Abstract
To improve the novel Bacillus thuringiensis insecticidal gene cry2Ah1 toxicity, two mutants cry2Ah1-vp (V354VP) and cry2Ah1-sp (V354SP) were performed. SWISS-MODEL analysis showed two mutants had a longer loop located between β-4 and β-5 of domain II, resulting in higher binding affinity with brush border membrane vesicles (BBMV) of Helicoverpa armigera comparing with Cry2Ah1. The cry2Ah1, cry2Ah1-vp, and cry2Ah1-sp were optimized codon usage according to plant codon bias, and named mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp. They were transformed into tobacco via Agrobacterium-mediated transformation and a total of 4, 8, and 24 transgenic tobacco plants were obtained, respectively. The molecular detection showed the exogenous gene was integrated into tobacco genome, and successfully expressed at the transcript and translation levels. Cry2Ah1 protein in transgenic tobacco plants varied from 4.41 to 40.28 μg g−1 fresh weight. Insect bioassays indicated that all transgenic tobacco plants were highly toxic to both susceptible and Cry1Ac-resistant cotton bollworm larvae, and the insect resistance efficiency to Cry1Ac-resistant cotton bollworm was highest in mcry2Ah1-sp transgenic tobacco plants. The results demonstrated that cry2Ah1 was a useful Bt insecticidal gene to susceptible and Cry1Ac-resistant cotton bollworm and had potential application for insect biocontrol and as a candidate for pyramid strategy in Bt crops.
Similar content being viewed by others
Introduction
Genetically engineered crops expressing insecticidal proteins from the Bacillus thuringiensis (Bt) have been used commercially since 19961,2,3. Bt crops have been proved that successfully apply plant genetic engineering technology to protect the economically important crops from infestation4. The wide use of transgenic Bt crops has reduced production costs and insecticide use, but analysis of more than a decade of global monitoring data reveals that the frequency of resistance alleles has increased substantially in some field population of Helicoverpa zea5, and some major pests which are resistant to Bt insecticidal proteins have been selected in the laboratory6,7. Thus the evolution of insects resistant to transgenic Bt crops presents a challenge.
Several deployment tactics design to delay resistance have been proposed. These strategies include the following: moderate toxin dosage to ensure survival of fraction of susceptible inserts, high toxin dosage to kill insects heterozygous for resistance, temporal or tissue-specific toxin expression, provision of nontoxic plants (refuge strategy) and combination/stacking/pyramid of toxins (pyramid strategy)2. The “refuge” and “pyramid” strategies are widely used among these strategies. To compare with “pyramid” strategy, “refuge” strategy is affected by more factors. At the present stage, “pyramid” strategy appears to be the best way to delay resistance2, but the stacking toxins should have different binding sites at pest midgut membrane to reduce the likelihood of cross-resistance8,9,10. Research indicated that the transgenic cotton Bollgard II (Monsanto 15985) expressing two Bt proteins (Cry1Ac+Cry2Ab) is found to be more toxic to lepidopterous pests than Bollgard (DP50B) expressing only one Bt protein (Cry1Ac)11. One of the most popular pyramided Bt traits in the commercial application is Genuity\(R\) SmartStaxTM which expresses six Bt proteins: Cry1A.105/Cry2Ab2 (MON 89034), Cry1F (TC1507), Cry3Bb1 (MON 88017), Cry34/35Ab1 (DAS-59122)12.
So far, there are a large number of Cry genes to be cloned, but only a few of them are used commercially in Bt crops13. Thus it is important to explore new highly toxic Bt genes for future Bt crop development. cry2Ah1 is a novel Bacillus thuringiensis insecticidal gene that was obtained by a pooled clone method from soil samples14. Previous study indicated that the Cry2Ah1 protein purified from Escherichia coli had a weight loss activity against Ostrinia furnacalis, and a growth inhibitory activity to both susceptible and Cry1Ac-resistant Helicoverpa armigera populations14. The Cry2Ah1 protein had a growth inhibitory activity to Cry1Ac-resistant cotton bollworm which implied the binding site of Cry2Ah1 might be different to the Cry1Ac in the cotton bollworm. Cry2Ah1-vp (V354VP) and Cry2Ah1-sp (V354SP) were mutanted from Cry2Ah1 by insertion of Proline behind Valine354 and replacement of Valine354 by Serine and Proline, respectively. Thus, they had the same carbon skeleton with Cry2Ab in mutation location. The Cry2Ah1 was 93.88% sequence identity with Cry2Ab which had been widely used commercially in Bt crops15. Similar to Cry2Ab, Cry2Ah1 also had a growth inhibitory activity to some key lepidopteran pests, such as H. armigera and O. furnacalis14. The cry2Ah1 gene and mutated cry2Ah1 genes have been patented.
