Abstract
High-pressure non-invasive positive pressure ventilation (NPPV) is a new strategy targeted at maximally reducing arterial carbon dioxide. However, high inspiratory positive airway pressure (IPAP) might cause respiratory adverse events likely to diminish the benefit of NPPV. In the setting of ventilatory support, monitoring NPPV efficacy and resolving problems promptly are critical. This study assessed the treatment effect of high and low-pressure NPPV in chronic hypercapnic COPD using home ventilator with built-in software. In this pilot study, we investigated 34 patients using NPPV for 3 months. 13 patients used high-pressure ventilation and 21 patients used low-pressure ventilation. The primary outcome was daytime partial pressure of arterial blood carbon dioxide (PaCO2). There were no between-group differences in daytime PaCO2 and FEV1, but a trend favouring high-pressure NPPV was observed. Significant between-group differences were found in the transition dyspnoea index (TDI) (high-pressure, 1.69 ± 1.75, versus low-pressure, −0.04 ± 2.71, p = 0.044). No differences were found in usage time, leakage, health-related quality of life, spirometry, or 6-minute walk test. High-pressure NPPV with built-in software monitoring in patients with chronic hypercapnic COPD is associated with improvement in TDI scores and a positive trend in favour of high-pressure NPPV for improving PaCO2 is observed.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic airway disease characterized by incompletely reversible airflow limitation. Currently, COPD is the fourth major cause of death in the world. Advanced-stage COPD is the most common cause of dyspnoea and respiratory failure, with hypoxemia or hypercapnia resulting from respiratory muscle fatigue and alveolar hypoventilation. Non-invasive positive pressure ventilation (NPPV) is considered for patients with chronic hypercapnic COPD (Evidence B)1. NPPV can help patients reduce the respiratory muscle workload and increase the alveolar ventilation volume, thus rectifying hypercapnia, improving oxygenation, and relieving shortness of breath1,2. However, different investigators have drawn various—even opposing—conclusions3,4,5,6,7,8. The reasons for these disputed results are unclear, perhaps they result from the lower inspiratory pressures that had been used in some randomized controlled trials.
High-intensity NPPV refers to particular NPPV settings that are using assist/control mode aimed at maximally improving the partial pressure of arterial blood carbon dioxide (PaCO2), with inspiratory positive airway pressure (IPAP) 20–30 cm H2O9,10,11,12,13,14. Compared with low-intensity NPPV, high-intensity NPPV could significantly reduce PaCO2 and even improve pulmonary function11. Results from one study showed that high-intensity NPPV was able to better improve the gas exchange and reduce inspiratory effort, and it led to nearly a complete rest of the diaphragm15. Some authors have pointed out that high inspiratory pressure played a significant role in high-intensity NPPV and there was no additional benefit adding a high back-up rate to high-pressure NPPV16. So far, no definitive conclusion reached about whether high-pressure NPPV is the best approach for the long-term treatment of patients with hypercapnic COPD. In addition, when using high-pressure NPPV, high IPAP might lead to excessive leakage, patient-ventilator asynchrony, and other respiratory adverse events, as well as adverse effects on cardiac performance. These factors probably reduce patient compliance with treatment11,15,17,18. Hence, monitoring the efficacy of home NPPV and fixing problems promptly are of great significance.
The objective of this pilot study was to test the feasibility and compare the efficacy of high-pressure NPPV with that of low-pressure NPPV in patients with chronic hypercapnic COPD using a non-invasive home ventilator equipped with built-in software.
Results
A total of 34 patients were included in this study, 13 patients in the high-pressure group and 21 patients in the low-pressure group. Baseline demographics were similar in both treatment groups (Table 1). The mean IPAPs in the high-pressure and low-pressure groups were 21.15 ± 1.34 cm H2O and 14.93 ± 0.87 cm H2O, respectively. Treatment compliance was good in both groups. Non-invasive ventilator (NIV) use time in both groups was similar (high-pressure group, 362.41 ± 99.69 minutes versus low-pressure group, 343.55 ± 74.23 minutes; p = 0.538). More leakage was detected in the high-pressure group; however, no significant difference was discovered between groups (high-pressure group, 40.57 ± 12.52 L/min versus low-pressure group, 37.11 ± 11.95 L/min; p = 0.169).
