Abstract
G-alpha (Gα) and ‘Regulator of G-protein Signaling (RGS)’ proteins are the two key components primarily involved in regulation of heterotrimeric G-proteins signaling across phyla. Unlike Arabidopsis thaliana, our knowledge about G-protein regulation in polyploid Brassica species is sparse. In this study, we identified one Gα and two RGS genes each from three species of Brassica ‘U’ triangle and assessed the effects of whole genome triplication on the divergence of gene sequence and structure, protein-protein interaction, biochemical activities, and gene expression. Sequence and phylogenetic analysis revealed that the deduced Gα and RGS proteins are evolutionarily conserved across Brassica species. The duplicated RGS proteins of each Brassica species interacted with their cognate Gα but displayed varying levels of interaction strength. The Gα and the duplicated RGS proteins of Brassica species exhibited highly conserved G-protein activities when tested under in-vitro conditions. Expression analysis of the B. rapa RGS genes revealed a high degree of transcriptional differentiation across the tested tissue types and in response to various elicitors, particularly under D-glucose, salt and phytohormone treatments. Taken together, our results suggest that the RGS-mediated regulation of G-protein signaling in Brassica species is predominantly governed by stage and condition-specific expression differentiation of the duplicated RGS genes.
Similar content being viewed by others
Introduction
Signaling through heterotrimeric G-protein (hereafter G-protein) complexes plays a fundamental role in controlling various cellular processes both in plants and animals1,2. The core G-protein functional complex comprises three different components i.e. G-alpha (Gα), G-beta (Gβ) and G-gamma (Gγ) subunits, where only Gα subunit can bind and dissociate guanine nucleotides (GTP/GDP). In animals, binding of a ligand to G-protein coupled receptor (GPCR), stimulates its guanine exchange factor (GEF) activity which promotes the release of GDP for GTP from Gα subunit, dissociating the inactive heterotrimer into two functionally independent components i.e. Gα-GTP and Gβγ dimer3,4. These two signaling units independently interact with various effector proteins which further validate their ability to participate in numerous biological functions. The intrinsic GTPase activity of the Gα subunit hydrolyzes the bound GTP and allows GDP-Gα form to reunite with Gβγ dimer, subsequently making the heterotrimer inactive5. In addition, GAP activity of the ‘Regulator of G-protein Signaling (RGS)’ protein is also known to accelerate the GTP-hydrolysis of Gα subunit and deactivating the G-protein cycle6.
Although the core components of G-protein signaling are highly conserved across phyla, the plant and animal systems are known to have enormous diversity in their quantitative repertoire and regulation of G-protein cycle. For example, the human genome encodes >800 GPCRs, 35 RGS, 23 Gα, five Gβ, and 12 Gγ proteins, regulating a wide range of biological processes1. In contrast, plants, in general, contain a simple repertoire of G-protein components encoding only up to four Gα and Gβ, 10 Gγ and two RGS proteins, having no prototypical GPCR7. Identification of multiple members of G-protein subunits has been attributed to inherent polyploidy in the angiosperm lineage7,8,9,10.
In addition, the activation of plant G-protein signaling is quite contrasting to the classical G-protein paradigm present in metazoans and relies on the self-activating properties of plant Gα subunit, independent of GPCR2. Structural and enzyme kinetic analysis of the Arabidopsis AtGPA1 protein plausibly explain its GPCR independent activation11,12,13. The GDP to GTP nucleotide exchange rate on AtGPA1 is approximately 100-fold faster than its rate of GTP-hydrolysis, suggesting that the plant Gα is predominantly present in the GTP-bound form11,14. Since G-proteins are signaling molecules, it is important to turn-off the continuing activation state of Gα-GTP after stimulation in plants. Identification of RGS proteins was primarily an important finding in plant G-protein research. In plants, the RGS protein acts as a GTPase-activating protein (GAP), accelerating the rate-limiting GTP-hydrolysis of GTP-bound Gα and so neutralizing the fast nucleotide exchange rate6. Interestingly, the RGS proteins reported from the plant lineage contain an N-terminal seven transmembrane (7-TM) structure which is unique and absent in their animal counterparts11,15. The GAP activity of plant RGS proteins is shown to be confined to the ‘RGS-domain’ present at its C-terminal region16. Thus, the interplay between RGS and Gα proteins is quite important in regulating overall G-protein mediated biological processes in plants. Although the plant G-protein cycle is principally known to be controlled at the deactivation step through RGS proteins; in recent years phosphorylation-dependent regulation of the G-protein cycle involving receptor-like kinases (RLKs) and their associated kinases has also been reported17,18,19,20,21,22.
The limited G-proteins repertoire in plants can yet control wide range of biological processes encompassing plant morphology and architecture, defence responses, abiotic stress response, sugar and phytohormone response, and yield related traits23,24,25,26,27,28,29,30,31,32,33,34. Although structurally similar across plant lineage, both Gα and RGS proteins interestingly possess distinct and species-specific functions. For example, the Gα mutation led to the dwarfing phenotype in rice d1 mutant24,35 and maize ct2 mutant17, whereas the Arabidopsis mutant (gpa1) did not show any significant change in plant height36. The Gα-RGS interplay is also known to regulate species-specific traits in plants, such as nodulation in soybean37. Species-specific roles of Gα and RGS proteins in plant lineage could be attributed to their distinct transcriptional and biochemical properties, as well as the involvement of their upstream regulators and downstream effectors.
Brassica species play an important role in global agriculture and horticulture, and share a close relationship to the model plant A. thaliana. The cultivable diploids (Brassica rapa, B. nigra and B. oleracea) and their natural allotetraploid (B. juncea, B. napus and B. carinata) species belonging to Brassica ‘U’ triangle have been well studied for their several agronomical traits like seed-yield, oil-quality, phyto-remediation, secondary metabolites, resistance against pests and pathogens38. The Brassica species are known to possess enormous genome complexity and diverse morpho-types, shaped by lineage-specific whole genome triplication (WGT) event, allopolyploidization and genomic rearrangements39,40,41. As a result, the so-called diploid Brassica species are paleohexaploid containing three sub-genomes and possess multiple gene homologs having variable gene expression patterns, gene-silencing effects, and neo- and sub-functionalization42. Although complex networks of G-protein signaling have been recently reported in few Brassica species10,43, detailed studies on the expression, biochemical, interaction and functional variance of the Gα and RGS proteins, arising from polyploidy, are fundamentally important for a better understanding of the regulation of G-protein signaling from globally cultivated Brassica crops.
To study the RGS-mediated regulation of G-protein signaling in Brassica genus, isolation of the full-length coding DNA sequence of Gα and RGS genes from three divergent species belonging to ‘Brassica U-triangle’ was carried out. Subsequently, the GTP-binding/hydrolysis activities of Gα orthologs; GAP activity of Brassica RGS proteins on Gα; and interaction selectivity between Gα and RGS proteins was examined. Later, in-depth expression profiling of RGS genes in various tissue types, plant developmental stages and environmental stress conditions in the Brassica model genome, B. rapa was also investigated. This work suggests that the RGS-mediated regulation of G-protein signaling in Brassica species is highly complex and predominantly governed by stage and condition-specific expression differentiation of duplicated RGS genes to control diverse growth and development processes.