In this study, we transformed codon-optimized cry2Ah1, cry2Ah1-vp, and cry2Ah1-sp genes into tobacco plants, respectively. Analysis showed that over-expression of mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp genes conferred transgenic tobacco plants with a high resistance to both susceptible and Cry1Ac-resistant cotton bollworm and the insect resistance efficiency to Cry1Ac-resistant cotton bollworm was highest in mcry2Ah1-sp transgenic tobacco plants. This suggested that cry2Ah1 gene and mutants, especially cry2Ah1-sp, could potentially be candidate gene to develop novel stacking traits Bt crops.
Results
Toxicity and binding affinity of Cry2Ah1 protein and mutants with BBMV
In previous studies14,16, the effective concentration 50 (EC50) of the Cry2Ah1 protein purified from E. coli was 8.74 μg mL−1 to O. furnacalis, 8.70 μg mL−1 to susceptible H. armigera, and 15.30 μg mL−1 to Cry1Ac-resistant H. armigera, respectively. The EC50 of Cry2Ah1-vp and Cry2Ah1-sp was 5.68 μg mL−1 and 1.63 μg mL−1 to susceptible H. armigera, respectively (Table 1). These results demonstrated Cry2Ah1-sp protein had the highest toxicity to susceptible H. armigera among three proteins. Furthermore, the binding of Cry2Ah1, Cry2Ah1-vp or Cry2Ah1-sp proteins with BBMV from H. armigera was measured by enzyme-linked immunosorbent assay (ELISA). The binding curve of Cry2Ah1 showed that there was no binding with BBMV, while Cry2Ah1-vp and Cry2Ah1-sp showed significant binding with BBMV but they were unsaturable (Fig. 1). This results suggested that Cry2Ah1 did not interact with BBMV, but its two mutants increased binding affinity with BBMV partially by insertion of Proline behind Valine354 and replacement of Valine354 by Serine and Proline.
SWISS-MODEL analysis of Cry2Ah1 and mutants three-dimensional structure
To localize the mutation region of Cry2Ah1-vp or Cry2Ah1-sp in structure, SWISS-MODEL (https://swissmodel.expasy.org) was employed to construct three-dimension structure by using the crystal structure of Cry2Aa (Fig. 2). The structures showed that Cry2Ah1-vp and Cry2Ah1-sp mutants had a longer loop located between β-4 and β-5 of domain II, which might contribute the higher growth inhibitory activity to H. armigera comparing with Cry2Ah1. These data suggested loop region between β-4 and β-5 of domain II was important but not sufficient for Cry2Ah1 binding with BBMV.
Simulation of three-dimensional structure of Cry2Ah1, Cry2Ah1-vp, and Cry2Ah1-sp proteins constructed by SWISS-MODEL (https://swissmodel.expasy.org). The homology gene Cry2Aa sequence was used as template for modeling. The images are licensed under the CC BY-SA 4.0 Creative Commons Attribution-ShareAlike 4.0 International License (https://creativecommons.org/licenses/by-sa/4.0/legalcode) and no change was made.
Acquisition and identification of transgenic tobacco plants
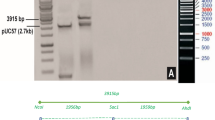
Agrobacterium tumefaciens EHA105 carrying pCSm2Ah1N-LR, pCSm2Ah1vpN-LR, pCSm2Ah1spN-LR were used for transformation of tobacco NC89 leaf disks (Fig. 3). A total of 29, 45, and 62 regenerated tobacco plants were obtained, respectively. Out of these, 15, 28, and 46 regenerated tobacco plants were found to carry the mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp gene as can be detected by PCR assays (Table 2 and Fig. 4a). Genomic DNA of wild-type and transgenic tobacco plants was digested with Hind III, for which there were no recognition sites in the probe region (Fig. 3). The Southern hybridization result showed that the mcry2Ah1 genes were integrated into different sites of tobacco genome as a single copy (Fig. 4b, m2Ah1vp-21) or multiple copies (Fig. 4b, m2Ah1sp-15, 78,79, m2Ah1vp-34, m2Ah1–57) in transgenic tobacco plants and hybridization signal was not detected in the wild-type plants (Fig. 4b, N). PCR and southern hybridization results confirmed that the exogenous genes were transformed and integrated into the tobacco genome.
Schematic diagram of the transformation vectors. RB: right border of the T-DNA, 35 S: cauliflower mosaic virus 35 S promoter, mcry2Ah1, mcry2Ah1-vp, mcry2Ah1-sp represent modified cry2Ah1 alleles, NOS: nopaline synthase gene terminator, LB: left border of the T-DNA, nptII: neomycin phosphotransferase II, a 862-bp fragment of the mcry2Ah1 gene used as probe in Southern blot analysis.
