Abstract
The Plasmodium falciparum apical asparagine (Asn)-rich protein (AARP) is one of malarial proteins, and it has been studied as a candidate of malaria subunit vaccine. Basic characterization of PvAARP has been performed with a focus on its immunogenicity and localization. In this study, we further analyzed the immunogenicity of PvAARP, focusing on the longevity of the antibody response, cross-species immunity and invasion inhibitory activity by using the primate malaria parasite Plasmodium knowlesi. We found that vivax malaria patient sera retained anti-PvAARP antibodies for at least one year without re-infection. Recombinant PvAARP protein was strongly recognized by knowlesi malaria patients. Antibody raised against the P. vivax and P. knowlesi AARP N-termini reacted with the apical side of the P. knowlesi merozoites and inhibited erythrocyte invasion by P. knowlesi in a concentration-dependent manner, thereby suggesting a cross-species nature of anti-PvAARP antibody against PkAARP. These results can be explained by B cell epitopes predicted in conserved surface-exposed regions of the AARP N-terminus in both species. The long-lived anti-PvAARP antibody response, cross-reactivity, and invasion inhibitory activity of anti-PvAARP support a critical role of AARP during the erythrocyte invasion and suggest that PvAARP induces long-lived cross-species protective immunity against P. vivax and P. knowlesi.
Similar content being viewed by others
Introduction
Plasmodium vivax, the most widely distributed malaria parasite, globally contributes to 16 million cases outside of Africa, and affects the economy of most developing countries1. Vivax vaccine candidates have been selected on the basis of the analysis of orthologous P. falciparum vaccine candidate antigens2. Protective immune responses to the blood stage through inhibition of merozoite invasion or phagocytosis3,4 involve complex interactions of humoral immune responses and cell-mediated immune responses5. The development and maintenance of immune responses are crucial for malaria vaccine development6. Several studies have evaluated humoral immune responses for blood-stage antigens, including merozoite surface proteins (PvMSPs)7, apical membrane antigen 1 (PvAMA1), Duffy-binding protein (PvDBP)6,7, and tryptophan-rich antigens (PvTRAgs)8 of P. vivax. Antibody titers against the high molecular weight rhoptry protein 2 (PvRhopH2)9 have been found to be high, and one against rhoptry-associated membrane antigen (PvRAMA) is stably maintained9,10.
In addition to four well-known species of Plasmodium causing malaria in humans, Plasmodium knowlesi has been confirmed to naturally infect humans and is considered an emerging threat11,12. Plasmodium knowlesi infections are prevalent in all Southeast Asian countries, and Malaysia serves as an epicenter accounting for up to 80% of human infection13. Recent genetic and genomic studies have revealed at least three P. knowlesi sub-populations infecting humans, thus adding to the complexity of treating and managing this disease13,14,15. Because of the phylogenetically close relationship between P. vivax and P. knowlesi, drug targets and cross-species vaccine candidates for these Plasmodium species would be of great interest16. Cross-species immune response has been observed by some malarial antigens in different species17,18,19, and a robust protective response for one malaria species can be sufficiently strong to protect against multiple malaria species19,20. Thus, a new vaccine strategy targeting both P. vivax and P. knowlesi via cross-species immunity might be a safe and cost-effective strategy20.
The P. falciparum apical asparagine(Asn)-rich protein (PfAARP, PF3D7_0423400 in gene ID of PlasmoDB) was originally identified as a merozoite rhoptry neck protein with a predicted signal peptide sequence at its N-terminus and a size of 219 amino acids21. The recombinant N-terminus of PfAARP binds to erythrocytes in a trypsin- or neuraminidase-treatment sensitive manner, thus suggesting that the receptor contains protein components and sialic acids21. The sequence of this region is highly conserved among P. falciparum field isolates, and sera from endemic areas recognize this region, thus indicating that this region is immunogenic21. Moreover, antibodies raised against this region inhibit erythrocyte invasion by merozoites in a concentration-dependent and strain-transcending manner, thus suggesting a role for the PfAARP N-terminus during invasion and a benefit in including this region in the subunit malaria vaccine21,22. PfAARP has orthologs in all reported Plasmodium species, including the P. vivax apical Asn-rich protein which has been reported as PvARP from previous study, here we refer as PvAARP (PVX_090210, Sal-1 strain)23 and P. knowlesi apical Asn-rich protein (PkAARP, PKNH_0515300, H strain). Recent studies have shown that PvAARP localizes on the surfaces of merozoites with accumulation on the apical side21,24, in contrast to the rhoptry neck localization of PfAARP. Both PvAARP and PkAARP also contain a signal peptide sequence at their N-terminus and Asn- and proline (Pro)-rich regions toward the C-terminus. Previous studies have also shown that recombinant PvAARP protein is immunogenic in natural vivax infection24,25.
Because the N-terminus of PvAARP shows high homology with PkAARP, we hypothesized that this N-terminal region would be suitable for inducing cross-species protective immunity between P. vivax and P. knowlesi. Thus, in this study, we first used bioinformatics programs for B-cell epitope prediction in both species to find shared epitopes in the N-terminal region with the highest sequence identity. We then generated recombinant PvAARP and PkAARP protein to produce a specific antibody with cross-species reactivity and erythrocyte invasion inhibitory activity, by using P. knowlesi. Recombinant PvAARP and PkAARP proteins were recognized by sera from vivax and knowlesi malaria patients. The antibody response against PvAARP was observed for up to one year in vivax malaria patients without re-infection. These findings support a critical role of AARP during the erythrocyte invasion process by these parasites and suggest that PvAARP induces long-lived cross-species protective immunity against P. vivax and P. knowlesi.