Results
Identification and sequence analysis of RGS and Gα subunit genes from diploid Brassica species
The full-length coding DNA sequences (CDS) of RGS genes from B. rapa (A genome), B. nigra (B) and B. oleracea (C) were amplified using the degenerate primers (Table S1). Two CDS for RGS genes from each Brassica species were isolated and designated as BraA.RGS1 and BraA.RGS2 (B. rapa); BniB.RGS1 and BniB.RGS2 (B. nigra); BolC.RGS1 and BolC.RGS2 (B. oleracea), based on the standardized nomenclature adopted for Brassica genus44. Full length coding RGS sequences isolated from different Brassica species ranged from 1368 to 1386 bp, encoding proteins of 455 to 461 amino acids in length, with an estimated molecular weight of approximately 52 kDa (Table S2). Deduced RGS proteins of B. rapa, B. nigra and B. oleracea shared 83.8–89.3% identity with the Arabidopsis AtRGS1 (Table S2). Nucleotide sequences of Brassica RGS genes showed 87.6–89.1% identity with AtRGS1 (Fig. S1, Table S3). Sequence analysis of the deduced RGS proteins of Brassica lineage on TMHMM server (http://www.cbs.dtu.dk/services/TMHMM/) revealed the presence of an N-terminal ‘seven trans-membrane domain (7-TM)’ and a C-terminal located ‘cytosolic RGS-domain’ (Fig. 1A), similar to that reported for the Arabidopsis and soybean RGS proteins15,45. The Glu320 residue of AtRGS1 protein necessary for the GAP activity was highly conserved in all the Brassica RGS proteins11. Moreover, most of the amino acid residues recently described for the plant Gα-RGS contact interface were also found to be conserved46 (Fig. 1A). For example, corresponding sites for Cys316, Try317, Ala318, Glu361, Asp363, Ser365, His366, Lys367, Asp389, Met392, Gln393, Leu394, Lys396, Asp398, Leu399 and Asp402 of AtRGS1, were all found to be highly conserved across the deduced RGS proteins of Brassica species. Interestingly, Brassica lineage-specific substitution was observed at the 362nd amino acid position, wherein Leu was replaced by Val. In addition, a Met397Thr substitution was also observed for the BniB.RGS2, localized in the Gα-RGS contact interface46.
Multiple sequence alignment of Brassica RGS and Gα proteins. (A) Amino acid sequence alignment of RGS proteins from B. rapa (BraA), B. nigra (BniB), B. oleracea (BolC) and Arabidopsis (AtRGS1) was performed using ClustalW (http://www.clustal.org). The predicted 7-TM domains are marked within the horizontal lines and RGS-domain is shown within the box. The critical Glu (E) residue for GAP activity of RGS protein is indicated with a filled circle. (B) Amino acid sequence alignment of Gα proteins from B. rapa, B. nigra, B. oleracea and Arabidopsis (AtGPA1). Position of consensus regions for GTP-binding and GTP-hydrolysis are marked within black horizontal lines (G1–G5); P/M, the predicted site for palmitoylation/myristoylation (MGXXCS); open square shows the important Thr (T) residue for RGS-Gα interaction; and the Gln (Q) residue for GTPase activity of Gα proteins is marked as filled square. The asterisks represent the important contact sites at RGS and Gα interfaces.
Earlier, we reported single Gα homolog of AtGPA1 from B. rapa10 and B. nigra43 genomes. Likewise, in this study, only one Gα homolog (BolC.Gα1) was identified and isolated from B. oleracea. The pairwise sequence alignment of BolC.Gα1 showed high sequence identity with AtGPA1 both at nucleotide (91.8%) and protein (96.4%) levels (Fig. 1B, Table S2, Fig. S2 and Table S4). Amino acid sequence analysis of Gα proteins isolated from three diploid Brassica species showed conservation of characteristics guanine nucleotide binding and hydrolysis domains (G1–G5). In addition, N-terminal palmitoylation and myristoylation sites (MGXXCS) required for plasma membrane anchoring, amino acid residue for GTPase activity of Gα protein (Gln222), and most of the contact sites including Thr194 essential for Gα-RGS interaction as described by Temple and Jones47 and Hackenberg et al. (2016)46 were also found to be conserved in all the Brassica Gα orthologs (Fig. 1B).
Evolutionary analysis of Brassica RGS and Gα genes
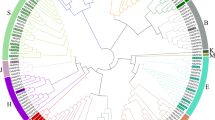
A high level of amino-acid sequence identity and domain conservation of Brassica-specific RGS and Gα proteins led us to investigate their evolutionary relationship with the sequences reported from other plant genomes. Phylogenetic analysis showed that all RGS and Gα proteins belonging to Brassicaceae family were clustered together with AtRGS1 and AtGPA1, respectively (Fig. 2A,C). Interestingly, the RGS sequences identified in this study were separated into two distinct clades, named as orthologous set-I (RGS1) and set-II (RGS2), suggesting duplication of RGS genes in Brassica lineage. The RGS proteins belonging to orthologous set-I were evolutionarily closer to the AtRGS1. Further, within each orthologous set, the RGS proteins from B. rapa and B. oleracea showed a close phylogenetic relationship, compared to its B. nigra counterpart. Comparison of synonymous substitution rate (Ks) value between the duplicated RGS genes isolated from each Brassica genome showed that RGS1 orthologs have lower Ks (0.34–0.36) values than RGS2 orthologs (0.41–0.47), signifying differential divergence of the duplicated RGS proteins (Table S5). Divergence time analysis showed that the duplicated RGS1 and RGS2 genes of each Brassica species diverged around 11.62–15.68 mya, very soon after the Arabidopsis-Brassica split event, estimated around 13–17 mya40. Interestingly, our data revealed that the duplicated RGS genes of B. nigra have higher Ks values compared to their B. rapa and B. oleracea counterparts. The Gα sequences identified in all the three Brassica species shared highly similar Ks values (0.39–0.42), estimated to diverge around 13.07–14.23 mya from the AtGPA1 (Table S5).
Evolutionary relationship of Brassica RGS and Gα proteins. The phylogenetic analysis of B. rapa, B. nigra and B. oleracea (A) RGS and (C) Gα proteins with other eudicots species was performed using maximum likelihood method in MEGA5.1. The names of RGS and Gα proteins used for the phylogenetic analysis are abbreviated followed by their locus ID which includes A. thaliana (At), B. rapa (BraA), B. nigra (BniB), B. oleracea (BolC), Capsella rubella (Cru), Cucumis sativus (Csa), Citrus clementine (Ccl), Eucalyptus grandis (Egr), Glycine max (Gma), Gossypium raimondii (Gra), Linum usitatissimum (Lus), Manihot esculenta (Mes), Medicago truncatula (Mtr), Oryza sativa (Os), Phaseolus vulgaris (Pvu), Physcomitrella patens (Ppe), Populus trichocarpa (Ptr), Prunus persica (Ppe), Ricinus communis (Rco), Setaria italica (Sit), and Theobroma cacao (Tca). The percentage of replicate trees in which the associated proteins clustered together in the bootstrap test (1000 replicates) is shown next to the branches. Gene organization of (B) RGS and (D) Gα genes in Brassica species. The full-length genomic sequences of B. rapa, B. nigra and B. oleracea RGS and Gα genes were retrieved from BRAD (http://www.brassicadb.org/) and phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) databases. The exons and introns are shows in black box and open triangle, respectively. Number denotes the length (in bp) of respective exon and intron, drawn to scale.
Based on the RGS and Gα coding sequences isolated in this study, we retrieved their genomic counterparts from the recently assembled Brassica database (http://brassicadb.org/brad/). The BraA.RGS1 (corresponds to Bra025181), BraA.RGS2 (Bra017336) and BraA.Gα1 (Bra007761) were localized onto A06, A09, and A09 chromosomes in the model B. rapa (A) genome (Table S6). Similarly, the RGS and Gα orthologs from Brassica ‘B’ (B. nigra) and ‘C’ (B. oleracea) were also identified and summarized in Table S6. Gene structure analysis revealed that the RGS genes contain 10 exons and 9 introns, whereas the Gα genes harbour 13 exons and 12 introns (Fig. 2B,D). Intron-exon organization of the G-protein orthologs present in B. rapa and B. oleracea was quite similar compared to their B. nigra counterparts, where the size of introns was found to be variable.