PCR and Southern blot analysis of the modified cry2Ah1 alleles in transgenic tobacco plants. (a) PCR analysis of the mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp genes in corresponding transgenic tobacco plants. M: DNA marker, P: positive control, N: wild-type tobacco plants, (b) Southern blot analysis of six transgenic and wild-type tobacco plants. P: positive control, N: wild-type tobacco plants. The original full-length pictures were shown in Supplementary Figure S1.
Quantitation of Cry2Ah1 and mutants proteins in transgenic tobacco plants
ELISA was used to quantitative detection of Cry2Ah1 protein expression in the above-mentioned 89 PCR-positive transgenic tobacco plants. Each treatment sample was carried on three technical replicates. Quantitative analysis revealed that 4 of mcry2Ah1, 8 of mcry2Ah1-vp, and 24 of mcry2Ah1-sp transgenic tobacco plants had a high Cry2Ah1 protein expression levels and the quantity of Cry2Ah1 endotoxin varied from 4.41 to 40.28 μg g−1 fresh weight (Table 3 and Fig. 5). The statistical analysis indicated that the Cry2Ah1 protein expression in mcry2Ah1-sp and mcry2Ah1-vp plants was not significantly different, but they were significantly higher than mcry2Ah1 plants (Table 4).
Relative quantitation of mcry2Ah1 transcript levels
The mcry2Ah1 transcript levels of 36 transgenic lines which had a high Cry2Ah1 protein expression were confirmed by quantitative RT-PCR. The tobacco actin1 gene was used as a reference gene; the m2Ah1sp-43 transgenic tobacco plant was used as a calibrator. Each sample was carried on three technical replicates. The data were analyzed by 2−△△Ct method. Quantitative RT-PCR results showed that the mcry2Ah1 alleles were successfully transcripted in transgenic tobacco plants and the transcript levels were varied among the different transgenic lines (Fig. 6).
Bioassay of transgenic tobacco plants harboring cry2Ah1 gene and mutants
To study whether transgenic tobacco producing Cry2Ah1 toxin could show the toxicity to the susceptible or Cry1Ac-resistant cotton bollworm, a total of 36 transgenic lines which had a high Cry2Ah1 protein expression were assessed along with two wild-type tobacco plants for control. The numbers of the living and dead cotton bollworm larvae were recorded every day and photographed the leaf damage at the end. The corrected mortality of cotton bollworm larvae was analyzed to assess the effect of the Cry2Ah1 protein on the larvae. After three days of infestation with the neonatal larvae, the transgenic tobacco plants showed significant resistance to both susceptible and Cry1Ac-resistant cotton bollworm compared to wild-type plants (Fig. 7). The wild-type leaves were badly damaged and most larvae were survived (Fig. 7, WT-1, 2). On the contrary, all transgenic plants leaves exhibited slightly damage and the vast majority of larvae were died and the growth of surviving larvae was significantly inhibited (Fig. 7). The corrected mortality rates of susceptible cotton bollworm larvae feeding on mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp plants were 95.30–100%, 100%, and 100%, respectively (Table 3). The statistical analysis showed that the corrected mortality rates of susceptible cotton bollworm larvae feeding on mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp plants were not significantly different (Table 4). To Cry1Ac-resistant cotton bollworm, the corrected mortality rates of larvae feeding on mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp plants after 3 days of infestation were 90.84–100%, 92.09–100% and 96.34–100%, respectively (Table 3). The statistical analysis showed that the corrected mortality rates of Cry1Ac-resistant cotton bollworm larvae feeding on mcry2Ah1 and mcry2Ah1-sp plants were significantly different; the mcry2Ah1-sp plants had a higher toxic than mcry2Ah1 plants. The corrected mortality rates of Cry1Ac-resistant cotton bollworm larvae were no significant difference between mcry2Ah1-vp and mcry2Ah1, mcry2Ah1-vp and mcry2Ah1-sp, but the corrected mortality rates feeding on mcry2Ah1-vp plants were higher than mcry2Ah1, lower than mcry2Ah1-sp. (Table 4). The results of the insect bioassays indicated that all transgenic tobacco plants were toxic to both susceptible and Cry1Ac-resistant cotton bollworm, the insect resistance efficiencies to susceptible cotton bollworm were not different among mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp plants, the insect resistance efficiency to Cry1Ac-resistant cotton bollworm was highest in mcry2Ah1-sp plants followed by mcry2Ah1-vp and mcry2Ah1 plants, respectively.