Results
Structures and B-cell epitope prediction for PvAARP and PkAARP
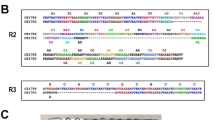
PvAARP (PVX_090210) and PkAARP (PKNH_0515300) contain the expected signal peptide sequence at their N-terminus and consist of 285 and 239 amino acids, respectively. No transmembrane region and GPI-anchor motif were predicted for PvAARP and PkAARP or PfAARP, according to several robust transmembrane prediction algorithms such as TMHMM ver 2.0, Phobius, and OCTOPUS26,27,28, despite a previous report of a predicted transmembrane domain in PfAARP. Thus, we inferred that AARP proteins probably do not possess a transmembrane domain. The apical Asn-rich region in PvAARP (118–239 aa) is longer than that in PkAARP (118–193 aa) (Fig. 1a). In addition to the Asn-rich region, we found that the amino acid sequences of the N-terminal region (aa 21–112 for both without the predicted signal peptide sequence) and the C-terminal region (aa 240−285 for PvAARP and 194–239 for PkAARP) of the Asn-rich region are highly conserved between PvAARP and PkAARP (74.7% and 84.8% identity, respectively). A total of four B-cell epitopes (#1−#4) were predicted for both PvAARP and PkAARP by two methods, Bcpred and IEDB web servers. The surface-exposed regions conserved between the two species were identified as the N-terminus-probable B-cell epitope regions (Fig. 1b,c). Fifty-six pvaarp sequences encoding the PvAARP N-terminal regions that originated from 10 countries (Brazil, China, Columbia, India, Mauritania, Mexico, North Korea, Peru, Papua New Guinea, and Thailand) were found to be 100% identical. Twenty-nine pkaarp sequences encoding the PkAARP N-terminal region, including 26 sequences originating from Sarawak, Malaysia29 and sequences from the H strain, SRA (SRS1051522), were used for the analysis (Supplementary Table S1). For 273 nucleotide positions, 20 segregating sites were found, and the nucleotide diversity was 0.01620, thus indicating that PkAARP shows limited polymorphism (Fig. 1d). Among 20 segregating sites, 9 were synonymous substitutions, and 11 were non-synonymous substitutions (Supplementary Table S2). dN and dS were not significantly different (Supplementary Table S3). Natural sequence variations were further tested using codon-based site-by-site analysis using FEL, IFEL, REL and MEME methods for 29 pkaarp sequences. A100S was identified to be under strong positive selection (Supplementary Table S3).
Schematic structure, B-cell epitope prediction and sequence diversity of PvAARP and PkAARP. (a) Schematic structures of PvAARP (285 amino acids [aa]) and PkAARP (239 aa). Asparagine (Asn)- and proline (Pro)-rich regions are indicated in yellow and blue, respectively. Regions used to generate PvAARP N-terminus (rPvAARP-N, aa 21−112), PkAARP N-terminus (rPkAARP-N, aa 21−112) and full-length PvAARP (rPvAARP-FL) and PkAARP (rPkAARP-FL) recombinant proteins are indicated under each structure. Four predicted B-cell epitopes (#1−#4) are highlighted with arrows and aa sequences. (b and c) SP, signal peptide. B-cell epitope prediction of PvAARP-N (b) and PkAARP-N (c). Yellow areas above the threshold (red line) were predicted to be part of the B-cell epitope, and green areas were unlikely to be a part of the B-cell epitope. (d) Sliding window plot of the nucleotide diversity of the pkaarp gene encoding the N-terminus using 29 pkaarp sequences with a window size of 60 and a step size of 3.
Recombinant protein expression and recognition of native parasite protein
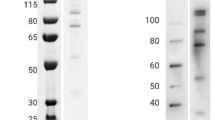
The rPvAARP-FL and rPkAARP-FL were expressed as a 37-kDa protein by the WGCF system, and a single specific band was detected with an anti-His antibody (Fig. 2a). The recombinant PvAARP-N (rPvAARP-N) was successfully expressed and purified; it migrated as a 35-kDa single band on SDS-PAGE and was recognized by the anti-GST antibody (Fig. 2b,c, lane Gst). Anti-PvAARP-N and anti-PkAARP-N serum recognized rPvAARP-N and rPkAARP-N, respectively, as determined by western blotting analysis (Fig. 2c, lane M and R). Anti-PvAARP-N and anti-PkAARP-N antibody specifically recognized schizont parasite lysate as 35 kDa (Fig. 2d) in similar of PfAARP21. IFA of P. knowlesi with anti-PvAARP-N and anti-PkAARP-N antibody revealed that signals on merozoite surface with accumulation on one side of the merozoites (Fig. 2e, Supplementary Fig. S1A) in a similar pattern to that reported previously for PvAARP24.