Brassica species are mesohexaploids, containing three sub-genomes (LF, MF1 and MF2) formed due to the whole-genome-triplication event48. Sub-genomic distribution analysis of the G-protein candidate genes in B. rapa and B. oleracea genomes (http://brassicadb.org/brad/) revealed that the Gα and RGS1 orthologs were present on LF (least fractionized) sub-genome, whereas RGS2 was present on MF2 (most fractionized) sub-genome (Fig. 3). Homologs of RGS and Gα genes were occupied within the Brassica ancestor genomic block ‘L’ and ‘I’, respectively. To get evolutionary insight into this differential gene-retention, we further analyzed the gene content within the genomic blocks ‘L’ and ‘I’ shared between A. thaliana and the three sub-genomes of sequenced B. rapa and B. oleracea genomes (Table S7). The gene-retention frequencies of ‘L’ and ‘I’ genomic blocks belonging to LF sub-genome were almost similar (42.1–47.0%), whereas within the MF2 sub-genome the gene retention of genomic block ‘L’ (19.8–20.7%) was comparably higher than genomic block ‘I’ (10.3–10.5%), thereby suggesting uneven gene-fractionation of the triplicated sub-genomes in Brassica species.
Comparison of gene organization in the genomic blocks ‘I’ and ‘L’ of A. thaliana, B. rapa and B. oleracea, as obtained from BRAD database (http://www.brassicadb.org/). Gene arrangement of B. rapa and B. oleracea syntenic orthologs of 25–30 representative A. thaliana genes flanking each side of (A) AtGPA1 within genomic block ‘I’, and (B) AtRGS1 within genomic block ‘L’. The syntenic position of AtGPA1 (At2g26300) and AtRGS1 (At3g26090) genes are marked within green boxes in (A) and (B), respectively. Syntenic genes shared between the three species are represented with black circle; Brassica lineage specific genes are marked with red circle showing their gene ID. The line nomenclature describes the ‘subgenome-genomic block-linkage group-Brassica species’.
Protein-protein interaction between duplicated RGS and Gα proteins of Brassica species
In metazoans, where multiple members of RGS and Gα subunit are present, interaction specificity between various Gα-RGS proteins controls the kinetics of nucleotide (GTP/GDP) cycling and sensitivity of G-protein signaling49. In plants, studying the Gα-RGS interaction is quite important considering that Gα protein has self-activating property and so far RGS and PLDα1 are the only well-studied modulators of G-protein signaling50,51. In this study, two divergent RGS proteins were identified each from the three diploid Brassica genomes. To analyse the interaction strength and specificity between the duplicated RGS proteins and Gα subunit, a mating based split ubiquitin system (mbSUS) assay was performed, wherein the Gα and RGS proteins were used as prey and bait proteins, respectively. Based on the growth of mated yeast cells on selection medium, we observed that duplicated RGS proteins of each Brassica species interacted with their cognate Gα protein (Fig. 4). In B. rapa and B. oleracea, RGS1 protein showed strong interaction with their respective Gα subunit in both the orientations, while RGS2 protein could interact only in one orientation of Gα (as a C-terminal fusion of Nub, Nub-Gα) when tested on three different concentrations of Met (Fig. 4A,C). The duplicated RGS proteins of B. nigra interacted with its cognate BniB.Gα1 specifically in one orientation (Nub-Gα), wherein the BniB.RGS2 showed relatively weak interaction (Fig. 4B).
Interaction between Gα and RGS proteins of Brassica species namely (A) B. rapa, (B) B. nigra, and (C) B. oleracea using mating based split ubiquitin system. Gα subunit was cloned in both the orientation of Nub vector (containing N-terminal half of ubiquitin), and RGS proteins were cloned in Cub vector (C-terminal half of ubiquitin), as fusion proteins. The interaction was examined by the growth of mated diploid yeast cells on selection plates (SD-AHLT) containing 0, 500 and 1000 μM Met. The NubWt-Gα protein and empty NubG vector were used as positive and negative control, respectively. Two biological replicates of the experiment were performed with identical results.
In Arabidopsis, the ‘RGS-domain’ present at the C-terminal region of RGS protein is known to interact with the AtGPA116. Therefore, to further validate our observation, we analysed the interaction of the C-terminal cytosolic domain containing the RGS-box of the duplicated RGS proteins of Brassica origin with their cognate Gα protein by utilizing the conventional GAL4 based yeast two hybrid (Y2H) system. The RGS-domains of duplicated RGS proteins of B. rapa showed strong and comparable interaction with BraA.Gα1 even up to 25 mM of 3AT (Fig. S3). However, as also observed for the full-length RGS proteins, the RGS-domains of the two B. nigra proteins showed differential interaction specificity with BniB.Gα1. The RGS-domain of the BniB.RGS1 showed strong interaction with BniB.Gα1, compared to BniB.RGS2 showing weak interaction. Hence, data obtained from both the mbSUS and Y2H assays, clearly suggest that duplicated RGS proteins interacted with their cognate Gα subunit in Brassica species, although showing a varying level of interaction specificity.
GTP-binding/hydrolysis activity of Gα protein and GAP activity of duplicated RGS proteins in Brassica species
Among the different G-protein core components, the biochemical properties of Gα subunit are contrasting between plants and animals thereby making the paradigm of G-protein signaling quite interesting. In order to get a primary insight into the regulation of G-protein in Brassica species, recombinant Gα proteins containing N-terminal His-tag were purified using Ni-NTA affinity chromatography (Fig. S4A) and an in-vitro activity assay of recombinant Gα proteins was carried out using BODIPY-GTP FL fluorescent dye in a real time fluorescent assay. The rates of GTP-binding (increase in fluorescence) and GTP-hydrolysis (decrease in fluorescence) of all the three Brassica Gα proteins was found to be highly comparable, and similar to that observed for the Arabidopsis AtGPA1 (Fig. 5A). In general, the intrinsic GTP-hydrolysis activity of the Brassica Gα proteins was found to be very slow.
Biochemical characterization of Brassica Gα and RGS proteins. (A) GTP-binding/hydrolysis activity of the recombinant Gα protein using BODIPY fluorescent dye in real-time fluorescence assays; (B) Effect of the recombinant BraA.RGS1 and BraA.RGS2 domains (expressed as C-terminal region containing RGS-box) on GTP-hydrolysis of BraA.Gα1 using BODIPY fluorescent dye in real-time fluorescence assays; and (C) In-vitro Pi release activity of BraA.Gα1 in the presence of different concentration of BraA.RGS1 and BraA.RGS2 domains. Inset table shows the kinetic parameters of Pi release from GTP-bound Gα protein, in the presence of RGS domains of the duplicated B. rapa RGS proteins. Experiments were carried out three times and data was averaged. Error bars represent the mean (±)SE. Data were analyzed using GraphPad Prism version 6.0.
Slow GTP-hydrolysis activities of Gα proteins indicate the important role of RGS proteins in regulating G-protein cycle in genus Brassica. Due to a high level of sequence similarity between RGS1 and RGS2 orthologs across Brassica species, we initially selected duplicated RGS proteins of the model Brassica genome, B. rapa, and for biochemical characterization of the same. The C-terminal region containing RGS-domain of the B. rapa RGS proteins i.e. BraA.RGS1-box (296–417 amino acids) and BraA.RGS2-box (284–415 amino acids) containing N-terminal His-tag were heterogeneously expressed in E. coli and purified using Ni-NTA based affinity chromatography (Fig. S4B). Real-time assays using BODIPY-GTP FL showed that both BraA.RGS1 and BraA.RGS2 accelerated the GTP-hydrolysis of their cognate BraA.Gα1, and the GTPase (GAP) activity was found to be somewhat similar for both of the duplicated BraA.RGS1/2 proteins (Fig. 5B). Likewise, the GAP activity of the duplicated RGS proteins of B. nigra and B. oleracea also showed similar trend on their cognate Gα proteins (Fig. S5A,B). Further, to determine an accurate rate of GAP activity of the duplicated BraA.RGS proteins, steady state kinetics of Pi release was carried out using 1 μM of BraA.Gα1 and 0.1–2.0 μM of BraA.RGS proteins. BraA.Gα1 showed a marginal difference in its rate of Pi release when tested using the BraA.RGS1 (Km 0.244 ± 0.03; Vmax 2.70 ± 0.09) and BraA.RGS2 (Km 0.311 ± 0.04; Vmax 2.55 ± 0.11) proteins (Fig. 5C). Overall, the presence of highly similar GTP-binding/hydrolysis activities of Gα proteins, and somewhat similar GAP activities of the duplicated RGS proteins, in all possibility, suggest that the RGS-mediated G-protein regulation is biochemically conserved in Brassica lineage.