The appearance of control and transgenic tobacco leaves after insect bioassays with susceptible and Cry1Ac-resistant cotton bollworm. Leaves WT-1 and WT-2 were from wild-type tobacco plants, leaves m2Ah1-27 and m2Ah1-36 were from mcry2Ah1 plants, leaves m2Ah1vp-9, m2Ah1vp-10, m2Ah1vp-21, and m2Ah1vp-34 were from mcry2Ah1-vp plants, leaves m2Ah1sp-6, m2Ah1sp-15, m2Ah1sp-78, and m2Ah1sp-79 were from mcry2Ah1-sp plants. Photographs were taken after 3 days of infestation.
Discussion
The cry2Ah1 is a novel Bacillus thuringiensis insecticidal gene that was obtained by a pooled clone method from soil samples14. It encoded a polypeptide of 632 amino acids with three domains which had 18.50%, 19.62%, and 93.88% sequence identity with Cry1Ab (AAA22561.1), Cry1Ac (AAA22331.1), and Cry2Ab (ABC95996.1), respectively. The previous research show that Cry2Ah1 protein purified from E. coli has a growth inhibitory activity to lepidopteran pests, including O. furnacalis and H. armigera14. Two mutants of Cry2Ah1, Cry2Ah1-vp and Cry2Ah1-sp, also have a growth inhibitory activity to H. armigera16.
In order to verify the insecticidal activity of Cry2Ah1 in plants, we modified the cry2Ah1 gene according to the plant codon bias (named mcry2Ah1) and created its two mutants (mcry2Ah1-vp, mcry2Ah1-sp) by overlap-PCR. We generated transgenic tobacco lines expressing the mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp alleles via Agrobacterium-mediated transformation. The results of molecular detection showed that the cry2Ah1 gene was inserted into the genome of tobacco and could be expressed normally (Figs 4, 5 and 6). In our study, we found the transcript and translation levels did not show a similar trend in transgenic tobacco plants. The correlation of mRNA-protein is not strictly linear due to the synthesis and degradation rates may affect the amount of mRNA and protein differentially17. Insect bioassays showed the transgenic tobacco plants harboring the mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp were highly toxic to the susceptible cotton bollworm larvae, the corrected mortality rates were not significantly different among them (Table 4). The results indicated that the cry2Ah1 was a highly toxic Bt gene, it could be used for the development of Bt crops. In addition, unlike purified from E. coli, the Cry2Ah1 protein expressing in tobacco plants had a high insecticidal activity to cotton bollworm. It was possible that the Cry2Ah1 protein purified from E. coli did not have the right active form.
In the two decades leading up to the first commercial release of Bt plants, an unprecedented cumulative hectarage of 2 billon hectares are successfully cultivated globally3. In China, Bt cotton producing Cry1Ac has been commercialized for 20 years18, and now the adoption rate of transgenic Bt cotton is nearly 96%3. As a result of Bt genes were used commercially, the evolution of resistance by pests is the primary threat to the continued efficacy of Bt crops5,19,20,21,22. Many reports have shown that mutations in midgut receptors are related to Cry1A resistance in Heliothis virescens, H. armigera, and Pectinophora gossypiella7,23. Therefore, it is important to identify new Bt proteins for the sustainable use of Bt crops, which should have different modes of action. Although the EC50 of Cry2Ah1 is lower than Cry1Ac to O. furnacalis or H. armigera24, the previous research showed Cry2Ah1 protein purified from E.coli is toxic to Cry1Ac-resistant cotton bollworm14. In the study, the transgenic tobacco lines producing the Cry2Ah1, Cry2Ah1-vp, and Cry2Ah1-sp were highly toxic to Cry1Ac-resistant cotton bollworm and Cry2Ah1-sp had the highest insecticidal activity. It confirmed that Cry2Ah1 did not compete for the Cry1Ac binding site in the cotton bollworm. The Cry1Ac-resistant population of H. armigera was provided by Wu’s lab. The mechanisms of resistance to Cry1Ac were some receptors’ mutation, such as mutation disrupting an ATP-binding cassette protein (ABCC2), mutations disrupting the domains of cadherin, mutation reducing aminopeptidase N (APN) activity, and unidentified genes25,26,27,28. This suggested that cry2Ah1, especially cry2Ah1-sp, might be a good candidate for development of stack trait Bt crops.
