0.0, 1.63 and 1.29 eV respectively.
Formation energies of mono-S and Se doped α-MoO3(2 × 2 × 2) were computed using the first principle calculations. The formation energies (Eform) for a doped system from a pure system can be calculated by using Eq. (1):
where E(doped) and E(pure) are the total energies of doped and perfect α-MoO3(2 × 2 × 2) respectively, and μO and μS are the chemical potential of the O and S atoms respectively and n is the number of substituted atoms (n = 1 to 3) in the supercell. From the equation (1) the calculated Eform are 2.64 and 2.15 eV for the mono-S doped MoO2.97(St)0.03 and the mono-Se doped MoO2.97(Set)0.03 respectively (Table S5 in the SI). This suggests that the Se doping is more energetically preferable than the S-doping. We obtained the reaction energy for the solid S/Se doping reaction with α-MoO3 (to release O2) by correcting Eform with the experimental heats of formation data42 of gas-phase O, S, and Se, for which the details can be found in SI. The reaction energies for doping α-MoO3 with solid-phase S and Se are 2.95 and 1.92 eV respectively. Both doping reactions are found to be highly endothermic, and thus it might require high temperature for the doping reactions to occur.
Site-dependent doping effects
Previous experimental and theoretical studies have suggested that both the oxygen vacancies and dopants could give rise to the impurity bands (IBs, Fig. 3)43,44. We found that impurity bands (IBs, Fig. 3) situated above the VBM in the band structure of MoO3 upon the S- and Se- doping, even at low concentration. Similar IBs have been reported in a previous study on Sn-doped chalcopyrite’s45 by Yang et al. We also found the number of IBs of the doped structure is dependent on which oxygen site was doped into. Two IBs are observed in the band structure of MoO2.97(Sa)0.03, whereas one IB is observed in the band structure of MoO2.97(St)0.03 and MoO2.97(Ss)0.03.
The calculated DOS’s and band structures for the mono-S doped MoO2.97(St)0.03, MoO2.97(Sa)0.03, and MoO2.97(Ss)0.03, structures are shown in Fig. 4. The IBs for all of the mono-S doped structures are found close to the corresponding VBM. For the cases of MoO2.97(St)0.03 and MoO2.97(Ss)0.03, a single IB are observed at 0.26 and 0.1 eV above the VBM, respectively; whereas for the case of MoO2.97(Sa)0.03, two IBs were observed at 0.24 eV and 0.75 eV above the VBM. The band structure diagram shows there are in fact two IBs above the VBM for MoO2.97(St)0.03, but the lower energy one is only ~0.1 eV above the VBM of pure material at Γ, which is not obviously shown in DOS. From the DOS, we clearly observed that all of the impurity bands have contributions from the 3p orbitals of S atom which are largely mixed with the 2p orbitals of O atoms nearby. The energy levels of the IBs for the three mono-doped structures are different. The IB for the MoO2.97(Ss)0.03 structure is ~0.1 eV above the VBM of the pure MoO3 structure, being the closest to the VBM. The IB for the MoO2.97(St)0.03 and the lower energy IB for MoO2.97(Sa)0.03 is about 0.4 eV above the VBM of the pure MoO3, whereas the higher energy IB for MoO2.97(Sa)0.03 is ~0.7 eV above the VBM of the pure MoO3. We think that there might be a correlation between the coordination of the S dopant atom at different doping sites and the energy level of the IB. The St forms a double bond with Mo and Sa forms two single bonds with two Mo’s. In both cases, there is a lone pair of 3p electrons that has no significant interaction with the surrounding atoms, of which the energy levels are higher than the energy levels of the bonding orbitals. These lone pairs are most likely to be responsible for the higher-energy IB of MoO2.97(St)0.03 and MoO2.97(Sa)0.03. The higher-energy IB of MoO2.97(Sa)0.03 is at higher energy level than the higher-energy IB of MoO2.97(St)0.03, possibly because the destabilization effect of the excessively large Mo-Oa-Mo angle (~154°). The lower-energy IB of MoO2.97(St)0.03 is attributed to the Mo-St π orbital, which is almost buried by the valence band edge. The lower-energy IB of MoO2.97(Sa)0.03 is attributed to one of the Mo-Sa σ orbital whose energy is raised due to the John-Teller distortion as a consequence of the two unequal Mo-Sa bonds. The Ss is coordinated to 3 Mo’s with two short intralayer Mo-S bonds and one long interlayer Mo-S bond (Table S3 in the SI). Clearly, the lone pair of Ss forms an interlayer donor-acceptor bond with a Mo of the adjacent layer, and the involved p-electrons have higher level than the p states that forms the two intralayer bonds but have slightly lower energy level than the lone pair states of the St-doped systems. Therefore, there is only a single IB close in energy to the VBM for MoO2.97(Ss)0.03.