Transcript expression and sub-cellular localization of B. rapa duplicated RGS proteins
Further, to investigate the transcriptional regulation of the duplicated RGS genes, we carried out gene expression analysis in the model Brassica species, B. rapa. Real-time qRT-PCR analysis revealed that both the B. rapa RGS genes (paralogs), resulting from WGT event, were expressed, showing a contrasting difference in their expression patterns. In general, BraA.RGS1 had higher expression compared to BraA.RGS2 in most of the tissue types, except seedlings (Fig. 6A). The expression of BraA.RGS1 was also found to be higher than BraA.RGS2 during all stages of seed development (Fig. 6B). Interestingly, the expression of both BraA.RGS1 and BraA.RGS2 was found to be up-regulated during later stages of seed maturation (35 dap, days-after-pollination) suggesting their important during seed development in B. rapa. Sub-cellular localization studies in transgenic Arabidopsis hypocotyls revealed that the C-terminal YFP-fusion of both BraA.RGS1 and BraA.RGS2 proteins were localized in the plasma-membrane, along with FM4-64 (red), a dye used for staining cell membrane (Fig. 6C). Moreover, transient expression studies in N. benthamiana epidermal cells also established that the duplicated RGS proteins of B. rapa are localized in the plasma-membrane (Fig. S6), as also reported for the soybean RGS proteins45.
Transcript expression profiling of the duplicated RGS genes in (A) different tissue types, and (B) seed maturation stages of B. rapa. Real time PCR amplifications were performed for each target gene in three biological replicates with two technical replicates each. The expression of TIPS-41 for different tissue types and GAPDH for seed maturation stages were used to normalize the data (set at 100). Error bars represent the standard error. Significant expression differences were calculated at P < 0.05 using independent sample t-test using SPSS statistic version 17 software and marked with asterisk on the top of the error bars. (C) Sub-cellular localization of BraA.RGS1 and BraA.RGS2 proteins. Four days old Arabidopsis seedlings (dark grown) showing localization of BraA.RGS1-YFP and BraA.RGS2-YFP fusion proteins (yellow) in plasma membrane of hypocotyl cells. FM4-64 (red) was used to stain the plasma membrane, and merged images are shown in the right column.
Expression analysis of B. rapa duplicated RGS genes under various elicitor treatments
So far, information about the roles and regulation of plant RGS genes under various developmental and environmental cues is sparse and mostly limited to the model plant A. thaliana. To get an initial insight into the transcription regulation of the duplicated RGS genes, in-depth transcript expression profiling was carried out in B. rapa. Five-day-old uniform seedlings of B. rapa, grown on 0.5 × Murashige and Skoog (MS) medium containing 3% sucrose, were subjected to different elicitor treatments, including D-glucose, phytohormones, abiotic and biotic stress conditions for 1, 3, 6, 12 and 24 hours (h) as described previously43,52. qRT-PCR analysis showed a significant up-regulation of BraA.RGS2 transcript compared to BraA.RGS1 when treated with D-glucose for all the tested time points (Fig. 7A), thereby suggesting a differential transcriptional response of the B. rapa duplicated RGS genes under glucose treatment. The BraA.RGS genes also showed differential expression patterns in response to exogenously supplied phytohormones. Expression of BraA.RGS1 transcript was, in general, found to be up-regulated during most of the tested time points of phytohormone treatments, including IAA, GA, BAP, ABA and BR (Fig. 7A). However, the transcript abundance of BraA.RGS2 was found to be up-regulated only during early time points for most of the phytohormone treatments. The duplicated RGS genes also showed distinct expression patterns in response to various abiotic and biotic stress conditions. A profound up-regulation of both BraA.RGS1 and BraA.RGS2 was observed under SA treatment, whereas these transcripts showed significant down-regulation under heat and cold treatments (Fig. 7B). Further, differential transcriptional regulation of the duplicated RGS genes was also observed in response to both NaCl and MeJA treatments, wherein the BraA.RGS1 transcript showed a significant up-regulation upon these treatments.
Expression pattern of the duplicated RGS genes under various elicitor treatments in B. rapa. Heat map of genes showing the effects of (A) D-Glucose and phytohormones; (B) abiotic and biotic stress conditions; and (C) heavy metals on transcript level of RGS genes in B. rapa. Real-time PCR analysis was conducted at five time points (1, 3, 6, 12 and 24 h) of treatments, and the data was averaged (n = 4) and normalized using of Ubiquitin (UBQ) gene expression. The colors on heat map represent the up-regulation (≥2 fold, red) and down-regulation (≤2 fold, green) of the RGS transcripts compared with the untreated mock seedlings grown in liquid 0.5X MS medium for similar time points.
Brassica species are known to be the heavy metal accumulators and are globally used for phyto-remediation purposes. We, therefore, studied the expression patterns of the duplicated RGS genes under various heavy-metal ion toxicity in B. rapa. Heavy metal treatment showed substantial parallelism in the expression patterns of the duplicated RGS genes (Fig. 7C). Interestingly, we found a high accumulation of BraA.RGS1 and BraA.RGS2 transcript under Cu stress during all the tested time points (1 to 24 h). Likewise, up-regulation of the duplicated RGS genes was also observed under Cd, Mn and As stress although, showing a time-dependent transcript accumulation. Under Zn and Pb treatments, we found differential transcript responses of the duplicated RGS genes during different time points. The profound up-regulation of the duplicated RGS genes in all possibility suggests their key involvement during heavy-metal ion toxicity in Brassica crops.
Discussion
Over the recent years, it has become increasingly clear that plants have a unique mechanism of G-protein signaling, owing to limited repertoire of core G-protein components, fast GTP-binding with very slow intrinsic GTPase activity of Gα proteins, and most importantly the absence of functional GPCRs in plants. Among the various G-protein signaling components reported across the plant lineage, the physical interaction and biochemical activities of Gα and RGS proteins are quite crucial for regulating the G-protein cycle53.
Brassica genomes encode highly conserved Gα and RGS proteins
Various comparative genomics and genome sequence studies have unequivocally reported the existence of a WGT event in Brassica lineage, after its split from the model plant Arabidopsis, dating around 13–17 mya, as a result of which the so-called diploid Brassica species are paleohexaploid containing three sub-genomes (LF, MF1 and MF2)39,40,41. Although three copies of each of the Arabidopsis ortholog are quite expected, in the current study only one Gα and two RGS genes were identified in each of the three Brassica genomes, present in LF and MF2 sub-genomes only (Fig. 3, Table S7). The uneven expansion of the candidate G-protein genes in Brassica species could be attributed to the biased gene fractionation (gene-loss) frequency across the three sub-genomes41,48. Notably, within the sub-genome MF2, the genomic block containing Gα (I) encountered higher gene-loss than the RGS containing genomic block (L). Our observation was quite in agreement with earlier reports describing the uneven expansion of key signaling genes in B. rapa, particularly involved in G-protein and 14-3-3 signaling pathways10,52.