SDS-PAGE and western blot analysis of PvAARP and PkAARP. (a) Crude recombinant proteins of full-length PvAARP and PkAARP [rPvAARP-FL and rPkAARP-FL] were expressed with the WGCF expression system. (b) Recombinant PvAARP-N [Pv, 0.5 µg] and PkAARP-N [Pk, 0.5 µg] proteins were expressed in E. coli and purified to a single band [arrowhead]. (c) Specific band have been detected with anti-glutathione S-transferase antibody [Gst], mouse serum [M] and rabbit immunized [R] with PvAARP-N or PkAARP-N. (d) P. knowlesi A1-H.1 parasite lysate was recognized with anti-PvAARP-N [α-Pv] and anti-PkAARP-N [α-Pk] antibody, nor non-immunized rabbit (α-NI). The full length of SDS-PAGE and western blots are presented in the Supplementary Fig. S2. (e) Immunofluorescence assay of anti-PvAARP-N and PkAARP-N with P. knowlesi A1-H.1. pRBC, parasitized-red blood cells; uRBC, uninfected-red blood cells; DAPI, 4’,6-diaminidino-2-phenylindole. Bars indicate 5 μm.
Cross-immunoreactivity and longevity of antibody response in patients
Protein microarray analysis revealed that both pooled vivax malaria and knowlesi malaria patient sera reacted significantly more strongly with both rPvAARP-FL and rPkAARP-FL than did sera from healthy individuals (Fig. 3a, Table 1). Notably, the reactivity of the antibody in heterologous combination was as high as that in the homologous combination. These data indicated that anti-PvAARP or PkAARP antibodies generated during the former infection probably recognize PkAARP or PvAARP in the new knowlesi or vivax infection, respectively.
(a) IgG responses of pooled vivax patient serum [V], pooled knowlesi patient serum [K] or pooled healthy individual serum [H] to the full-length PvAARP-FL [rPvAARP-FL] or PkAARP [rPkAARP-FL] recombinant proteins. (b) IgG responses of 32 sets of archived vivax malaria patient sera to rPvAARP-FL. The IgG response is represented by normalized mean fluorescence intensity [MFI]: MFI of the test sample/[MFI + 2 standard deviations].
To determine the longevity of antibodies against PvAARP, 32 sets of vivax malaria patient sera from D0 (acute), D28 (sub-acute), and one year (1 yr, after infection) were evaluated (Table 1). The antibody response against PvAARP was significantly higher than that for the control healthy individuals’ sera and the response of sera collected at D0 and after 1 yr did not show significant differences (Fig. 3b, Table 2), thus suggesting that the anti-PvAARP antibody response was not diminished within one year.
Cross-invasion inhibitory activity
To determine the cross-invasion inhibitory activity, the anti-PvAARP-N IgG antibody was administered to the P. knowlesi A1-H.1 and H strain culture with human and monkey erythrocytes, respectively. The efficiency of inhibition was compared to the anti-PkAARP-N antibody. We found that P. knowlesi invasion into human erythrocytes was significantly inhibited with purified anti-PvAARP-N IgG as efficient as anti-PkAARP-N IgG when 2 or 4 mg/mL IgG was administered (Fig. 4, no significant different (ns) p > 0.05). Whereas invasion inhibitory activity of anti-PvAARP-N antibody was found higher in P. knowlesi H strain at concentration <1 mg/mL (Supplementary Fig. S1B).
Invasion inhibition activity of anti-PvAARP-N and anti-PkAARP-N against P. knowlesi A1-H.1 into human erythrocytes. Purified IgG [0.25, 0.5, 1.0, 2.0 and 4.0 mg/mL] from rPvAARP-N and rPkAARP-N-immunized, non-immunized [NI], and His-GST-immunized rabbit, as well as anti-DARC [2C3] monoclonal antibodies [25 μg/mL] were examined for their inhibitory activity against erythrocyte invasion by P. knowlesi A1-H.1 into human erythrocytes. ns, not significant different. p > 0.05; single asterisk, p < 0.05; double asterisks, p < 0.01; triple asterisks, p < 0.001.
Discussion
With recent advances in bioinformatics, robust algorithms have been designed and widely used to predict malaria vaccine candidates23,30,31. On the basis of the predictions, functional assays and antibody responses have been evaluated in P. vivax and P. falciparum10,23,32. One antigen is P. falciparum AARP, for which orthologous members have been reported in other Plasmodium species21,24. The AARP ortholog of P. vivax shows high homology to the primate malaria parasite P. knowlesi, approximately 74.7% at the N-terminal region upstream of the Asn-rich domain. Previous studies have shown that malarial antigens with high sequence homology can induce a cross-immune response20. We successfully expressed and purified N-terminal region of AARP in P. vivax and P. knowlesi and used for producing antibody. Indeed, we observed cross-reactivity between P. vivax and P. knowlesi via AARP proteins. The P. vivax and P. knowlesi AARP-N termini specifically recognized the merozoite surface of P. knowlesi with accumulation in apical end surface. The difference with PfAARP localization in apical end organelle might due to the timing of secretion. Our result by using parasite lysate also showed specific band around 35 kDa by western blot as shown in PfAARP21. It suggests that cross-reactivity was truly observed in AARP of P. vivax and P. knowlesi parasite level. Moreover, it might also be thought that function of AARP is conserved among Plasmodium spp.