According to the calculated PDOS for the bands attributed to the three S 3p orbitals (Fig. S4 in the SI) for the mono-S doped MoO3 structures, the average energy levels of the three S 3p orbitals with respect to the VBM of the pure α-MoO3 are: MoO2.97(St)0.03 < MoO2.97(Ss)0.03 < MoO2.97(Sa)0.03, which is consistent with the stability ranking of the three mono-S doped structures. This can be expected by treating the electrons as non-interacting electrons and writing the total energy as the integral of the population-weighted valence band energies (i. e. the band energy).
From the band-structure calculations, the band gap of bulk α-MoO3(2 × 2 × 2) is 2.5 eV using PBE+U. For mono-S doped structures band gaps are ~2.2, ~2.1 and ~2.45 eV for the St, Sa and Ss doping, respectively. The decreases in the band gaps with respect to the band gap of the pure MoO3 (calculated to be 2.6 eV) are mainly due to the emergences of the unoccupied gap states closely below CBM upon the doping, and also due to the distorted local geometries of the doped materials that shift the whole VBM towards lower energy, as indicated by the band structures of the doped materials (Fig. 4). Among the three doping positions, S doping at Oa position is most suitable for the reduction of band gap.
Similar IBs were also observed in the mono-Se doped MoO3 structures as shown in Fig. 5. Two IBs were observed for both MoO2.97(Set)0.03 and MoO2.97(Sea)0.03 structures, whereas in the case of MoO2.97(Ses)0.03 only a single IB was observed in the band-gap area. For MoO2.97(Set)0.03 the newly-introduced bands are ~0.25 and 0.75 eV above the VBM of the pure α-MoO3 (cf. Fig. 5(d)). For MoO2.97(Sea)0.03, the two IBs are ~0.5 eV and 1.1 eV above the VBM. The latter IB is almost located in middle of the VBM and CBM. Similar explanations as what we gave for the cases of the mono-S doped structures can be applied to explain the rises of the IBs for the mono-Se doped structures. The IBs that appear in the electronic structures of the mono-Se doped structures are found to be higher in energy than the IBs that appear for the mono-S doped structures, as the valence p states of Se are higher in energy than the valence p states of S. Comparing to the S mono-doped systems, the impurity band in MoO2.97(Ses)0.03 is slightly higher than that of in MoO2.97(Ss)0.03. For MoO2.97(Ses)0.03 the single impurity band is found at 0.25 eV above to the VBM, whereas for MoO2.97(Ss)0.03 the IB is 0.1 eV above the VBM. As for MoO2.97(Set)0.03 and MoO2.97 (Sea)0.03, a slight reduction was observed in the band gap. Overall, for the mono- S and Se doped systems, similar kind of impurity bands were observed, but the position of IB bands are different in both cases. The energy gap between the highest energy IB and the CBM for a mono-Se doped system is therefore smaller than the gap for the corresponding mono-S doped system. This might result in different optical absorption behaviors for the two classes of doped MoO3 material in the visible and infrared ranges. The band gap of mono-Se doped systems are 2.24, 2.20 and 2.35 eV for the Set, Sea and Ses doping, respectively, which are comparable to the band gaps of the corresponding mono-S doped systems.