Sequence and phylogenetic analysis of both RGS and Gα proteins suggest that these proteins are evolutionarily conserved in Brassica lineage (Figs 1 and 2), wherein the Gα orthologs have significantly high sequence conservation compared to the duplicated RGS proteins. This in all possibility suggests that the canonical Gα proteins might have retained highly conserved biochemical activities vis-à-vis biological functions during the evolution of extant Brassica species. In such scenario, the presence of divergent RGS or other regulatory proteins could play an important role in regulating the G-protein cycle and signaling in these mesohexaploid Brassica species. The differential synonymous base substitution (Ks) rates observed for the duplicated RGS proteins suggests their differential functional specificity and interaction selectivity with the Gα and other effector proteins, which needs detailed investigation.
Brassica Gα and RGS proteins display differential interaction specificity
Expansion of the repertoire of G-protein components revealed the presence of complex signaling network in plants7. These components tend to interact in various combinations to govern the functional selectivity in various cell and/or tissue types. Various studies in animal systems, clearly established the significance of RGS and Gα interactions in regulating the GAP activity54. Our data suggest that during evolution, the canonical Gα protein present in each Brassica species has retained strong interaction with the ancestral RGS1 protein compared to the recently evolved RGS2 protein (Figs 4, S3). Divergent residues present between the duplicated RGS proteins (Fig. 1) could govern this differential interaction specificity with the canonical Gα protein. Interestingly, among the RGS2 orthologs present across Brassica species, the B. nigra BniB.RGS2 showed comparatively weaker interaction with its cognate BniB.Gα1. A comparably higher Ks value of the BniB.RGS2 and various invariant residues, preferentially present at the C-terminally located RGS-domain (including Met397Thr substitution), might alter the stability and interaction of the Gα-RGS contact interface46, which needs further investigation. The predominance of BniB.RGS1-BniB.Gα1 interaction in all possibility suggested that the regulation of G-protein signaling is quite distinct in B. nigra, and somewhat different from the Brassica A/B genomes. Noteworthy, the gene-structure analysis in our study also revealed that the intron-exon attributes of both Gα and RGS genes in B. nigra varied somewhat from their B. rapa and B. oleracea counterparts (Fig. 2). These observations could be best explained by the fact that B. nigra has evolved separately from the B. rapa/B. oleracea lineage in the tribe Brassicaceae55. Nonetheless, the differential RGS-Gα interaction specificity suggests distinct RGS-mediated regulation of G-protein signaling in Brassica lineage. Detailed functional studies using gain- and loss-of-function strategies of G-protein genes in each of these Brassica species need to be undertaken to uncover the significance of this differential interaction.
The canonical Gα and duplicated RGS proteins display similar G-protein activities in Brassica lineage
The unusual self-activating property of Gα protein makes the plant G-protein signaling unique from that of metazoans. The plant Gα proteins posses fast rate of GTP-binding and slow GTP-hydrolysis ability, which are quite contrasting to their animal counterparts2. In this study, we observed that the canonical Gα orthologs of three Brassica species are biochemically active and display highly similar GTP-binding and GTP-hydrolysis activities, similar to the Arabidopsis AtGPA1 (Fig. 5). This indicates that during the evolution of extant Brassica species, changes in few amino acid residues do not seem to impart any significant differences on the activities of these Gα proteins, at least under the tested in-vitro conditions. In plants, GTP-hydrolysis of Gα is the rate limiting step of G-protein cycle and is modulated by the GAP activity of RGS proteins11. Our in-vitro data based on fluorescence assays and steady state kinetics experiments show that the cytosolic RGS-domain of the duplicated RGS proteins increases the rate of GTP-hydrolysis of the cognate Gα, and are active GAP proteins (Fig. 5). Further, comparable GAP activities of the active RGS-box of duplicated RGS proteins in Brassica lineage suggest that the RGS-mediated regulation of G-protein cycle in Brassica crops is biochemically conserved, although other modes of regulation may be expected.
Our observation is different from that reported in allotetraploid soybean genome, where the four Gα and duplicated RGS protein exhibit distinct G-protein activities8,45. The distinct activities of G-protein regulatory elements can be best explained by the fact that the soybean genome has experienced two rounds of whole genome duplication (WGD) events dating around 58–60 mya (ancient duplication) and a recent duplication ca. around 13 mya56. Since the paralogs created during the ancient WGD event are expected to diverge out considerably, the G-protein members in soybean have retained distinct G-protein activities and functional divergence to regulate various plant growth and developmental traits37. However, multiple homologs formed in Brassica lineage have resulted from a very recent WGT event (~13–15 mya), as a result of which the duplicated/triplicated genes (paralogs) could have retained comparably higher sequence identity vis-à-vis similar biochemical activities and biological functions in the extant Brassica species, as evident from our current study.
Differential transcriptional regulation of duplicated RGS genes under plant developmental stages and elicitor treatments
Over time, the duplicated genes formed as a result of WGD and WGT events in polyploids are known to alter their gene expression, as a result of which these genes undergo different evolutionary fates including neo-functionalization, sub-functionalization and pseudogenization57,58. The transcriptional differentiation of multiple gene homologs is well documented in polyploid plant species59. In this study, the duplicated RGS genes of B. rapa showed a high degree of transcriptional bias across various developmental stages wherein BraA.RGS1 was found to be transcriptionally more active compared to BraA.RGS2 (Fig. 6). Considering only one canonical Gα subunit, and conserved biochemical activities of both Gα and duplicated RGS proteins present across Brassica species, it is quite possible that the RGS-mediated G-protein regulation could be dependent on the differential transcriptional response of duplicated RGS genes to regulate various assets of plant growth and development, as also proposed for soybean45. Moreover, in Brassica lineage where WGT is an inherited norm the sub-genome dominance also shapes the expression and functional dominance of paralogous gene within a gene family42,52. The sub-genome dominance effect is also evident in this study, wherein the highly expressed BraA.RGS1 is localized in the transcriptionally active least-fractionized (LF) sub-genome of B. rapa.
Both loss- and gain-of-function studies in plants under phytohormones and other elicitor treatments show the significance of G-proteins in controlling various assets of plant growth and development. The Arabidopsis G-protein mutants exhibit differential phenotypic response to auxin and ABA treatments. For example, gpa1 mutant displayed hypersensitivity towards ABA during seedling and root development60, whereas, it showed wild-type like inhibition of seedling growth; and hyposensitivity in lateral root development to auxin treatment25,28. Among the two RGS1 genes of B. rapa, a higher up-regulation of BraA.RGS1 during IAA and ABA treatments is quite evident and might suggest its preferential cross-talk with auxin and ABA-mediated seedling development. The Arabidopsis G-protein mutants show hypersensitive response to D-glucose during early stages of plant development like seed germination, seedling development and root growth2. In recent years, it has been well documented that upon D-glucose and NaCl treatments, AtRGS1 undergoes endocytosis from the plasma-membrane to the endosomes which lead to physical uncoupling of AtRGS1 from AtGPA1, thereby allowing the AtGPA1 to self-activate61,62. Among the duplicated RGS genes, the up-regulation of BraA.RGS1 and BraA.RGS2 expression in response to NaCl and D-glucose treatment, respectively, suggest their specific roles in regulating various salt and sugar-responsive phenotypes in B. rapa. The involvement of G-protein components in plant defence is quite established in Arabidopsis26,27,29,34. In addition, the up-regulation of the duplicated BraA.RGS genes under SA treatments possibly suggests their coordinated involvements in defence signaling in B. rapa.