In addition of cross-immunoreactivity might exist in clinical patients, immunoscreening assay was conducted. Wheat germ cell-free system was chosen for protein production as there is no glycosylation affect for incorrect immune response33,34,35. We successfully expressed the full-length domain by WGCF and unfortunately, we failed to express by E. coli system. The recombinant PvAARP-FL was specifically recognized by vivax- and knowlesi-infected patients. Moreover, the immunogenic strength of AARP was observed in vivax-infected patients that the anti-PvAARP could be maintained up to one year without reinfection. We also demonstrated cross-reactivity and cross-species inhibitory activity of the anti-PvAARP-N antibody against P. knowlesi invasion into human erythrocytes at a concentration-dependent manner, with a maximum inhibition of more than 70% when the 4 mg/mL antibody was administered. The efficiency of anti-PvAARP-N antibody to block the P. knowlesi invasion was as high as anti-PkAARP-N antibody. This result was consistent with the previously reported ability of the anti-PfAARP-N antibody to inhibit erythrocyte invasion by two independent P. falciparum strains in a concentration-dependent manner21,36,37. This result might prove the recognition of shared or common epitope of P. vivax and P. knowlesi. Hence, we propose that the AARP N-terminus is a putative efficacious blood stage vaccine candidate that may confer protection from both P. vivax and P. knowlesi infection in humans.
In a previous study, mice immunized with the N-terminus of PfAARP (aa 20–107) have been found to show high-titer, long-lived antibody responses for more than 4 months after immunization38. In the case of P. vivax, PvAARP has been shown to be immunogenic in most vivax patients24. In this study, we observed that anti-PvAARP was retained even one year after the P. vivax infection was resolved. In natural malaria infection, specific antibodies are often induced rapidly but also decline rapidly following parasite clearance after acute clinical malaria infection6,32,39. In this regard, PvAARP appears to have high potential as a vaccine candidate. B-cell epitopes predicted in the N-terminal region might induce long-lasting responses as PfAARP38. This finding may also suggest that memory B-cells play a role in the observed stably maintained antibody response against PvAARP in the absence of re-infection. In this study, we found that the N-terminus of pvaarp is completely conserved among parasites circulating in many countries in the world, and even though the pkaarp N-terminus contains polymorphisms, the degree of polymorphism is not high. Nonetheless, bioinformatic analyses detected one codon in the PkAARP N-terminus that was under positive selection to increase diversity, probably because of host immunity. Similar B and T cell epitope regions and natural selection have been described for other malaria antigens, such as P. falciparum circumsporozoite protein and apical membrane antigen proteins40. The PkAARP C-terminal polymorphic region was not analyzed in this study, but it may also contain codons under positive selection, and thus further investigation is needed.
In summary, we found that vivax malaria patients retained anti-PvAARP antibody responses up to one year after the infection was resolved. The cross-reactivity between P. vivax and the P. knowlesi AARP N-terminus may be due to shared B-cell epitopes identified in this study. We also showed that the anti-PvAARP antibody inhibited P. knowlesi erythrocyte invasion as efficient as anti-PkAARP antibody. These results support a critical role of AARP during erythrocyte invasion by these parasites and suggest that PvAARP induces long-lived cross-species protective immunity against P. vivax and P. knowlesi.
Materials and Methods
P. vivax- and P. knowlesi-infected patient samples
Vivax malaria patient blood samples were collected from Shwegyin Township in Myanmar on the day that the patient visited (D0, acute infection), day 28 after proper treatment (D28, sub-acute) and one year later without re-infection (1 yr). Pooled vivax-patient sera from Republic of Korea (ROK) was used after species-specific PCR of vivax-malaria was confirmed. Blood was also collected from healthy individuals living in non-endemic areas of the ROK; after malaria negativity was confirmed by microscopy and PCR; these samples were used as a negative control. Sera were separated from whole blood and used for protein microarray screening. All experiments were performed in accordance with relevant guidelines and regulations and all experimental protocols involving human samples approved by the Institutional Ethical Committee of the Department of Medical Research, Myanmar (Approval number 49/Ethics-2014) and the Kangwon National University Hospital Ethical Committee (IRB No. 2014-08-008-002). Knowlesi malaria patient sera were obtained in Malaysia between 2010 and 2013. All P. knowlesi and P. vivax patient sera chosen for this study have been confirmed by patient record files with no malaria infection history and the malaria status was also confirmed by microscopy and species-specific PCR. All experiments were performed in accordance with relevant guidelines and regulations and all experimental protocols involving human samples approved by the University of Malaya Medical Ethics Committee (Ref No. 817.18) and the Medical Research Ethic Committee (MREC), Ministry of Health, Malaysia (National Medical Research Register ID No. 13079). Informed consent was obtained from all subjects.
In vitro culture of P. knowlesi
P. knowlesi H strain (a kind gift from Duraisingh MT, Harvard University)41 was maintained with fresh rhesus monkey erythrocytes in RPMI-1640-based medium (Invitrogen Life Technologies, Grand Island, NY) containing L-glutamine, 25 mM HEPES (Invitrogen Life Technologies) and 0.5% Albumax II (Invitrogen Life Technologies)42. P. knowlesi A1-H.1 (a kind gift from Robert W. Moon, LSHTM) was maintained with fresh human erythrocytes in RPMI-1640 in addition of human AB serum43. One cycle lasted approximately 28 h. Erythrocytes for P. knowlesi H strain in vitro culture were obtained from rhesus monkeys approved by the Korea National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB) (Cheongju, Korea) and the Kangwon National University Hospital Ethical Committee (IRB No. KWNUIRB-2017-05-009-001).