(a,c,e) The total density of states (TDOS) and the Mo 3d, O 2p and S 3p projected density of states (PDOS) for MoO2.97(Set)0.03, MoO2.97(Sea)0.03 and MoO2.97(Ses)0.03; (b,d,f) the calculated band structures for MoO2.97(Set)0.03, MoO2.97(Sea)0.03 and MoO2.97(Ses)0.03 at the PBE+U level (Ueff = 8.6 eV) with zero energy set to the VBM of the pure α-MoO3.
Tuning the doping effects with doping concentration
To explore the S and Se atoms doping about the same Mo-site, which is related to the practical situations where the dopant concentration is high and the local concentration (about a Mo) increases, we have carried out the geometry optimization calculations for the bi-S and tri-S doped systems, namely MoO2.94(StSa)0.03 and MoO2.91(StSaSs)0.03, and the resulting optimized structures are shown in Fig. 1(e,f). For the tri-S doped MoO2.91(StSaSs)0.03 systems, we have all three variants of oxygens (OtOaOs) sites replaced about the same Mo atom by S atoms. Unlike the cases of the mono-S doped MoO3 structures and the bi- doped MoO2.94(StSa)0.03, where all the S dopant atoms sit essentially at the original O sites, the Ss dopant in the optimized geometry of MoO2.91(StSaSs)0.03 is found to be out of the position of Os and form a S-S bond (2.045 Å) with Sa. The Ss no longer form two equal intralayer Mo-Ss bonds with two Mo atoms; instead, the two intralayer Mo-Ss bond distances are found to be 2.352 and 2.479 Å in MoO2.91(StSaSs)0.03. The interlayer Mo-Ss distance is found to be essentially the same as the interlayer Mo-Ss distance in the mono-S doped MoO2.97(Ss)0.03, indicating that the displacement of Ss in MoO2.91(StSaSs)0.03 is approximated confined in the xy-plane. The formation of the Ss-Sa bond also affects the bonding between Sa and Mo’s. The bond distance of the two Mo-Sa bonds in MoO2.91(StSaSs)0.03 are 2.391 and 2.357 Å, which are approximately equal.
The calculated densities of states and band structures of MoO2.94(StSa)0.03 and MoO2.91(StSaSs)0.03 are shown in Fig. 6. Two impurity bands are observed in the band gap for MoO2.94(StSa)0.03, whereas only a single impurity band is found lying close to the VBM for MoO2.91(StSaSs)0.03. The IBs of the MoO2.94(StSa)0.03, as shown in PDOS, are essentially the superposition of the IBs of the St and Sa mono-doped structures, which is reasonable as the local geometries around the St and Sa dopants of the bi-doped structure are similar to the corresponding local geometries in the mono-doped structures. The higher energy IB for MoO2.94(StSa)0.03 is mainly ascribed to the S 3p lone pair on Sa, and the lower energy IB is the superposition of the S 3p lone pair on St and the 3p state of Sa that contributes to the longer Mo-Sa bond whose energy level is raised due to the John-Teller effects caused by two unequal Mo-Sa bonds (Fig. S4). The number of the IB decreases from two to one when an additional S dopant is added to the bi-doped MoO2.94(StSa)0.03 structure to form local Mo surrounded by three dopant atoms in the resulting tri- S doped MoO2.91(StSaSs)0.03 structure. This suggests that the IB of the tri-doped structure is no longer a simple superposition of the IBs of the mono- and bi- doped structures, in contrast to the cases with lower local dopant concentrations. This is due to the substantial changes of the local geometry and electronic structure upon the introduction of the third S dopant onto the same Mo site. The PDOS projected to the dopant atoms (Fig. S5 in the SI) shows that, the Sa dopant atoms only give rise to one IB in MoO2.91(StSaSs)0.03. In MoO2.91(StSaSs)0.03, Sa forms two Mo-S bonds and one S-S bond, so its valence p lone pair state must be involved in a donor-acceptor type bond, which gives rise to an IB. The energy level of this IB is expected to be comparable with the energy level of the IB of MoO2.97(Ss)0.03 due to the similar bonding natures. Similarly, the over-coordinated Ss of MoO2.91(StSaSs)0.03 will give rise to an IB at approximately the same energy level. We have learnt earlier that the IB of the MoO2.97(St)0.03 is close in energy than the IB of MoO2.97(Ss)0.03. Therefore, the Sa, Ss and St of MoO2.91(StSaSs)0.03 each give rise to an IB above the VBM, and the three IBs with similar peak positions overlap with each other to appear as one IB.