Quite interestingly, both the duplicated RGS genes are highly up-regulated in response to Cu and Cd treatments, suggesting their potential roles during heavy-metal toxicity. Recently, Kunihito et al.63 also showed the involvement of G-proteins in conferring Cd tolerance in yeast and Arabidopsis. Thus RGS-mediated G-protein signaling could represent a novel pathway for phyto-remediation of heavy-metal ions in Brassica species, although other, yet unknown, mechanism may also exist, which warrants further investigation.
Overall, our study shows that the transcriptional differentiation of the biochemically conserved RGS proteins could be quite important to condition-specific Gα-RGS interaction vis-à-vis biological functions in the Brassica lineage. A detailed characterization of Gα and RGS genes could be carried out to integrate the multiple molecular connections that co-ordinately regulate the strength and/or duration of G-protein signals in controlling the various assets of plant growth and development in the globally important Brassica crops.
Materials and Methods
Plant material and growth conditions
Three Brassica species namely, B. rapa L. (cv. YIDI), B. nigra L. (cv. IC257) and B. oleracea L. (cv. Golden Acre) used in the present study were grown under controlled growth conditions at day (24 °C; 10 h; ca. 300 μmol.m−2.s−1) and night (18 °C; 14 h) photoperiod with 55–60% relative humidity. Tissue types representing different developmental stages of B. rapa including five-day old seedlings, fully developed leaves, root, stem, flower and different stages of developing siliques (7 to 35 days-after-pollination) were collected and stored at −80 °C.
Amplification and cloning of Gα and RGS CDS from Brassica species
The standard PCR amplification conditions were deployed with an annealing temperature of 55 °C (30 sec) to obtain the full-length coding DNA sequence of Gα and RGS genes from B. rapa (A genome), B. nigra (B genome) and B. oleracea (C genome). The cloning of Gα CDS from B. rapa and B. nigra has been reported in our earlier studies10,43, whereas from B. oleracea was performed in the present study. For cloning RGS genes, the primers were designed based on the Arabidopsis ortholog (AtRGS1) and annotated B. rapa genes available in the phytozome database (Locus ID: Brara.F03296.1 and Brara.I02169.1) (Table S1). Subsequently, PCR products were cloned into the pENTR/D-TOPO vector (Invitrogen, USA) and sequenced to confirm their fidelity. At least, three independent PCR amplifications were carried out to confirm the gene sequences.
Sequence alignment, phylogenetic and divergence analysis
Phylogenetic analysis of the deduced RGS and Gα protein sequences isolated from B. rapa, B. nigra, B. oleracea, and those retrieved from different plant species (https://phytozome.jgi.doe.gov/pz/portal.html) was carried out using the maximum likelihood method in MEGA5.1 with 1,000 bootstrap iterations in MEGA5.164. The full-length genomic sequences and the chromosomal attributes of the G-protein candidate genes of Brassica origin was retrieved from BRAD database (http://brassicadb.org/brad/). To estimate the divergence time, ClustalW was used for the pairwise alignments of coding DNA sequences of Brassica-specific RGS and Gα genes with their Arabidopsis orthologous counterparts. Ks (synonymous substitution rate) and Ka (non-synonymous substitution rate) were calculated using the DnaSP v5 program bases on the multiple sequence alignment. The divergence time (T) was calculated using the equation: T = Ks/2λ, where λ is the synonymous mutation rate, reported as 1.5 × 10−8 substitution per site per year for Brassica genes65.
Gα and RGS protein-protein interaction assays
Mating based split ubiquitin system (mbSUS) was utilized to study the interaction between Gα subunit and RGS proteins66. Full-length CDS of Brassica Gα proteins were cloned in both the orientation of Nub vector (N- and C-terminal of Nub vector) and RGS proteins in Cub vector. Nub-Wt and empty Nub-vector were used as positive and negative controls, respectively and transformation and mating were performed as described10,43. Finally, strength and selectivity of Brassica Gα and RGS subunit protein interactions were determined by the growth of mated yeast cells on the selection medium lacking adenine, histidine, leucine and tryptophan (SD–AHLT), having 0, 500 and 1000 µM of methionine.
The interaction between Gα subunit and the cytosolic RGS-domain (RGS-box + C-terminal) of RGS proteins was tested using GAL4 based yeast two hybrid system. Full length CDS of Brassica Gα and RGS-domain were cloned into pENTR/D-TOPO entry vector. Thereafter, Brassica Gα proteins (bait) and RGS-domain (prey) were mobilized in pDEST-GBKT7 gateway (containing DNA binding domain) (ABRC stock: CD3-764) and pDEST-GADT7 gateway (containing having activation domain) (ABRC stock: CD3-763) vectors67, respectively using gateway based cloning strategy. The independent sets of bait and prey plasmids were then co-transformed into yeast strain Y2HGold. Five-six colonies were pooled and inoculated into 3 ml of liquid minimal medium deficient with leucine and tryptophan (−LT) and incubated for 16 h at 30 °C with shaking. Cultures were equalized to an OD600 = 0.8 and 10 μl of culture was placed on the selection medium. The interaction strength and selectivity were determined by the ability of diploid yeast cells to grow on SD–AHLT selection medium (3–5 days post inoculation), having different concentration of 3-amino-1,2,4-triazole (3-AT).
Expression and purification of recombinant Gα and RGS proteins
The coding regions of AtGPA1, BraA.Gα1 (Gα subunit of B. rapa), BniB.Gα1 (Gα subunit of B. nigra), BolC.Gα1 (Gα subunit of B. oleracea), BraA.RGS1box + Ct and BraA.RGS2box + Ct (cytosolic RGS-domain with C-terminal region) were cloned into pET28a expression vector (Novagen, USA) and transformed into E. coli Rosetta-gami2 (DE3) cells (Novagen, USA). The N-terminal His-tagged recombinant Gα proteins were purified by Ni2+-NTA affinity chromatography68. Under the condition described for Gα protein purification, the recombinant RGS2-domain (RGS2box + Ct) was accumulated in the inclusion bodies (IBs). In order to maintain the purification similarities, the recombinant RGS-domain of duplicated RGS proteins were purified under denaturation condition from inclusion bodies. The pellet fraction containing the expressed protein was resuspended in extraction buffer (50 mM Tris-HCl pH 7.5; 8 M Urea; 1 mM DTT; 1 mM PMSF) and kept for 60 min at room temperature for solubilization. Solubilized protein was diluted 10 fold with extraction buffer and dialyzed overnight in wash buffer (50 mM Tris-HCl; pH 7.5; 200 mM NaCl; 1% Triton X-100 and 1 M Urea). Dialyzed protein was pooled and centrifuged at 12,000 rpm for 30 min at 4 °C and the supernatant was purified using Ni2+-NTA affinity chromatography similar to Gα proteins.
G-protein activity assay of recombinant Gα and RGS proteins
In-vitro G-protein activity assay of the purified Gα proteins was carried out using 4,4-difluoro-4-bora-3α,4α-diaza-s-indacene-GTP Fluorophore (BODIPY-GTP FL, Invitrogen) dye in real time fluorescent assays as described previously69. Further to determine the GAP activity of the recombinant RGS-domain, in-vitro Pi release activity was also carried out using ENZchek phosphate assay kit (Invitrogen) as described previously45. Briefly, BraA.Gα1 protein (1 µM) was pre-loaded with GTP (1 mM) and incubated with 0.1 to 2 µM of purified RGS-domain of BraA.RGS1 and BraA.RGS2 proteins. Phosphate (Pi) released was measured as the absorbance at 360 nm using a spectrophotometer (FLUOstar Optima, BMGLab Technologies).
Total RNA isolation, cDNA synthesis and real-time qRT–PCR
Total RNA isolation from different developmental tissues of Brassica species, first strand cDNA synthesis and real-time qRT-PCR were performed as described previously43. cDNA samples representing various growth and developmental stages were diluted 1:25 in nuclease-free water, and real-time PCRs were performed using gene-specific primers (Table S1).