B-cell epitope prediction
The full-length pvaarp (PVX_090210, Sal-1 strain) and pkaarp (PKNH_0515300, H strain) gene sequences retrieved from PlasmoDB (www.plasmodb.org)44 were aligned to locate regions with high sequence identity at the nucleotide and amino-acid levels. On the basis of the highest sequence identity, the N-terminus of the PvAARP and PkAARP were chosen for B-cell epitope prediction, expression and antibody production. The in silico prediction of linear B-cell epitopes in the N-terminus was conducted by using the Bcpred server (http://www.imtech.res.in/raghava/bcepred/bcepred_team.html)45, as well as the antibody epitope prediction server at the IEDB Analysis resource, by using the Emini Surface Accessibility Prediction model (http://tools.immuneepitope.org/bcell)46.
Recombinant protein expression and purification
The DNA fragment encoding the full-length pvaarp was amplified from a Korean vivax isolate with the primers PvAARP-FL_F (gggcggatatctcgagAGTATTTTCCGAAAAAGGAAAATT) and PvAARP-FL_R (gcggtacccgggatccCTACGGCACGCCAAACAG). The DNA fragment encoding the full-length pkaarp was amplified from the P. knowlesi H strain with the primers PkAARP-FL_F (gggcggatatctcgagAGTATTCTCCGAAAGAGGAAAATTATT) and PkAARP-FL_R (gcggtacccgggatccTTACGGCACGCCAAACAACTT). Small letters indicate the plasmid-derived sequence, and italicized and underlined letters indicate enzyme restriction sites, XhoI and BamHI, respectively. PCR was run with an initial denaturation at 94 °C for 2 min, followed by 35 cycles of 94 °C for 20 sec, 60 °C for 30 sec, and 58 °C for 1 min and a final extension at 68 °C for 10 min. The amplified PCR products were purified and cloned into the pEU-E01-His-TEV-N2 plasmid vector (CellFree Sciences, Matsuyama, Japan) by using an In-fusion cloning kit (Clontech, Palo, Alto, CA) in a wheat-germ cell-free (WGCF) protein expression system. Expressed crude recombinant proteins rPvAARP-FL and rPkAARP-FL were used directly for immunoscreening by protein array.
The glutathione S-transferase (GST)-tagged PvAARP and PkAARP N-terminus (rPvAARP-N and PkAARP-N, respectively) were expressed in an Escherichia coli system as follows. Genomic DNA from a Korean vivax isolate was used as a template for PCR amplification with specific primer pairs PvAARP-N_F (ggatccccaggaattcatAAAAGTATTTTCCGAAAAAGGAAAA) and PvAARP-N_R (gatgcggccgctcgagTGGCCATCCTCCTCCTCT). Genomic DNA from P. knowlesi H strain was used to amplify PkAARP-N with specific primers PkAARP-N_F (ggatccccaggaattcatAAAAGTATTCTCCGAAAGAGGAAAA) and PkAARP-N_R (gatgcggccgctcgagTGACTCTCCTCCTCTTCTTCCTC). Small letters indicate the plasmid-derived sequence, and italicized and underlined letters indicate EcoRI and XhoI restriction enzyme sites, respectively. PCR was run using a high-fidelity KDO-plus Kit (Toyobo Co., Osaka, Japan) with initial denaturation at 94 °C for 2 min, followed by 35 cycles of 94 °C for 15 sec, 60 °C for 30 sec, and 58 °C for 1 min and a final extension at 68 °C for 10 min. The final amplicon was purified using a gel extraction kit and ligated into the pGEX-4T-2 expression vector (GE Healthcare, Upsala, Sweden). The obtained plasmid was confirmed by DNA sequencing analysis and transformed into E. coli BL21(DE3) competent cells (Invitrogen Life Technologies). GST-fused recombinant proteins were induced with 0.1 mM isopropyl-β-D-thiogalactopyranoside (IPTG; Sigma-Aldrich Co., St. Louis, MO) and purified using glutathione Sepharose 4B (GE Healthcare) according to the manufacturer’s instructions. The purity was confirmed by SDS-PAGE and western blot analysis.
Animal antibody production
Mouse antibodies were raised against rPvAARP-N and PkAARP-N in 6- to 8-week female BALB/c mice (DBL, Seoul, Korea). Thirty micrograms of recombinant protein in PBS was intraperitoneally injected into mice with complete Freund’s adjuvant (Sigma-Aldrich Co.) in a final volume of 100 µL at first, and this was followed by two rounds of boosting with incomplete Freund’s adjuvant. Rabbit antibodies were generated in Japanese white rabbit with 250 ug purified recombinant proteins. All animal immunizations were conducted three times at two-week intervals. Sera were collected two weeks after the last boost. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Kangwon National University and the experiments were conducted according to the Ethical Guidelines for Animal Experiments of Kangwon National University (KIACUC-16-0158).