The band gap of MoO2.94(StSa)0.06 is ~2.0 eV, slightly smaller than the band gaps of MoO2.97(Sa)0.03 and MoO2.97(St)0.03. A larger band gap reduction was observed for MoO2.91(StSaSs)0.09, with the band gap calculated to be ~1.88 eV. The positions of the IB of MoO2.91(StSaSs)0.09 is close to the VBM, and therefore the energy gap between the IB and CBM is larger than the IB-CBM gaps for the mono- and bi-S doped MoO3.
For bi-/tri- Se doped structures MoO2.94(SetSea)0.03 and MoO2.97(SetSeaSes)0.03, optimized structures are shown in Fig. S1. In MoO2.94(SetSea)0.03, the Se dopants are essentially located at the original O sites. In MoO2.97(SetSeaSes)0.03, no Sea-Ses bond is found, in contrast to the case of MoO2.97(StSaSs)0.03 where a dopant-dopant bond is found; instead, a Sea-Oa bond is formed with a bond distance of 1.890 Å. In addition, the Ses dopant is found to be out of the original Os position into a new position on the Mo layer plane by rotating about the adjacent Mo-Mo axis (Fig. S1). The calculated DOS’s and band structures for MoO2.94(SetSea)0.03 and MoO2.91(SetSeaSes)0.03 are shown in Fig. 7. Two IBs were found for both MoO2.94(SetSea)0.03 and MoO2.97(SetSeaSes)0.03 (by omitting the new bands that are too close to the VBM and CBM), but the origins for the IBs of the two cases are different. For the MoO2.94(SetSea)0.03, the DOS of the IBs is approximately a superposition of the DOS’s of the IBs of MoO2.94(Set)0.03 and MoO2.94(Sea)0.03. Such superposition is supposed to give rise to three IBs at 0.2, 0.5 and 0.8 eV above the VBM, but the lowest energy IB is too close to the VBM and thus appears to be absorbed therein. For MoO2.97(SetSeaSes)0.03 the composition of the IBs is totally different. The lower energy IB of the tri-Se doped structure is approximately equally contributed by the p states of Set, Sea, and Ses. The higher energy IB is contributed by the p states of Sea and Set (Fig. S4 in the SI). The distinct difference between the electronic structures of MoO2.94(SetSea)0.03 and MoO2.97(SetSeaSes)0.03 are apparently raised by the local geometry difference (Fig. S1(c,d); Table S4 in the SI) although the reason for the energy upshifts of the p states of Set from the bi-Se doped structure to the tri-Se doped structure is not clear, which is plausibly due to the stronger interactions between the Se dopants in the tri-S doped structure than in the bi-S doped structure. The band gaps of bi-Se and tri-Se doped systems are ~1.9 eV and ~2.25 eV respectively, both smaller than the band gap of the pure material. The maximum edges of the highest energy IBs of the bi-Se and tri-Se doped systems are much higher in energy than VBM, and the energy difference between the maximum edge of highest energy IB and the CBM is ~1 eV for MoO2.94(SetSea)0.03 and is 1.5 eV for MoO2.97(SetSeaSes)0.03. This suggests that MoO2.94(SetSea)0.03 may have the best absorption properties in the red and infrared regions among all the studied systems.