Sub-cellular localization of B. rapa RGS genes
In order to study the sub-cellular localization, the full-length coding regions of BraA.RGS1 and BraA.RGS2 were mobilized into destination binary vector pEarleyGate101 (ABRC stock CD3-683)70 from Gateway entry vector pENTR/D-TOPO using LR recombination strategy (Invitrogen, USA). Thereafter, stable Arabidopsis lines (Col-0 background) were generated independently expressing BraA.RGS genes fused to C-terminal YFP tag using Agrobacterium-mediated transformation. Hypocotyl cells of four-day old seedlings (T2 generation) grown under continuous dark conditions on 0.5 × (MS) medium without any exogenous sugar source were used to study the localization of BraA.RGS proteins. Localization of B. rapa RGS proteins was also tested in Nicotiana benthamiana epidermal leaf cells using Agro-infiltration, as described previously71. The overnight grown culture of Agrobacterium containing desired constructs were pelted down and resuspended in infiltration buffer (10 mM MgCl2; 10 mM MES and 100 µM Acetosyringone, pH 5.6) to an OD600 of 0.6. Thereafter, infiltration was carried out on the abaxial side of 4-weeks old N. benthamiana leaves. Infiltrated plants were kept under dark for 24 h followed by 18 h light and 6 h dark cycle for 2 days in a plant growth chamber. Fluorescence was detected using following parameters: YFP (λ ex514 nm, λ em530-560) and FM4-64 (λ ex543 nm, λ em560). Confocal images were analysed using LAS AF Lite software (Leica Microsystems). At least two independent lines were tested to establish the localization.
Elicitor treatments
Seeds of B. rapa were sterilized using 0.05% HgCl2 and washed thoroughly using sterile distilled water. Sterile seeds were then transferred on 0.5 × (MS) medium containing 0.8% (w/v) agar and 3% (w/v) sucrose. Seeds were germinated under controlled Brassica growth conditions for four days under light and dark. Subsequently, uniformly grown seedlings were then adapted for 24 h in 0.5X liquid MS medium containing 1% sucrose (except for D-glucose treatment) before feeding with different phytohormones (100 µM IAA, 100 µM GA, 100 µM BAP, 100 µM ABA, 100 µM ACC and 1 µM BR); D-glucose (3%); stress and elicitors (42 °C heat, 4 °C cold, 200 mM NaCl, 200 μM MeJA, 200 μM SA, 300 μM Cu2+ as CuCl2, 500 μM Zn2+ as ZnCl2, 500 μM Mn2+ as MnCl2, 80 μM Cd2+ as CdCl2, 300 μM Pb2+ as PbCl2 and 300 μM As (V) as Na2HAsO4) each for 1, 3, 6, 12 and 24 h as described previously41,53. The untreated seedlings of each time points served as respective controls.
References
Wettschureck, N. & Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiology Review 85, 159–1204 (2005).
Urano, D., Chen, J. G., Botella, J. R. & Jones, A. M. Heterotrimeric G protein signalling in the plant kingdom. Open Biol. 3, 120186 (2013).
Gilman, A. G. G proteins: transducers of receptor-generated signals. Ann. Rev. Biochem. 56, 615–649 (1987).
Offermanns, S. G-proteins as transducers in transmembrane signalling. Prog. Biophys. Mol. Biol. 83, 101–130 (2003).
Sprang, S. R. G protein mechanisms: insights from structural analysis. Ann. Rev. Biochem. 66, 639–678 (1997).
Siderovski, D. P. & Willard, F. S. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int. J. Biol. Sci. 1, 51–66 (2005).
Urano, D. et al. G protein activation without a GEF in the plant kingdom. PLoS Genetics 8, e1002756 (2012).
Bisht, N. C., Joseph, M. J. & Pandey, S. An elaborate heterotrimeric G-protein family from soybean expands the diversity of plant G-protein networks. New Phytol. 190, 35–48 (2011).
Trusov, Y., Chakravorty, D. & Botella, J. R. Diversity of heterotrimeric G-protein γ subunits in plants. BMC Research Note 5, 608 (2012).
Arya, G. C., Kumar, R. & Bisht, N. C. Evolution, expression differentiation and interaction specificity of heterotrimeric G-protein subunit gene family in the mesohexaploid Brassica rapa. PLoS ONE 9, e105771 (2014).
Johnston, C. A. et al. GTPase acceleration as the rate-limiting step in Arabidopsis G protein coupled sugar signaling. Proc. Natl. Acad. Sci. USA 104, 17317–17322 (2007).
Jones, J. C. et al. The crystal structure of a self-activating G protein α subunit reveals its distinct mechanism of signal initiation. Sci. Signal. 4, ra8 (2011).
Jones, J. C., Jones, A. M., Temple, B. R. & Dohlman, H. G. Differences in intradomain and interdomain motion confer distinct activation properties to structurally similar Gαproteins. Proc. Natl. Acad. Sci. USA 109, 7275–7279 (2012).
Johnston, C. A., Willard, M. D., Kimple, A. J., Siderovski, D. P. & Willard, F. S. A sweet cycle for Arabidopsis G-proteins: Recent discoveries and controversies in plant G-protein signal transduction. Plant Signal. Behav. 3, 1067–1076 (2008).
Chen, J. G. et al. A seven transmembrane RGS protein that modulates plant cell proliferation. Science 301, 1728–1731 (2003).
Willard, F. S. & Siderovski, D. P. Purification and in vitro functional analysis of the Arabidopsis thaliana regulator of G-protein signaling-1. Methods Enzymol. 389, 320–38 (2004).
Bommert, P., Je, B. I., Goldshmidt, A. & Jackson, D. The maize Gα gene COMPACT PLANT2 functions in CLAVATA signalling to control shoot meristem size. Nature 502, 555–558 (2013).
Liu, J. et al. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol. 161, 2146–2158 (2013).
Ishida, T. et al. Heterotrimeric G proteins control stem cell proliferation through CLAVATA signaling in. Arabidopsis. EMBO Rep. 15, 1202–1209 (2014).
Aranda-Sicilia, M. N. et al. Heterotrimeric G proteins interact with defense-related receptor-like kinases in Arabidopsis. J. Plant Physiol. 188, 44–148 (2015).
Yu, T. Y. et al. The Arabidopsis Receptor Kinase ZAR1 is required for zygote asymmetric division and its daughter cell fate. PLoS Genet. 12, e1005933 (2016).
Liang, X. et al. Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. Elife 5, e13568 (2016).
Ashikari, M., Wu, J., Yano, M., Sasaki, T. & Yoshimura, A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 96, 10284–10289 (1999).
Ueguchi-Tanaka, M. et al. Rice dwarf mutantd1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 97, 11638–11643 (2000).
Ullah, H. et al. The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15, 393–409 (2003).
Llorente, F., Alonso-Blanco, C., Sanchez-Rodriguez, C., Jorda, L. & Molina, A. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J. 43, 165–180 (2005).
Trusov, Y. et al. Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol. 140, 210–220 (2006).
Chen, J. G., Gao, Y. & Jones, A. M. Differential roles of Arabidopsis heterotrimeric G-Protein subunits in modulating cell division in roots. Plant Phsyiol. 141, 887–897 (2006).
Trusov, Y. et al. Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell 19, 1235–1250 (2007).
Trusov, Y. et al. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J. 58, 69–81 (2009).
Chakravorty, D. et al. An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J. 67, 840–851 (2011).
Utsunomiya, Y. et al. Suppression of the rice heterotrimeric G protein β-subunit gene, RGB1, causes dwarfism and browning of internodes and lamina joint regions. Plant J. 67, 907–916 (2011).
Thung, L., Trusov, Y., Chakravorty, D. & Botella, J. R. Gγ1+Gγ2+Gγ3 = Gβ: the search for heterotrimeric G-protein γ subunits in Arabidopsis is over. J. Plant Physiol. 169, 542–545 (2012).