SDS-PAGE and western blot analysis
PkA1-H.1 schizont was lysed with tetanolysin 100 U/mL47 and incubated for 50 min in 37 °C. Cells were separated from supernatant and pellet. Pellet was washed with 1X complete protease inhibitor cocktail in PBS. Parasite antigen was used for western blot analysis. Recombinant proteins rPvAARP-FL, rPkAARP-FL, rPvAARP-N (0.5 µg) and rPkAARP-N (0.5 µg) were separated by 13% SDS-PAGE under reducing conditions and stained with 0.25% Coomassie brilliant blue R-250 (Sigma-Aldrich Co.). The proteins were also transferred to a 0.45-µm polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA) in a semi-dry transfer apparatus at a current of 360 mA for 40 min. After being blocked with 5% skim milk overnight at 4 °C, membrane-transferred proteins were reacted with primary anti-GST (1:10,000) or anti-His (1:2,000) monoclonal antibodies, animal immune sera (1:1000) and then reacted with secondary IRDye goat anti-mouse or goat anti-rabbit (1:10,000) antibodies (LI-COR Bioscience, Lincoln, NE). The Odyssey infrared imaging system (LI-COR Bioscience) and Odyssey software (LI-COR Bioscience) were used to visualize the bands.
Immunofluorescence assay (IFA)
Parasites on the IFA slides were fixed with ice-cold acetone for 10 min and blocked with 5% BSA at 37 °C for 30 min as describe somewhere else7. Anti-PvAARP-N and PkAARP-N (1:50 dilution) polyclonal antiserum were used as a primary antibody and Alexa Fluor 488-conjugated anti-mouse or anti-rabbit (H + L) antibody (1:500, Invitrogen Life Technologies) was used as a secondary antibody.
Protein microarray
Glass slides were coated with amine solution as described in a previous report25. Evaluation of the acquired immune response was assessed in 32 pairs of vivax sera samples (D0, D28, and 1 yr). Sera pooled from eight vivax malaria patients or eight knowlesi malaria patients were used to evaluate cross-immunoreactivity. The crude rPvAARP-FL and rPkAARP-FL proteins were spotted in duplicate, incubated at 37 °C for 2 h, blocked with 5% BSA in PBS 0.1% Tween-20, and incubated at 37 °C for 1 h. The rPvAARP-FL-coated slides were reacted with sera from malaria patients or healthy individuals (1:25 dilution) at 37 °C for 1 h. Alexa Fluor 546-conjugated goat anti-human IgG (10 µg/mL; Invitrogen Life Technologies) was used as a secondary antibody, and the fluorescence signal was detected with a scanner (InnoScan scanner, Carbonne, France). The cut-off values were set to the mean fluorescence intensity (MFI) plus 2 standard deviations (SDs). The normalized MFI was calculated by dividing the MFI by the cut-off value.
Invasion inhibition assay
P. knowlesi schizonts were purified by using MACS technology (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in 96-well plates in a total 100-µL volume in each well. Hematocrit and initial parasitemia were adjusted to 2% and 1.0–1.5%, respectively. Purified anti-PvAARP-N and PkAARP-N IgG antibodies at different concentrations were added to the 96 well plate (test wells). Control wells without antibody (normal invasion well) were set to validate the normal invasion ability of the parasite. The culture was incubated at 37 °C in a humidified culture chamber for approximately 10 h until newly invaded ring stage parasites were found. Each assay was performed independently in duplicate thrice. The non-immunized mouse or rabbit IgG, anti-His-GST IgG, and anti-Fy6 monoclonal antibody against Duffy antigen receptor for chemokines (DARC) (2C3; 25 μg/mL; a kind gift from Renia L, Singapore Immunology Network-BMSI-A STAR)48 were used as a baseline control. The parasites were stained with SYBR Green I (Sigma-Aldrich Co.). Briefly, cultures were centrifuged at 500× g for 5 min, and pellets were washed two times with filtered 1× PBS and fixed with 0.05% glutaraldehyde (Sigma-Aldrich Co.) for 10 min. Fixed samples were washed twice, stained with SYBR Green I at 0.2× dilution for 10 min, and washed twice. The samples were analyzed with an Accuri C6 flow cytometer (Accuri cytometers Inc., Ann Arbor, MI). In total, 200,000 cells were recorded. Percent inhibition was calculated using the following formula: 100 – (100× (test well/normal invasion well)).
Genetic analysis of N-terminal region of pvaarp and pkaarp
Pvaarp sequences were obtained through PlasmoDB44. All raw sequences were analyzed and trimmed using SeqMan software Lasergene ver. 7.0 (DNASTAR Co., Madison, WI) to contain nucleotide positions 61–333, based on the Sal-1 pvaarp sequence. Sequences were aligned using CLUSTAL-W in MegAlign, Lasergene ver. 7.0 (DNASTAR Co.). Nucleotide diversity (π), defined as the average number of nucleotide differences per site between the two sequences, and the numbers of polymorphic sites (S) and haplotypes (H) were calculated using DnaSP ver. 5.049. Non-synonymous substitutions per non-synonymous sites (dN) and synonymous substitutions per synonymous sites (dS) were compared by using a codon-based z-test implemented in MEGA ver. 7.050. Natural selection acting at the N-terminal epitope region was also tested by using codon-based site-by-site analysis to detect codon sites under positive selection at the population level by determining the differences between dN and dS per site using fixed-effects likelihood (FEL)51, internal fixed-effects likelihood (IFEL)52, random-effects likelihood (REL), and mixed-effects model of episodic selection (MEME) methods53.