We have learnt that the Ot site is the most energetically favorable doping site for the mono-S and Se doping of MoO3. In real situation when the doping concentration is low, the newly introduced dopant tends to replace another Ot site rather than the Oa and Os sites about the St (or Set). Thus, the bi-St and tri-St doped systems with Ot being exclusively substituted were also calculated using the MoO2.94(St)0.06 (Mo32O94S2) and MoO2.91(St)0.09 (Mo32O94S2) models, to show the effects of the dopant concentration on the electronic structures, corresponding S/Se doped at different Ot position results are shown in the Figs S2 and S6 in the SI. According to the DOS diagrams (Fig. S6 in the SI), the same number of impurity bands are observed for both cases (S at same Mo-site and S doped at different Ot positions). The band gap remains the same as the doping concentration increases with the dopant atoms replacing the same type of O site. As expected, the populations of the impurity bands increase as the doping concentration increases, which leads to the high absorption strength for the peaks that are associated with the optical excitations involving the impurity bands (Fig. S7 in the SI).
Optical proprieties of the doped MoO3
The optical absorption spectra for the pure and doped (S- and Se-) α-MoO3 systems were calculated, and the results are shown in Fig. 8. The optical absorption spectra of pure and doped systems are shown for wavelength >400 nm. The pure material has negligibly small absorption in this wavelength region. Notable increases in the absorption strength are found for the optical absorption spectra of the doped materials. All the S-doped structures in Fig. 8a have stronger absorption in the blue light region (450–495 nm), but only MoO2.97(Sa)0.03, MoO2.94(StSa)0.03 and MoO2.91(StSaSs)0.03 exhibit slightly enhanced absorption in the red light (620–750 nm) and infrared regions. The optical absorption strengths for the locally tri-doped MoO2.91(StSaSs)0.03 are overall weaker than those for the locally bi-doped MoO2.94(StSa)0.03. This is because the change of the local geometry at high local dopant concentration lowers the energy of the IBs, and hence increases the gap between the maximum edge of the highest energy IB and CBM, which reduces the excitations in red and infrared regions for MoO2.91(StSaSs)0.03. Se-doped systems show better absorption enhancements in the visible and infrared region than do the S-doped systems, in terms of the absorption strength increases as compared to absorption spectrum of the pure material. The Se-doped MoO2.97(Sea)0.03, MoO2.94(SetSea)0.03 and MoO2.91(SetSeaSes)0.03 structures also show good absorption strengths in the infrared region, which is not found for the S-doped structures. The superior optical properties (in the visible and infrared region) of the Se-doped systems are likely due to the higher energy levels of the valence p orbitals of Se than the p orbitals of S (that leads to higher energy maximum IB edges). Similar to the S-doping cases, the optical performance of S-doped increases as the local dopant concentration increases, until it reaches a point that the Mo sites of the material incorporate two Se dopants, as found in the MoO2.94(SetSea)0.03 structure model. Further increasing the doping concentration will generate Mo sites that incorporate over three Se dopant atoms, as found in MoO2.91(SetSeaSs)0.03, which leads to a local geometry change that the dopants are displaced from the original O sites. As expected, for all the doped structures, the number of distinct absorption peaks in the infrared region are consistent with the number of distinct IBs between the VBM and CBM, since the excitations from each IB to conduction band levels near the CBM account for a new absorption peak in the optical spectra. The wavelength of the new adsorption peak can be approximately estimated as the energy difference between certain IB level and the center of the low-lying CB of the doped material. Overall, the optical absorption enhancements ascribed to the emergence of IBs were observed for the S and Se doped materials. The correlations between the new adsorption peaks and the dopant-induced IBs are analyzed and explained. Our results provide new insight for tuning the electronic and optical properties of MoO3.
Conclusions
Using the first-principles calculations, we have studied the electronic structures and optical properties of sulfur and selenium doped α-MoO3. Doped structures with S or Se replacing several distinct O sites were looked into. From the formation energies, the S and Se prefer being doped into the Ot sites than the Oa and Os sites. The reaction energies for doping α-MoO3 with solid-phase S and Se are 2.95 and 1.92 eV per dopant, respectively. Both doping reactions are found to be highly endothermic; the Se-doping reaction is more favorable than the S-doping reaction.