The insect resistance efficiency to Cry1Ac-resistant cotton bollworm was highest in mcry2Ah1-sp transgenic tobacco plants (Table 4), indicated that the Cry2Ah1-sp protein had the highest toxicity among Cry2Ah1, Cry2Ah1-vp, and Cry2Ah1-sp. The differences were 354th amino acid located in loop between β-4 and β-5 of domain II among Cry2Ah1, Cry2Ah1-vp, and Cry2Ah1-sp. The loops of Cry protein domain II are always involved in the specificity of toxin binding to the receptor29,30. Mutations of amino acids in domain II may have either a negative or positive effect on binding and toxicity31. Substitution of domain II residue N372 with Ala or Gly (N372A, N372G) increase the toxicity of Cry1Ab against gypsy moth larvae 8-fold and enhance binding affinity to gypsy moth midgut BBMV about 4-fold32. In this study, we found Cry2Ah1 was no binding affinity with BBMV of H. armigera, while Cry2Ah1-vp (V354VP) and Cry2Ah1-sp (V354SP) showed significant binding affinity with BBMV (Fig. 1), resulting in significantly increased the toxicity of Cry2Ah1-vp and cry2Ah1-sp against Cry1Ac-resistant cotton bollworm larvae. This change made the mutants had a longer loop located between β-4 and β-5 of domain II and enhance the binding affinity to BBMV (Fig. 2).
Overall, this study clearly showed that cry2Ah1 and mutants was a useful Bt insecticidal gene to lepidopteran larve. Its high level of toxicity to lepidopteran larve and no cross-resistance with Cry1Ac made it potentially useful for insect biocontrol and as a candidate for pyramid strategy in Bt crops.
Methods
Expression and purification of Cry2Ah1 protein and mutants
Expression of Cry2Ah1 protein and Cry2Ah1-vp and Cry2Ah1-sp in 300 mL LB medium of E. coli was induced with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at 16 °C overnight, and then cells were collected by centrifugation at 10,000 g for 8 min and the cell pellet was resuspended in 30 mL of binding buffer (20 mM Tris-HCl with 0.5 M NaCl and 50 mM imidazole, pH 8.0). Cells were then lysed by ultrasonication (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) for 6 min (70% power, 3-s pulse on, 5-s pulse off) and then centrifuged at 13,000 g for 15 min at 4 °C. Cry2Ah1 toxin and Cry2Ah1-vp and Cry2Ah1-sp were solubilized from inclusion bodies with 5 mL urea buffer (8 M urea, 10 mM Tris-HCl and 100 mM NaH2PO4). These three proteins were purified by Ni-affinity chromatography (GE Healthcare, Uppsala, Sweden), then preequilibrated with urea buffer. Nonspecifically adsorbed proteins were removed by washing with decreased concentration urea buffer (6 M urea, 4 M urea and 2 M urea) orderly. Proteins bound to the column were eluted with elution buffer (20 mM Tris-HCl, 500 mM NaCl, 250 mM imidazole, pH 8.0).
Preparation of brush border membrane vesicles (BBMV)
H. armigera (Hübner) midgut tissues from third instar larvaes were dissected and stored immediately at −70 °C. BBMV were prepared by the magnesium precipitation method as described by Wolfersberger et al.33 and stored at −70 °C until used. Purity of BBMV preparations was determined by estimating the enrichment of aminopeptidase N (APN) specific activity in the BBMV compared to that in initial midgut tissue homogenates as described by Lorence et al.34. Representative APN activity enrichment in the final BBMV preparations was 3- to 4-fold compared to initial midgut homogenates.
ELISA analysis of Cry2Ah1 protein and mutants with BBMV binding
In binding assay, ELISA plates, 96 wells, were incubated 12 h at 4 °C with 1 µg BBMV in PBS (pH 7.4), followed by 5 times wash with PBS. The plates were then incubated with PBST with 2% BSA for 2 h at 37 °C. The ELISA plates were incubated, subsequently, with different concentrations of Cry2Ah1, Cry2Ah1-vp or Cry2Ah1-sp proteins (0, 2.5, 5, 10, 20, 40 and 80 nM) for 1 h at 37 °C, after 5 times wash with PBST, then detected with anti-Cry2Ah antibody (1: 5,000 dilution) for 1 h at 37 °C. Goat-anti mouse secondary antibody was incubated with plate in dilution of 1: 20, 000 (Sigma-Aldrich, St. Louis, USA). The enzymatic activity was revealed with TMB Single-Component Substrate solusion (Solarbio, Beijing, China). The enzymatic reaction was stopped with 2 M HCl, and the absorbance was read at 450 nm. Binding data were analyzed and plotted with SigmaPlot v.12.5 software.
SWISS-MODEL analysis of Cry2Ah1 and mutants three-dimensional structure
The three-dimensional structures of Cry2Ah1 and mutants were calculated with the SWISS-MODEL Server (Swiss Institute of Bioinformatics, Lausanne, Switzerland, https://swissmodel.expasy.org)35,36. The homology gene Cry2Aa sequence was used as template for modeling. The images are licensed under the CC BY-SA 4.0 Creative Commons Attribution-ShareAlike 4.0 International License (https://creativecommons.org/licenses/by-sa/4.0/legalcode) and no change was made.