For the primary outcome, no significant between-group difference could be found in daytime PaCO2 (high-pressure group, 47.40 ± 5.23 mmHg versus low-pressure group, 51.67 ± 7.40 mmHg, p = 0.058). A positive trend in the difference between both groups was noted and the same trend was seen in FEV1 (high-pressure group, 0.62 ± 0.11 L versus low-pressure group, 0.55 ± 0.21 L, p = 0.065). Moreover, the transition dyspnoea index (TDI) of the high-pressure group was improved, and a significant difference between both groups was observed (high-pressure group, 1.69 ± 1.75 versus low-pressure group, −0.04 ± 2.71, p = 0.044). In addition, the health-related quality of life (HRQL) (Severe Respiratory Insufficiency (SRI) and the COPD assessment test (CAT)) improved in both groups; however, no between-group differences were seen (SRI: high-pressure group, 54.43 ± 13.74 versus low-pressure group, 52.95 ± 10.28, p = 0.722; CAT: high-pressure group, 21.77 ± 5.92 versus low-pressure group, 22.24 ± 6.67, p = 0.837). (Table 2) There were no differences in arterial oxygen saturation, FVC, pH, or partial pressure of arterial blood oxygen (PaO2) between the high-pressure and low-pressure groups. Furthermore, the percentage of the changes in the 6-minute walk distance (6MWD) reached minimal clinically important difference of 30 m was similar in both groups: seven patients in the high-pressure group (53.85%) versus eight patients in the low-pressure group, (38.10%); p = 0.484.
Discussion
To the best of our knowledge, this is the first pilot study comparing the treatment effect between high-pressure NPPV and low-pressure NPPV in chronic hypercapnic COPD patients using a non-invasive home ventilator equipped with built-in software in Asia. The software allowed early identification and prompt resolution of adverse events during use. In this study, the association between high-pressure NPPV and the improvement in TDI scores was found when compared with low-pressure NPPV, however, no significant between-group differences were detected in leakage, compliance, daytime PaCO2, pulmonary function, HRQL, and exercise tolerance.
Interestingly, a between-group difference was observed in TDI. Also, FEV1 was shown to have a positive trend in improvement, probably because of airway dilatation or the anti-inflammatory effect of using NPPV19,20. Another essential reason might be the mitigation of airway oedema. Carbon dioxide retention can lead to vasodilation, which may result in oedema. Decreasing PaCO2 level after using home NPPV, this is especially true for high-pressure NPPV, which could restore daytime PaCO2 to the normal range in this study, thus might obtain the relevance of reducing airway oedema and improving the TDI2,19.
As expected, higher IPAP would lead to higher leakage; however, in contrast to other studies, no difference could be found between the two groups in our study. This observation probably was because a lower IPAP level had been used compared with other investigations11,15. Another reason might be that we closely monitored the usage of the ventilator at home and instructed or helped the patient to deal with the problem during the follow-up period if the leakage was too large. Similar to results reported by Dreher et al.11 and Duiverman et al.10, no significant between-group difference was noted in daytime PaCO2, but a positive tendency in favour of high-pressure NPPV was observed in our study. In addition, the mean values of daytime PaCO2 in the high-pressure group were in the normal range, while patients in the low-pressure group were still hypercapnic. Similar findings were seen in the HRQL, which was improved in both groups, but no significant difference could be detected. As for exercise tolerance, the percentage of the changes in the 6-minute walk distance got minimal clinically important difference21,22 with the values for high-pressure and low-pressure groups being 53.85% and 38.10%, respectively. Although we did not observe statistically significant differences between these two groups, probably because of the small sample size and the short follow-up period, our findings imply that high-pressure NPPV might be relevant to improve HRQL and exercise tolerance.