Statistical analysis
Data analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA). The student’s t-test was used to compare mean from two groups or a one-way ANOVA with Tukey post hoc test was used to evaluate the significant differences between the means from more than two groups. The values of p < 0.05 were considered significantly different.
References
World Health Organization. World malaria report (2016). (2017).
Sauerwein, R. W., Roestenberg, M. & Moorthy, V. S. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol 11, 57–64 (2011).
Baird, J. K. Host Age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today 11, 105–111 (1995).
Dechavanne, C. et al. Acquisition of natural humoral immunity to P. falciparum in early life in Benin: impact of clinical, environmental and host factors. Sci Rep 6, 33961 (2016).
Beeson, J. G., Osier, F. H. A. & Engwerda, C. R. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol 24, 578–584 (2008).
Wipasa, J. et al. Long-lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog 6, e1000770 (2010).
Muh, F. et al. Identification of a novel merozoite surface antigen of Plasmodium vivax, PvMSA180. Malar J 16, 133 (2017).
Wang, B. et al. Immunoprofiling of the tryptophan-rich antigen family in Plasmodium vivax. Infect Immun 83, 3083–3095 (2015).
Lu, F. et al. Profiling the humoral immune responses to Plasmodium vivax infection and identification of candidate immunogenic rhoptry-associated membrane antigen (RAMA). J Proteomics 102, 66–82 (2014).
Changrob, S. et al. Naturally-acquired immune response against Plasmodium vivax Rhoptry-Associated Membrane Antigen. PLoS One 11, e0153110 (2016).
Singh, B. et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. The Lancet 363, 1017–1024 (2004).
Ahmed, M. A. & Cox-Singh, J. Plasmodium knowlesi - an emerging pathogen. ISBT Sci Ser 10, 134–140 (2015).
Yusof, R. et al. Phylogeographic evidence for 2 genetically distinct zoonotic Plasmodium knowlesi parasites, Malaysia. Emerg Infect Dis 22, 1371–1380 (2016).
Ahmed, M. A., Fong, M. Y., Lau, Y. L. & Yusof, R. Clustering and genetic differentiation of the normocyte binding protein (nbpxa) of Plasmodium knowlesi clinical isolates from Peninsular Malaysia and Malaysia Borneo. Malaria J 15, 241 (2016).
Pinheiro, M. M. et al. Plasmodium knowlesi genome sequences from clinical isolates reveal extensive genomic dimorphism. PLoS One 10, e0121303 (2015).
Cornejo, O. E. & Escalante, A. A. The origin and age of Plasmodium vivax. Trends Parasitol 22, 558–563 (2006).
Miller, L. H. et al. Determinants on surface proteins of Plasmodium knowlesi merozoites common to Plasmodium falciparum schizonts. J Exp Med 151, 790–798 (1980).
Yadava, A., Nurmukhambetova, S., Pichugin, A. V. & Lumsden, J. M. Cross-species immunity following immunization with a circumsporozoite protein-based vaccine for malaria. J Infect Dis 205, 1456–1463 (2012).
Sedegah, M., Weiss, W. W. & Hoffman, S. L. Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites. Parasite Immunol 29, 559–565 (2007).
Douradinha, B., Mota, M. M., Luty, A. J. F. & Sauerwein, R. W. Cross-Species Immunity in malaria vaccine development: two, three, or even four for the price of one? Infect Immun 76, 873–878 (2008).
Wickramarachchi, T., Devi, Y. S., Mohmmed, A. & Chauhan, V. S. Identification and characterization of a novel Plasmodium falciparum merozoite apical protein involved in erythrocyte binding and invasion. PLoS One 3, e1732 (2008).
Kalra, A., Edula, J. R., Gupta, P. K., Pandey, A. K. & Chauhan, V. S. Antigenicity of a bacterially expressed triple chimeric antigen of Plasmodium falciparum AARP, MSP-311 and MSP-119: PfAMSP-Fu35. PLoS One 11, e0165720 (2016).
Restrepo-Montoya, D. et al. Identification of Plasmodium vivax proteins with potential role in invasion using sequence redundancy reduction and profile hidden Markov models. PLoS One 6, e25189 (2011).
Moreno-Perez, D. A., Saldarriaga, A. & Patarroyo, M. A. Characterizing PvARP, a novel Plasmodium vivax antigen. Malar J 12, 165 (2013).
Chen, J.-H. et al. Immunoproteomics profiling of blood stage Plasmodium vivax Infection by high-throughput screening assays. J Proteome Res 9, 6479–6489 (2010).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. L. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J Mol Biol 305, 567–580 (2001).
Käll, L., Krogh, A. & Sonnhammer, E. L. L. Advantages of combined transmembrane topology and signal peptide prediction—the Phobius web server. Nucleic Acids Res 35, W429–W432 (2007).
Viklund, H. & Elofsson, A. OCTOPUS: improving topology prediction by two-track ANN-based preference scores and an extended topological grammar. Bioinformatics 24, 1662–1668 (2008).
Assefa, S. et al. Population genomic structure and adaptation in the zoonotic malaria parasite Plasmodium knowlesi. Proc Natl Acad Sci USA 112, 13027–13032 (2015).
Scarselli, M., Giuliani, M. M., Adu-Bobie, J., Pizza, M. & Rappuoli, R. The impact of genomics on vaccine design. Trends Biotechnol 23, 84–91 (2005).