Codon-optimized of cry2Ah1 gene and construction of transformation vectors
According to plant codon usage pattern, we optimized the codon of cry2Ah1 (EU939453.1) gene. The variations of codon usage in the optimized cry2Ah1 gene are shown in Table 5. The GC content was increased from the original 34% to 61.6%. The sequence of optimized cry2Ah1 gene was synthesized by GenScript Corporation (Nanjing, China). The 1899 bp mcry2Ah1 gene was digested by BamH I (5′) and Kpn I (3′), and subsequently inserted into BamH I/Kpn I-digested binary vector pCSN-LR which was modified from pCAMBIA2300, with a CaMV35S promoter and Nos terminator. The resulting binary vector is named as pCSm2Ah1N-LR and has the nptII as plant selection marker gene. The mcry2Ah1-vp gene and mcry2Ah1-sp gene were cloned by overlap-PCR and constructed into pCSN-LR according to the protocol of mcry2Ah1, named pCSm2Ah1vpN-LR and pCSm2Ah1spN-LR, respectively.
Tobacco materials and transformation
Wild-type tobacco (NC89) was selected as experimental materials. The tobacco’s growth and tissue culture according to the protocol described by Li et al.37. Three plant expression vectors pCSm2Ah1N-LR, pCSm2Ah1vpN-LR, and pCSm2Ah1spN-LR were transformed into tobacco via Agrobacterium-mediated transformation38. Kanamycin (100 mg L−1) was the selection agent on Murashige and Skoog (MS) regeneration medium39. PhytoTechnology Laboratories (Shawnee Mission, KS, USA) provided the plant tissue culture reagents.
PCR and southern blot analysis
Genomic DNA was isolated from the leaf tissues by CTAB method40. The following PCR primers were used to detection of mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp genes: forward primer 5′-CCTCATCTTCCCGTC-3′, and reverse primer 5′-GTGTTGCTCTGCTCG-3′. The 20-μL reaction mixtures contained 100 ng of DNA template, 0.1 μM of each primer, 10 μL 2× Taq MasterMix (Dye) (CWBIO, Beijing, China). The PCR reaction conditions were as follows: 2 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 56 °C and 60 s at 72 °C. A final extension step (10 min at 72 °C) was added to all reactions. The PCR products were visualized and documented on Gel DocTM XR+ system (Bio-Rad Laboratories, California, USA). More than 30 μg of genomic DNA of wild-type and each transgenic tobacco plant were used for the southern blot analysis. They were digested with Hind III and electrophoresed on a 0.7% (w/v) agarose gel and blotted on HybondTM-N+ nylon membranes (GE Healthcare, UK). A 862-bp fragment of the mcry2Ah1 gene was used as probe in Southern blot analysis and it was amplified from pCSm2Ah1N-LR by PCR using the following primers: forward primer 5′- GAGTGGATGGAGTGGAAG-3′, and reverse primer 5′- CGATGTTTGGGAAGGTCT-3′. The probe DNA was labeled with DIG-11-dUTP using the PCR DIG Probe Synthesis Kit (Roche, Mannheim, Germany). The immunological detection was carried out as the protocol of DIG High Prime DNA labeling and Detection Starter Kit II (Roche, Mannheim, Germany). The image analysis was carried out in Tanon 5200 automatic chemiluminescence imaging analysis system (YPH-BIO, Beijing, China).
ELISA analysis of Cry2Ah1 protein expression in transgenic tobacco plants
QuantiPlateTM Kit for Cry2A (Envirologix, Portland, USA) was used for quantitative detection of Cry2Ah1 protein in fresh leaf tissues. 100 mg of tobacco leaf tissues were homogenized and used for detection of Cry2Ah1 protein as per the manufacturer’s protocol. The OD was measured at 450 nm using BioTek Elx808 (BioTek, Winooski, USA).
Quantitative RT-PCR analysis
Total RNA was isolated from 100 mg of leaf tissues using Trizol reagents (TransGen, Beijing, China). Approximately 2 μg of total RNA was used as a template for reverse transcription using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, USA) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using an Applied Biosystems® QuantStudio® 3 Real-Time PCR System (ThermoFisher Scientific, Waltham, USA). The tobacco actin (X63603.1) gene was used as a reference gene. All reactions were run as duplicates in 96-well plates. The 20-μL reaction mixtures contained 10 μL of 2× TransStart® Top Green qPCR SuperMix (TransGen, Beijing, China), 0.2 μM of each primer and 1 μL of cDNA template. The reaction conditions were as follows: 30 s at 95 °C, 40 cycles of 5 s at 95 °C and 34 s at 60 °C, followed by a melting curve analysis. Primer pair mcry2Ah1-qF1/R1 was used to detect mcry2Ah1, mcry2Ah1-vp, and mcry2Ah1-sp transcript, respectively, and primer pair actin-qF1/R1 was used to detect the actin1 transcript. The sequence of primers were mcry2Ah1-qF1: 5′-AGGGCGTACATGGTGAGC-3′, mcry2Ah1-qR1: 5′-GGATGGGGGAGATGGTGA-3′, actin-qF1: 5′-GGCATCATACATTTTACAACGAA-3′, actin-qR1: 5′-ATGGCGACATACATAGCAGGAGT-3′.