Significant changes were observed for the electronic structures as well as the optical absorption properties of α-MoO3 upon the S- and Se- doping treatments. The calculated band gap of the α-MoO3−xSx slightly decreases as the doping concentration increases mostly due to the dopants levels that are closed to the CBM. For all of the doped structures, IBs were found between the VBM and the CBM. For MoO2.97(Sa)0.03, MoO2.94(StSa)0.03, MoO2.97(Sea)0.03 and MoO2.94(SetSea)0.03, two distinct IBs were found. The energy levels and the number of the IBs greatly depend on the local molecular geometries and the local electronic structures. At low local doping concentration (number of S or Se < 3 about a Mo), in terms of number of dopant about a Mo center, the dopants will replace O atoms and stay on-site. As a consequence, the effects of the dopants in altering the electronic structures are additive. Hence, increasing the doping concentration at low concentration will enhance the optical absorption properties of the material in the visible and infrared regions. At high local doping concentration (number of S or Se ≥ 3 about a Mo), the net doping effect is no longer the superposition of the effects of the individual on-site dopant atoms as found for the low-concentration cases. The local geometries are greatly affected by the additional dopant atoms at high local doping concentration: dopant atoms are dislocated, Mo-S/Mo-Se bond distances are changed, and bonds between main group atoms, such as S-S and Se-O, are formed. Such local geometry changes turn out to substantially affect the band structures, especially to increase the gap between the maximum IB edge and CBM, which in turn applies a negative effect on the optical absorption properties in the visible and infrared regions. The optical absorption spectra were calculated for the studied systems. New absorption peaks with moderate intensities were found in the visible and infrared regions for the doped structure, especially for MoO2.94(StSa)0.03 and MoO2.94(SetSea)0.03; such absorption peaks are beneficial for solar energy harvesting. Correlations between the IBs and the new absorption peaks in the visible and infrared region were found. Based on our first-principles calculation results, the designed S- and Se-doping at different concentrations appear to be effective for tuning of the band gaps and optical properties of MoO3. Our study suggests that the isovalent doped-MoO3 with economically viable dopants may be utilized in water oxidation and many other photochemical applications, although further computational and experimental efforts are required for more thorough investigations.
References
Pham, T. T. P., Nguyen, P. H. D., Vo, T. T., Nguyen, H. H. P. & Luu, C. L. Facile method for synthesis of nanosized β –MoO3 and their catalytic behavior for selective oxidation of methanol to formaldehyde. Adv. Nat. Sci.: Nanosci. Nanotechnol. 6, 045010 (2015).
Hassan, K. H. & Mitchell, P. C. H. Evaluation of different methods to prepare the Fe2O3/MoO3 catalyst used for selective oxidation of methanol to formaldehyde. Stud. Surf. Sci. Catal. 175, 475–478 (2010).
Meng, Y. et al. Selective oxidation of methanol to dimethoxymethane on V2O5-MoO3/γ-Al2O3 catalysts. Appl. Catal. B: Environ. 160, 161–172 (2014).
Madeira, L. M., Portela, M. F. & Mazzocchia, C. Nickel Molybdate Catalysts and Their Use in the Selective Oxidation of Hydrocarbons. Catal. Revs. 46, 53–110 (2008).
Cheng, L., Shao, M., Wang, X. & Hu, H. Single-Crystalline Molybdenum Trioxide Nanoribbons: Photocatalytic, Photoconductive, and Electrochemical Properties. Chem. Eur. J. 15, 2310–2316 (2009).
Lam, S. M., Sin, J. C., Abdullah, A. Z. & Mohamed, A. J. Investigation on visible-light photocatalytic degradation of 2, 4-dichlorophenoxyacetic acid in the presence of MoO3/ZnO nanorod composites. J.Mol. Catal. A: Chem. 370, 123–131 (2013).