In this pilot study, the average IPAP level of high-pressure NPPV was only 21.15 cm H2O, and this pressure was lower compared with those in previous studies10,11. While owing to that the somatotype and upper airway resistance of Asians are quite different from those of westerners and with respect to the conventional IPAP level used in clinical practice23, the IPAP level of high-pressure NPPV in our study was a relatively high pressure. Furthermore, the back-up rate was not set higher than the spontaneous respiratory frequency to reach a controlled mode, which differed from prior studies. However, Murphy et al.16 pointed out that no additional benefit resulted from supplementing high IPAP with a high back-up rate, and they suggested that high pressure played a significant role in high-intensity NPPV.
Some limitations of this study should be mentioned. First, the power calculation for daytime PaCO2 was only 0.62 which was less than 0.75, indicated that larger sample size and longer follow-up time were needed in the further clinical trial. Second, we did not perform night-time arterial blood gas analysis; therefore, we did not obtain patient PaCO2 during nocturnal NPPV.
In this current pilot study, it implied us that the association between high-pressure NPPV and the improvement in TDI scores might exist when compared with low-pressure NPPV. In addition, a positive trend favouring high-pressure NPPV for improving PaCO2 and FEV1 was noticed. However, the sample size was not large enough and the follow-up period was quite not long. In the near future, we will proceed a longer follow-up time (more than a year) clinical study with large sample size determined by an accurate power calculation to determine what the best settings are for long-term NPPV for hypercapnic COPD patients. The inclusive patients will be randomly assigned to high-pressure group or low-pressure group using stratified block randomization, via a computer-generated block randomization sequence, with a block size of four.
For now, no unified method for setting up high-pressure NPPV has been established. Most of the trials utilized gradually increased IPAP depending on the patient’s tolerance10,11,12. However, from a respiratory physiology point of view, excessive IPAP may lead to lung hyperinflation, increased intrinsic positive end expiratory pressures, increased oxygen consumption, and ineffective work of breathing17. Therefore, seeking a method to establish individualized high-pressure NPPV is of vital importance.
In conclusion, high-pressure NPPV used for patients with chronic hypercapnic COPD is associated with improvement in TDI scores and a positive trend in favour of high-pressure NPPV for improving PaCO2 is observed. Use of an NIV equipped with built-in software allowed monitoring of the efficacy of home NPPV. A large sample size studies with greater follow-up period are necessary to determine the long-term effect of high-pressure NPPV and a more advanced method for establishing this new NPPV strategy is needed.
Methods
The Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China approved this study, and all patients provided written informed consent before the study began. The trial was registered with ClinicalTrials.gov, number NCT02499718 (July 16, 2015). In this study, all methods were performed in accordance with the relevant guidelines and regulations.
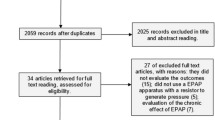
In this pilot study, we investigated 34 patients recruited in a prior, prospective, multicentre, randomized, controlled trial24, using high-pressure (IPAP ≥ 20 cm H2O9) or low-pressure ventilation (IPAP ≤ 16 cm H2O11) for 3 months. Patients were clinically stable with chronic hypercapnic COPD with severe to very severe airflow limitation. Patients were judged to be clinically stable if they had no acute exacerbation1,25, which was characterized as an acute worsening of more than one respiratory symptom (new onset of or increase in sputum volume or purulence, wheezing, cough, dyspnoea, or fever) lasting for at least 2 consecutive days and did not have any changes in their conventional therapy for 1 month3. Exclusion criteria were: (1) other lung/pleural diseases (for example, bronchiectasis, bronchogenic carcinoma, neuromuscular disease) or thoracic deformity; (2) severe heart failure (New York Heart Association class IV), severe dysrhythmia, unstable angina, or malignant comorbidity; (3) obesity (BMI ≥ 35 kg/m²); (4) severe obstructive sleep apnoea syndrome; and (5) previous NPPV or any form of invasive ventilation.