Cotton bollworm bioassays
To determine the resistance of transgenic tobacco plants to susceptible and Cry1Ac-resistant cotton bollworm (H. armigera). The neonatal larvae of susceptible and Cry1Ac-resistant cotton bollworm and the leaves of 1-month-old transgenic tobacco plants were used in the bioassay. The resistance level of Cry1Ac-resistant cotton bollworm reached 2917.33-fold41. Each fresh leaf was infested with 12 neonate insect larvae and kept in the rearing room at 25 °C, 16:8 light:dark period. The numbers of the living and dead cotton bollworm larvae were recorded every day. Each treatment was performed three biological replicates and wild-type tobacco plants (NC89) as a control. Mortality rates were presented as the proportion of dead larvae to total larvae applied (%). Corrected mortality rates were calculated in the equation (1).
References
Estruch, J. J. et al. Transgenic plants: an emerging approach to pest control. Nat Biotechnol. 15, 137–141 (1997).
Bates, S. L., Zhao, J. Z., Roush, R. T. & Shelton, A. M. Insect resistance management in GM crops: past, present and future. Nat Biotechnol. 23, 57–62 (2005).
James, C. 20th Anniversary (1996 to 2015) of the Global Commercialization of Biotech Crops and Biotech Crop Highlights in 2015 (ed. James, C.) 1, 73, (ISAAA, 2015).
Gatehouse, J. A. Biotechnological prospects for engineering insect-resistant plants. Plant physiol. 146, 881–887 (2008).
Tabashnik, B. E., Gassmann, A. J., Crowder, D. W. & Carriére, Y. Insect resistance to Bt crops: evidence versus theory. Nat Biotechnol. 26, 199–202 (2008).
Tabashnik, B. E. et al. Insect Resistance to Transgenic Bt Crops: Lessons from the Laboratory and Field. J Econ Entomol. 96, 1031–1038 (2003).
Tabashnik, B. E. et al. Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat Biotechnol. 29, 1128 (2011).
Roush, R. T. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philos Trans R Soc Lond B Biol Sci. 353, 1777–1786 (1998).
Pigott, C. R. & Ellar, D. J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev. 71, 255–281 (2007).
Pardo-Lopez, L., Soberon, M. & Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. Fems Microbiol Rev. 37, 3–22 (2013).
Chitkowski, R. L., Turnipseed, S. G., Sullivan, M. J. & Bridges, W. C. Jr. Field and laboratory evaluations of transgenic cottons expressing one or two Bacillus thuringiensis var. kurstaki Berliner proteins for management of noctuid (Lepidoptera) pests. J Econ Entomol. 96, 755–762 (2003).
Yang, F. et al. Occurrence, distribution, and ear damage of Helicoverpa zea (Lepidoptera: Noctuidae) in mixed plantings of non-Bt and Bt corn containing Genuity (R) SmartStaxTM traits. Crop Prot. 55, 127–132 (2014).
Bravo, A. & Soberón, M. How to cope with insect resistance to Bt toxins? Trends Biotechnol. 26, 573–579 (2008).
Shu, C. et al. Use of a pooled clone method to isolate a novel Bacillus thuringiensis Cry2A toxin with activity against Ostrinia furnacalis. J Invertebr Pathol. 114, 31–33 (2013).
Tabashnik, B. E. et al. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc Natl Acad Sci. 106, 11889–11894 (2009).
Shu, C. L. et al. Highly toxic to lepidoptera pests insecticidal gene cry2Ah-like and its application. CN 103204912 B (2013).
Jin, J. et al. Effect of Pre-Stressing on the Acid-Stress Response in Bifidobacterium Revealed Using Proteomic and Physiological Approaches. PLoS One. 10, e0117702 (2015).
Zhang, H. et al. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS One. 6, e22874 (2011).
Tabashnik, B. E. Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol. 39, 47–79 (1994).
Gould, F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol. 43, 701–726 (1998).
Soberón, M. et al. Engineering Modified Bt Toxins to Counter Insect Resistance. Science. 318, 1640–1642 (2007).
Meihls, L. N. et al. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc Natl Acad Sci. 105, 19177–19182 (2008).