Zhao, S., Li, J., Wang, L. & Wang, X. Degradation of Rhodamine B and Safranin‐T by MoO3:CeO2 Nanofibers and Air Using a Continuous Mode. Clean-Soil Air Water 38, 268–274 (2010).
Carcia, P. F. & McCarron, E. M. Synthesis and properties of thin film polymorphs of molybdenum trioxide. Thin Solid Films 155, 53–63 (1987).
Zheng, L., Xu, Y., Jin, D. & Xie, Y. Novel Metastable Hexagonal MoO3 Nanobelts: Synthesis, Photochromic, and Electrochromic Properties. Chem. Mater. 21, 5681–5690 (2009).
Kumar, V., Wang, X. & Lee, P. S. Oxygen ions mediated pseudo capacitive charge storage in molybdenum trioxide nanobelt. Chem.Nano.Mat. 1, 403–408 (2015).
Tang, Q., Wang, L., Zhu, K., Shan, Z. & Qin, X. Synthesis and electrochemical properties of H-MoO3/graphene composite. Mater. Lett. 100, 127–129 (2013).
Brezesinski, T., Wang, J., Tolbert, S. H. & Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 9, 146–151 (2010).
Sunu, S. S. et al. Electrical conductivity and gas sensing properties of MoO3. Sens. Actuators B 101, 161–174 (2004).
Lupan, O. et al. Versatile Growth of Freestanding Orthorhombic α-Molybdenum Trioxide Nano- and Microstructures by Rapid Thermal Processing for Gas Nanosensors. J. Phys. Chem. C 118, 15068–15078 (2014).
Lee, S. H. et al. Reversible Lithium-Ion Insertion in Molybdenum Oxide Nanoparticles. Adv. Mater. 20, 3627–3632 (2008).
Michailovski, A. et al. Studying the Solvothermal Formation of MoO3 Fibers by Complementary In Situ EXAFS/EDXRD Techniques. Angew. Chem., Int. Ed. 44, 5643–5647 (2005).
Hamwi, S., Meyer, J., Winkler, T., Riedl, T. & Kowalsky, W. P -type doping efficiency of MoO3 in organic hole transport materials. Appl. Phys. Lett. 94, 253307 (2009).
White, R. T., Thibau, E. S. & Lu, Z. H. Interface, Structure of MoO3 on OrganicSemiconductors. Sci. Rep. 6, 21109 (2016).
Qin, P. et al. Sulfur-Doped Molybdenum Oxide Anode Interface Layer for Organic Solar Cell Application. ACS Appl. Mater. Interfaces 6, (2963–2973 (2014).
Lei, Y. H. & Chen, Z. X. DFT+U Study of Properties of MoO3 and Hydrogen Adsorption on MoO3 (010). J. Phys. Chem. C 116, 25757–25764 (2016).
Agarwal, V. & Metiu, H. Oxygen Vacancy Formation on α-MoO3 Slabs and Ribbons. J. Phys. Chem. C 120, 19252–19264 (2016).
Yang, M., Zhang, L., Jin, B., Huang, L. & Gan, Y. Enhanced photoelectrochemical properties and water splitting activity of self-ordered MoO3-TiO2 nanotubes. Appl. Surf. Sci. 364, 410–415 (2016).
Yu, H. et al. Novel MoO3-TiO2 composite nanorods films with improved electrochromic performance. Mater. Lett. 169, 65–68 (2016).
Chen, C. Z., Li, Y. & Tang, X. D. Evidence of oxygen vacancy and possible intermediate gap state in layered α-MoO3 single-crystal nanobelts. Physica B 481, 192 (2016).
Martin, W. C., Zalubas, R. & Musgrove, A. Energy Levels of Sulfur, S I Through S XVI. J. Phys. Chem. Ref. Data 19, 821 (1990).
Morillon, C. & Vergès, J. Classification du Spectre d’Arc du Sélénium (Sel) entre 3900 et 11 844 cm−1. Phys. Scr. 10, 227 (1974).