Villard, V. et al. Rapid identification of malaria vaccine candidates based on α-helical coiled coil protein motif. PLoS One 2, e645 (2007).
Achtman, A. H., Bull, P. C., Stephens, R. & Langhorne, J. Longevity of the immune response and memory to blood-stage malaria infection. Curr Top Microbiol Immunol 297, 71–102 (2005).
Gowda, D. C. & Davidson, E. A. Protein glycosylation in the malaria parasite. Parasitol Today 15, 147–152 (1999).
Kedees, M. H. et al. Plasmodium falciparum: glycosylation status of Plasmodium falciparum circumsporozoite protein expressed in the baculovirus system. Exp Parasitol 101, 64–68 (2002).
Endo, Y. & Sawasaki, T. Cell-free expression systems for eukaryotic protein production. Curr Opin Biotechnol 17, 373–380 (2006).
Pandey, A. K. et al. Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies. Infect Immun 81, 441–451 (2013).
Reddy, K. S. et al. Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies. Infect Immun 82, 152–164 (2014).
Kalra, A., Mukherjee, P. & Chauhan, V. S. Characterization of fine specificity of the immune response to a Plasmodium falciparum rhoptry neck protein, PfAARP. Malar J 15, 457 (2016).
Akpogheneta, O. J. et al. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun 76, 1748–1755 (2008).
Escalante, A. A., Lal, A. A. & Ayala, F. J. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics 149, 189 (1998).
Lim, C. et al. Expansion of host cellular niche can drive adaptation of a zoonotic malaria parasite to humans. Nat Commun 4, 1638–1638 (2013).
Trigg, P. & Gutteridge, W. A minimal medium for the growth of Plasmodium knowlesi in dilution cultures. Parasitology 62, 113–123 (1971).
Moon, R. W. et al. Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc Natl Acad Sci USA 110, 531–536 (2013).
Aurrecoechea, C. et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res 37, D539–D543 (2009).
Saha, S. & Raghava, G. P. S. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins: Struct, Funct Bioinf 65, 40–48 (2006).
Emini, E. A., Hughes, J. V., Perlow, D. S. & Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol 55, 836–839 (1985).
Boddey, J. A., Moritz, R. L., Simpson, R. J. & Cowman, A. F. Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic 10, 285–299 (2009).
Wasniowska, K. et al. Structural characterization of the epitope recognized by the new anti-Fy6 monoclonal antibody NaM185-2C3. Transfus Med 12, 205–211 (2002).
Librado, P. & Rozas, J. DnaSPv5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33, 1870–1874 (2016).
Kosakovsky Pond, S. L. & Frost, S. D. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol Biol Evol 22, 1208–1222 (2005).
Pond, S. L. et al. Adaptation to different human populations by HIV-1 revealed by codon-based analyses. PLoS Comput Biol 2, e62 (2006).
Murrell, B. et al. Detecting individual sites subject to episodic diversifying selection. PLoS Genet 8, e1002764 (2012).
Acknowledgements
The authors are grateful to the Korea National Primate Research Center for providing rhesus erythrocytes; to Professor Laurent Renia, Laboratory of Pathogen Immunobiology, Singapore Immunology Network-BMSI-A STAR, Singapore for the 2C3 antibody against human DARC; to Robert W. Moon Department of Immunology and Infection, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London WC1E 7HT, United Kingdom for providing the P. knowlesi A1-H.1 strain and to Professor Manoj Duraisingh, Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, USA for providing the P. knowlesi H strain. Mr. Fauzi Muh gratefully acknowledges the Indonesia Endowment Fund for Education for the financial support of his scholarship. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2017R1A2A2A05069562), by Basic Science Research Programme through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1A4A1038666) and Indonesia Endowment Fund for Education (LPDP/ 20140812021475; scholarship to F.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
F.M. and E.T.H. designed the study. F.M., J.H.H., L.S.K. conducted the laboratory work and data analysis. M.A.H. and O.K. performed genetic analysis. M.H.N. and Y.L.L. collected patient sera. All authors contributed to writing, reading and approving the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muh, F., Ahmed, M.A., Han, JH. et al. Cross-species analysis of apical asparagine-rich protein of Plasmodium vivax and Plasmodium knowlesi. Sci Rep 8, 5781 (2018). https://doi.org/10.1038/s41598-018-23728-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23728-1
This article is cited by
-
Diagnostic performance of a 5-plex malaria immunoassay in regions co-endemic for Plasmodium falciparum, P. vivax, P. knowlesi, P. malariae and P. ovale
Scientific Reports (2022)
-
Updates on malaria incidence and profile in Malaysia from 2013 to 2017
Malaria Journal (2020)
-
Inhibition of parasite invasion by monoclonal antibody against epidermal growth factor-like domain of Plasmodium vivax merozoite surface protein 1 paralog
Scientific Reports (2019)
-
In vitro invasion inhibition assay using antibodies against Plasmodium knowlesi Duffy binding protein alpha and apical membrane antigen protein 1 in human erythrocyte-adapted P. knowlesi A1-H.1 strain
Malaria Journal (2018)
-
Within-population genetic diversity and population structure of Plasmodium knowlesi merozoite surface protein 1 gene from geographically distinct regions of Malaysia and Thailand
Malaria Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.