Delgado-Cerezo, M. et al. Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant. 5, 98–114 (2012).
Fujisawa, Y. et al. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci. USA 96, 7575–7580 (1999).
Urano, D. et al. Plant morphology of heterotrimeric G protein mutants. Plant Cell Physiol. 57, 437–445 (2016).
Choudhury, S. R. & Pandey, S. Specific subunits of heterotrimeric G proteins play important roles during nodulation in soybean. Plant Physiol. 162, 522–533 (2013).
Augustine, R., Arya, G. C., Nambiar, D. M., Kumar, R. & Bisht, N. C. Translational genomics in Brassica crops: challenges, progress, and future prospects. Plant Biotech. Rep. 8, 65–81 (2013).
Lysak, M. A., Cheung, K., Kitschke, M. & Bures, P. Ancestral chromosomal blocks are triplicated in Brassiceae species with varying chromosome number and genome size. Plant Physiol. 145, 402–410 (2007).
Mun, J. H. et al. Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biol. 10, R111 (2009).
Wang, X. X. et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genetics 43, 1035–1039 (2011).
Cheng, F., Wu, J. & Wang, X. Genome triplication drove the diversification of Brassica plants. Hort. Res. 1, 14024 (2014).
Kumar, R., Arya, G. C. & Bisht, N. C. Differential expression and interaction specificity of the heterotrimeric G-protein family in Brassica nigra reveal their developmental- and condition-specific roles. Plant Cell Physiol. 55, 1954–1968 (2014).
Ostergaard, L. & King, G. J. Standardized gene nomenclature for the Brassica genus. Plant Methods 4, 10 (2008).
Choudhury, S. R. et al. Two chimeric regulators of G-protein signaling (RGS) proteins differentially modulate soybean heterotrimeric G-protein cycle. J. Biol. Chem. 287, 17870–17881 (2012).
Hackenberg, D. et al. Gα and regulator of G-protein signaling (RGS) protein pairs maintain functional compatibility and conserved interaction interfaces throughout evolution despite frequent loss of RGS proteins in plants. New Phytol. 216, 562–575 (2017).
Temple, R. S. & Jones, A. M. The plant heterotrimeric G-protein complex. Ann. Rev. Plant Biol. 58, 249–266 (2007).
Cheng, F. et al. Biased gene fractionation and dominant gene expression among the subgenomes of Brassica rapa. PLoS ONE 7, e36442 (2012).
Kimple, A. J., Bosch, D. E., Giguère, P. M. & Siderovski, D. P. Regulators of G-protein signaling and their Gα substrates: promises and challenges in their use as drug discovery targets. Pharmacol. Rev. 63, 728–749 (2011).
Choudhury, S. R. & Pandey, S. The role of PLDα1 in providing specificity to signal-response coupling by heterotrimeric G-protein components in Arabidopsis. Plant J. 86, 50–61 (2016).
Choudhury, S. R. & Pandey, S. Phosphatidic acid binding inhibits RGS1 activity to affect specific signaling pathways in Arabidopsis. Plant J. 90, 466–477 (2017).
Chandna, R. et al. Class-specific evolution and transcriptional differentiation of 14-3-3 family members in mesohexaploid Brassica rapa. Front. Plant Sci. 7, 12 (2016).
Urano, D. & Jones, A. M. Heterotrimeric G protein-coupled signaling in plants. Ann. Rev. Plant Biol. 65, 365–384 (2014).
Lambert, N. A. et al. Regulators of G-protein signaling accelerate GPCR signaling kinetics and govern sensitivity solely by accelerating GTPase activity. Proc. Natl. Acad. Sci. USA 107, 7066–7071 (2010).
Sharma, S. et al. Two plastid DNA lineages–Rapa/Oleracea and Nigra–within the tribe Brassiceae can be best explained by reciprocal crosses at hexaploidy: evidence from divergence times of the plastid genomes and R-block genes of the A and B genomes of Brassica juncea. PLoS One 9, e93260 (2014).
Schmutz, J. et al. Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183 (2010).
Adams, K. L., Cronn, R., Percifield, R. & Wendel, J. F. Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. Proc. Natl. Acad. Sci. USA 100, 4649–4654 (2003).
Roulin, A. et al. The fate of duplicated genes in a polyploid plant genome. Plant J. 73, 143–153 (2013).
Yoo, M. J., Liu, X., Pires, J. C., Soltis, P. S. & Soltis, D. E. Nonadditive gene expression in polyploids. Ann. Rev. of Genet. 48, 485–517 (2014).
Pandey, S., Chen, J. G., Jones, A. M. & Assmann, S. M. G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and post germination development. Plant Physiol. 141, 243–256 (2006).
Urano, D. et al. Endocytosis of the seven-transmembrane RGS1 protein activates G-protein-coupled signalling in Arabidopsis. Nat. Cell Biol. 14, 1079–88 (2012).
Colaneri, A. C., Tunc-Ozdemir, M., Huang, J. P. & Jones, A. M. Growth attenuation under saline stress is mediated by the heterotrimeric G protein complex. BMC Plant Biol. 14, 129 (2014).
Kunihito, S. et al. Rice DEP1, encoding a highly cysteine-rich G protein γ subunit, confers cadmium tolerance on yeast cells and plants. J. Exp. Bot. 64, 4517–4527 (2013).
Tamura, K. et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Koch, M. A., Haubold, B. & Mitchell-Olds, T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol. Biol. Evol. 17, 1483–1498 (2000).
Obrdlik, P. et al. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA 101, 12242–12247 (2004).
Rossignol, P., Collier, S., Bush, M., Shaw, P. & Doonan, J. H. Arabidopsis POT1A interacts with TERT-V(I8), an N-terminal splicing variant of telomerase. J Cell Sci. 120, 3678–3687 (2007).
Jez, J. M. & Cahoon, R. E. Kinetic mechanism of glutathione synthetase from Arabidopsis thaliana. J. Biol. Chem. 279, 42726–42731 (2004).
Choudhury, S. R., Westfall, C. S., Hackenberg, D. & Pandey, S. Measurement of GTP-binding and GTPase activity of heterotrimeric Gα proteins. Methods Mol Biol. 1043, 13–20 (2013).
Earley, K. W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006).
Goodin, M. M., Dietzgen, R. G., Schichnes, D., Ruzin, S. & Jackson, A. O. pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31, 375–83 (2002).
Acknowledgements
The central instrumentation facility and plant growth facility at National Institute of Plant Genome Research, India is acknowledged. The work was supported by the core grant of National Institute of Plant Genome Research (NIPGR). RK was funded with the Senior Research Fellowship by the University Grant Commission (India) and NIPGR.
Author information
Authors and Affiliations
Contributions
R.K. and N.C.B. planned and designed the research; R.K. performed experiments, analyzed and interpreted data; R.K. and N.C.B. wrote and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, R., Bisht, N.C. Duplicated RGS (Regulator of G-protein signaling) proteins exhibit conserved biochemical but differential transcriptional regulation of heterotrimeric G-protein signaling in Brassica species. Sci Rep 8, 2176 (2018). https://doi.org/10.1038/s41598-018-20500-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20500-3
This article is cited by
-
Impact of biotic stresses on the Brassicaceae family and opportunities for crop improvement by exploiting genotyping traits
Planta (2024)
-
The multifaceted roles of heterotrimeric G-proteins: lessons from models and crops
Planta (2022)
-
A complex interplay of Gβ and Gγ proteins regulates plant growth and defence traits in the allotetraploid Brassica juncea
Plant Molecular Biology (2021)
-
Heterotrimeric Gα subunit regulates plant architecture, organ size and seed weight in the oilseed Brassica juncea
Plant Molecular Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.