Eriksson, K. B. S. & Isberg, H. B. S. Ark. Fys. (Stockholm) 37, 221 (1968).
Wang, F., Valentin, C. D. & Pacchioni, G. Doping of WO3 for Photocatalytic Water Splitting: Hints from Density Functional Theory. J. Phys. Chem. C 116, 8901–8909 (2012).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Liechtenstein, A. I., Castell, M. R., Briggs, G. A. D. & Sutton, A. P. Surface states on NiO (100) and the origin of the contrast reversal in atomically resolved scanning tunneling microscope images. Phys. Rev. B 56, 4900–4908 (1997).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B. 13, 5188–5192 (1976).
Gajdos, M., Hummer, K., Kresse, G., Furthmuller, J. & Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 73, 045112–9 (2006).
Kihlborg, L. Least squares refinement of crystal structure of molybdenum trioxide. Ark. Kemi 21, (357 (1963).
Erre, R., Legay, M. H. & Fropiat, J. J. Reaction of molecular hydrogen with the 100 face of MoO3: II. Kinetics initiated by atomic hydrogen and characterization of the surface electronic state. Surf. Sci. 127, 69–82 (1983).
Coquet, R. & Willock, D. J. The (010) surface of α-MoO3, a DFT+U study. Phys. Chem. Chem. 7, 3819–3828 (2005).
Krylov, O. Catalysis by Non-Metals, Academic Press, New York (1980).
Scanlon, D. O. et al. Theoretical and Experimental Study of the Electronic Structures of MoO3 and MoO2. J. Phys. Chem. C 114, 4636–4645 (2010).
Dasent, W. E. “Inorganic Energetics,” 2nd ed., Cambridge University Press, NY, p. 35 (1982).
Inzani, K., Grande, T., Bruer, F. V. & Selbach, S. M. J. Phys. Chem. C. A van der Waals Density Functional Study of MoO3 and Its Oxygen Vacancies. 120, 8959–8968 (2016).
Li, X. B. et al. Structures, stabilities, and electronic properties of defects in monolayer black phosphorus. Sci. Rep. 5, 10848 (2015).
Yang, C. et al. Observation of an Intermediate Band in Sn-doped Chalcopyrites with Wide-spectrum Solar Response. Sci. Rep. 3, 1286 (2013).
Acknowledgements
CY acknowledges the National Basic Research Program of China (No. 2014CB921402). BS acknowledges the financial support of the Natural Science Foundation of China (No. 21650110464). The authors gratefully acknowledge the funding from the Beijing Computational Science Research Center and National Natural Science Foundation of China (Grant No. U1530401) and the computational resources from the Beijing Computational Science Research Center. Dr. Li-Min Liu was acknowledged for many discussions.
Author information
Authors and Affiliations
Contributions
B.S., G.S. and M.C. initiated the work and designed the calculations with ideas supplied by N.E. and C.Y. during the working process. B.S. and G.S. performed all the calculations. B.S., G.S., N.E., C.Y. and M.C. analyzed the data. B.S., G.S. and M.C. wrote the manuscript. All authors were involved in the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bandaru, S., Saranya, G., English, N.J. et al. Tweaking the Electronic and Optical Properties of α-MoO3 by Sulphur and Selenium Doping – a Density Functional Theory Study. Sci Rep 8, 10144 (2018). https://doi.org/10.1038/s41598-018-28522-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28522-7
This article is cited by
-
First-principles analysis of how Cobalt doping affects the structural, electronic, and optical properties of α-MoO3
Indian Journal of Physics (2023)
-
MoO3/γ-In2Se3 heterostructure photoanodes for enhanced photoelectrochemical water splitting
Journal of Materials Science: Materials in Electronics (2023)
-
Emerging noble metal-free Mo-based bifunctional catalysts for electrochemical energy conversion
Nano Research (2022)
-
Advanced Strategies to Improve Performances of Molybdenum-Based Gas Sensors
Nano-Micro Letters (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.