Jurat-Fuentes, J. L. et al. Reduced levels of membrane-bound alkaline phosphatase are common to lepidopteran strains resistant to Cry toxins from Bacillus thuringiensis. PLoS One. 6, e17606 (2011).
Xue, J. et al. Cloning and characterization of a novel Cry1A toxin from Bacillus thuringiensis with high toxicity to the Asian corn borer and other lepidopteran insects. Fems Microbiol Lett. 280, 95–101 (2008).
Liu, C. X. et al. Cis-mediated down-regulation of a trypsin gene associated with Bt resistance in cotton bollworm. Sci Rep. 4, 7219 (2014).
Xiao, Y. T. et al. Mis-splicing of the ABCC2 gene linked with Bt toxin resistance in Helicoverpa armigera. Sci Rep. 4, 6184 (2014).
Chen, W. B. et al. A Toxin-Binding Alkaline Phosphatase Fragment Synergizes Bt Toxin Cry1Ac against Susceptible and Resistant Helicoverpa armigera. PLoS One. 10, https://doi.org/10.1371/journal.pone.0126288 (2015).
Xiao, Y. T. et al. Resistance to Bacillus thuringiensis Mediated by an ABC Transporter Mutation Increases Susceptibility to Toxins from Other Bacteria in an Invasive Insect. PLoS Pathog. 12, https://doi.org/10.1371/journal.ppat.1005450 (2016).
Zhou, Z. S. et al. Insecticidal Specificity of Cry1Ah to Helicoverpa armigera Is Determined by Binding of APN1 via Domain II Loops 2 and 3. Appl Environ Microbiol. 83, e02864–16 (2017).
Pacheco, S. et al. Domain II Loop 3 of Bacillus thuringiensis Cry1Ab Toxin Is Involved in a “Ping Pong” Binding Mechanism with Manduca sexta Aminopeptidase-N and Cadherin Receptors. J Biol Chem. 284, 32750–32757 (2009).
Schnepf, E. et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 62, 775–806 (1998).
Rajamohan, F., Alzate, O., Cotrill, J. A., Curtiss, A. & Dean, D. H. Protein engineering of Bacillus thuringiensis δ-endotoxin: Mutations at domain II of CryIAb enhance receptor affinity and toxicity toward gypsy moth larvae. Proc Natl Acad Sci. 93, 14338–14343 (1996).
Wolfersberger, M. et al. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp Biochem Physiol A Physiol. 86, 301–308 (1987).
Lorence, A., Darszon, A. & Bravo, A. Aminopeptidase dependent pore formation of Bacillus thuringiensis Cry1Ac toxin on Trichoplusia ni membranes. FEBS lett. 414, 303–307 (1997).
Biasini, M. et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42 (Web Server issue), W252–258, (2014).
Arnold, K., Bordoli, L., Kopp, J. & Schwede, T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006).
Li, X. et al. Chloroplast-targeted expression of the codon-optimized truncated cry1Ah gene in transgenic tobacco confers a high level of protection against insects. Plant Cell Rep. 32, 1299–1308 (2013).
Horsch, R. B. & Fraley, R. T. A simple and general method for transferring genes into plants. Science. 227, 1229–1231 (1985).
Gamborg, O. L., Murashige, T., Thorpe, T. A. & Vasil, I. K. Plant tissue culture media. In Vitro Cell Dev Biol – Plant. 12, 473–478 (1976).
Porebski, S., Bailey, L. G. & Baum, B. R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Report. 15, 8–15 (1997).
Zhang, W. et al. Fitness costs of reproductive capacity and ovarian development in a Bt-resistant strain of the cotton bollworm Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Pest Manag Sci. 71, 870–877 (2015).
Acknowledgements
The authors gratefully acknowledge the financial support of Genetically Modified Organisms Breeding Major Projects (No. 2016ZX08003-001) and China Postdoctoral Science Foundation funded project (No. 166721).
Author information
Authors and Affiliations
Contributions
Zhihong Lang, Shengyan Li and Zeyu Wang designed the experiments. Shengyan Li, Zeyu Wang, Yiyao Zhou, Changhui Li and Guiping Wang performed the experiments. Shengyan Li, Zeyu Wang and Hai Wang analyzed the data. Gemei Liang provided the cotton bollworm. Zhihong Lang, Shengyan Li, Zeyu Wang, Jie Zhang, and Gemei Liang wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, S., Wang, Z., Zhou, Y. et al. Expression of cry2Ah1 and two domain II mutants in transgenic tobacco confers high resistance to susceptible and Cry1Ac-resistant cotton bollworm. Sci Rep 8, 508 (2018). https://doi.org/10.1038/s41598-017-19064-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-19064-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.