All patients received regular, optimal pharmacologic treatments according to the GOLD guideline1. Patients were advised to use NIV at least 5 hours per day. They were recommended to use NPPV during sleep, but daytime usage was also acceptable. For this study, patients were provided with a Flexo ST 30 NIV ventilator (Curative Medical Technology Inc., Suzhou, People’s Republic of China) using a spontaneous/timed mode of ventilatory support. Expiratory positive airway pressure (EPAP) was set at a low level (4–6 cm H2O), and the IPAP was gradually increased according to the toleration of patient. The pressure support level (IPAP−EPAP difference) was more than 10 cm H2O6. The back-up rate was set at 16 breaths/min. The software built into the NIV could record parameters such as leakage, IPAP, EPAP, air flow, tidal volume, minute ventilation, and respiratory rate. Staffs monitored patients’ daily usage. If leakage was above 40 L/min, we guided the patients to tighten their head band or changed the mask with appropriate size. When patient’s daily usage below 5 hours, we reminded the patients to lengthen the usage time. Additionally, 24 h phone service was available for the patients in case of problems happened. Furthermore, if the problems could not be solved by phone, staff provided door-to-door service immediately. Also detailed data were extracted from the software and analysed every 4 weeks.
Outcomes
The primary outcome was daytime PaCO2. Daytime arterial blood gas samples were taken with patients resting in a sitting position and breathing room air without having used NPPV for at least 1 hour3. Secondary outcomes were HRQL, based on the SRI Questionnaire26 and the CAT27; pulmonary function; 6MWD; blood oxygen saturation; and baseline/transition dyspnoea index28.
Statistical analysis
The data analysis was conducted using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). 20 patients per group were needed in order to reveal a difference of 7 mmHg in daytime PaCO2 between high-pressure and low-pressure ventilation with an SD of 7 mmHg was assured from the previous study13. The study was designed to have a power of 80% and a two-sided significance level of 0.05. The data are presented as n (%), mean ± standard deviation (SD) or median ± range according to their distribution. P-values < 0.05 were considered statistically significant. For continuous variables, comparisons were performed using the independent samples t test for normally distributed data and a nonparametric test for data not normally distributed. For categorical variables, the percentages of patients in each group were compared using Pearson’s chi-squared test.
Data Availability
All data generated or analyzed during this study are included in this published article.
Change history
22 March 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) Available from: http://goldcopd.org (2017).
Windisch, W., Storre, J. H. & Köhnlein, T. Nocturnal non-invasive positive pressure ventilation for COPD. Expert Rev. Respir. Med. 9, 295–308 (2015).
Köhnlein, T. et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir. Med. 2, 698–705 (2014).
Struik, F. M. et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 69, 826–834 (2014).
Struik, F. M., Lacasse, Y., Goldstein, R. S., Kerstjens, H. A. M. & Wijkstra, P. J. Nocturnal noninvasive positive pressure ventilation in stable COPD: A systematic review and individual patient data meta-analysis. Respir. Med. 108, 329–337 (2014).
McEvoy, R. D. et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax 64, 561–566 (2009).
Windisch, W. Impact of home mechanical ventilation on health-related quality of life. Eur. Respir. J. 32, 1328–1336 (2008).
Duiverman, M. L. et al. Nocturnal non-invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax 63, 1052–1057 (2008).
Schwarz, S. B., Magnet, F. S. & Windisch, W. Why High-Intensity NPPV is Favourable to Low-Intensity NPPV: Clinical and Physiological Reasons. COPD J. Chronic Obstr. Pulm. Dis. 2555, 1–7 (2017).
Duiverman, M. L. et al. Impact of High-Intensity-NIV on the heart in stable COPD: a randomised cross-over pilot study. Respir. Res. 18, 76 (2017).
Dreher, M., Storre, J. H., Schmoor, C. & Windisch, W. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 65, 303–308 (2010).
Windisch, W., Haenel, M., STorre, J. H. & Dreher, M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int. J. Med. Sci. 6, 72–76 (2009).
Windisch, W., Kostić, S., Dreher, M., Virchow, J. C. & Sorichter, S. Outcome of Patients With Stable COPD Receiving Controlled Noninvasive Positive Pressure Ventilation Aimed at a Maximal Reduction of Paco2. Chest 128, 657–662 (2005).
Windisch, W. et al. Normocapnia during nIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2. Respir. Med. 96, 572–579 (2002).
Lukácsovits, J. et al. Physiological changes during low- and high-intensity noninvasive ventilation. Eur. Respir. J. 39, 869–875 (2012).
Murphy, P. B. et al. High pressure versus high intensity noninvasive ventilation in stable hypercapnic chronic obstructive pulmonary disease: a randomized crossover trial. Int. J. Chron. Obstruct. Pulmon. Dis. 7, 811–818 (2012).
de Wit, M. et al. Ineffective triggering predicts increased duration of mechanical ventilation*. Crit. Care Med. 37, 2740–2745 (2009).
Duiverman, M. L., Arellano-Maric, M. P. & Windisch, W. Long-term noninvasive ventilation in patients with chronic hypercapnic respiratory failure: assisting the diaphragm, but threatening the heart? Curr. Opin. Pulm. Med. 22, 130–7 (2016).
Elliott, M. W. Domiciliary non-invasive ventilation in stable COPD? Thorax 64, 553–556 (2009).
Dreher, M., Schulte, L., Müller, T., Ekkernkamp, E. & Zirlik, A. Influence of effective noninvasive positive pressure ventilation on inflammatory and cardiovascular biomarkers in stable hypercapnic COPD patients. Respir. Med. 109, 1300–1304 (2015).
Singh, S. J. et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 44, 1447–1478 (2014).
Holland, A. E. et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 44, 1428–1446 (2014).
Kadowaki, T. et al. Low-intensity noninvasive ventilation: Lower pressure, more exacerbations of chronic respiratory failure. Ann. Thorac. Med. 11, 141–5 (2016).
Zhou, L. et al. Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: A short-term prospective, multicenter, randomized, controlled trial. Int. J. COPD 12, 1279–1286 (2017).
Wedzicha, J. A. & Seemungal, T. A. COPD exacerbations: defining their cause and prevention. Lancet 370, 786–796 (2007).
Windisch, W., Budweiser, S., Heinemann, F., Pfeifer, M. & Rzehak, P. The Severe Respiratory Insufficiency Questionnaire was valid for COPD patients with severe chronic respiratory failure. J. Clin. Epidemiol. 61, 848–853 (2008).
Jones, P. W. et al. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 34, 648–654 (2009).
Mahler, D. A., Weinberg, D. H., Wells, C. K. & Feinstein, A. R. The measurement of dyspnea.Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 85, 751–758 (1984).
Acknowledgements
We thank Andrea Baird, MD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. The authors wish to thank Curative Medical Inc. (China) for providing the ventilators. The study was supported by the Science and Technology Project of Guangdong Province (2017A020211018) and the Guangzhou Healthcare collaborative innovation major project (201604040012) and State’s Key Project of Research and Development Plan (2017YFC1310600).
Author information
Authors and Affiliations
Contributions
R.C. Chen and L.L. Guan contributed to the conception and design of the study, drafting the submitted article and revising the draft critically for important intellectual content. W.L. Wu, X.Y. Li and J.W. Xu contributed to the data acquisition, the interpretation of outcomes, data analysis and drafting the submitted article. R.C. Chen and L.Q. Zhou contributed to the crucial revision of the draft for important intellectual content and providing final confirmation of the revised version to be published. Xin Chen, B.P. Guo, Y.T. Huo and Y.Q. Yang contributed to following up the patients, collecting, extracting and analysing the data. All authors contributed to data analysis, drafting the manuscript, amending the paper and being responsible for all aspects of the work. All the data could be accessed to all of the authors and all of the authors assured the accuracy of the reported data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A correction to this article is available online at https://doi.org/10.1038/s41598-018-22723-w.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, L., Guan, L., Wu, W. et al. High-pressure versus low-pressure home non-invasive positive pressure ventilation with built-in software in patients with stable hypercapnic COPD: a pilot study. Sci Rep 7, 16728 (2017). https://doi.org/10.1038/s41598-017-17142-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17142-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.