Abstract
A strategy to enhance the upconversion white-light intensity via Mg2+ ion doping was demonstrated in Ho3+/Yb3+/Tm3+/LiNbO3 single crystal. It is found Mg2+ ion doping affects the crystal field symmetry around RE3+ ions and enhance the upconversion emission intensity. Bright white-light is obtained when the Mg2+ ion concentration is 0.5 mol% in the melt. And the CIE coordinates are hardly changed with Mg2+ ion doping. In addition, the upconversion mechanism is discussed in detail. It is observed the longer lifetimes of intermediate levels result in the lower upconversion photon numbers, which are beneficial to the upconversion process. Therefore, Mg2+ ion doped Ho3+/Yb3+/Tm3+/LiNbO3 single crystals would have potential applications in stable white-light devices and photoelectric instruments.
Similar content being viewed by others
Introduction
Recently, trivalent rare earths (RE3+) doped upconverting white-light materials have attracted great attentions due to their potential applications in color display, bio-label, solar cell, optical temperature sensor, and so on1,2,3,4,5. As known, upconversion is an anti-stokes process which can convert two or more low-energy photons into a high-energy photon. To avoid the intrinsic color balance, device complication and high cost problems when using multiphosphors, the blue, green and red emissions are expected to be generated simultaneously in a host material. The single white-light phosphors are pursued6,7,8. At present, many researches focus on the tri-doped Yb3+, Ho3+ and Tm3+ system in different host materials9,10,11, where the Yb3+ ions act as sensitizers to efficiently absorb the pump photons and transfer their energy to Tm3+ or Ho3+ ions. As a result, the blue emission (Tm3+), green and red emissions (Ho3+) are achieved, which can further produce the white-light emission.
Since the upconversion emission intensity and chromaticity play key roles in practical applications for white-light materials, it is of technological and scientific importance to look for an effective way to enhance the upconversion emission intensity and obtain a stable white-light emission. During the past few years, in order to improve the properties of upconversion white-light materials, much attentions have been focus on the modulation of RE3+ ion concentration, controlled synthesis of host material, suitable selection of excitation source, and the optimization for temperature and some other affecting factors, etc12,13,14,15. However, the obtained results are not satisfying so far because of their relevant limitations. Generally, high RE ion concentrations are required to guarantee the upconversion emission intensity but detrimental for the crystal quality of host material, too high RE3+ ion concentrations may induce the quenching of upconversion luminescence. And meanwhile, the adjustment of host material or affecting factors may result in the application difficulty and operation complexity.
Worthy of notice, in addition to the presence of RE3+ ions in upconverting white-light materials, the codoping of various non-luminous ions may cause the improvement of luminescence behavior by modifying the local environment around the emitters16. Recently, a few reports have focused on the enhancement of upconversion emission by codoping non-lanthanides (Li+, Mg2+, Na+, Sc3+ etc.) in RE3+ ions doped upconverting materials17,18,19. Luitel et al. have studied the effects of M+ (M = Li, Na, K, Rb) ion in CaMoO4:RE3+,Yb3+ (RE = Er, Ho, Tm) phosphors20. Guo et al. revealed that the Li+ doping could enhance the emission intensity of Yb3+/Ho3+ codoped Lu6O5F8 nanoparticles21. Many researches show that the codoping of non-luminous ions into RE3+ ions doped upconverting materials is a promising way to increase the intensity of upconversion emission. But the relative studies are still limited, to the best of our knowledge, few reports concerned about the influence of non-luminous ions on upconversion white-light properties in RE3+ ions doped materials to date.
In this article, LiNbO3 single crystal was used as host material, its lower phonon energy guarantees the higher upconversion efficiency. Mg2+ ion was introduced into Ho3+/Yb3+/Tm3+ tri-doped LiNbO3 single crystals due to its small ionic radius. Here, we represent a new strategy to improve the properties of upconversion white-light emission. Under 980 nm excitation, the influences of Mg2+ ion on the intensity and color tunability of upconversion white-light emission were demonstrated and the rational explanation was given. Preferably, the multi-function of LiNbO3 single crystal will create sufficient conditions for opening up new perspectives to the studies of integration and tiny devices.
Results and Discussion
Table 1 presents the molar compositions of cations in the melt or crystal for Mg2+ doped Ho3+/Yb3+/Tm3+/LiNbO3 crystals. It can be seen that the Mg2+ and RE3+ ions are introduced into the crystals successfully. With increasing Mg2+ ion concentrations in the melt, Mg2+ ion concentrations in the crystals are increased evidently. However, the total concentrations of RE3+ ions in the crystals are decreased slightly with increasing Mg2+ ion concentrations, which could be considered as unchanged. In addition, Fig. 1(a) shows the powder XRD patterns of pure LiNbO3 and Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with different Mg2+ ion concentrations. As shown, all the diffraction peaks of the samples can be well indexed to the standard LiNbO3 phase (JCPDS file no. 20-0631), no secondary phases were identified. It can be concluded that the doping ions do not alter the phase structure of host material, and the ionic radius differences between doping ions and host ions result in the variations of diffraction peak intensities. To further investigate the effect of Mg2+ ions on the structure of Ho3+/Yb3+/Tm3+/LiNbO3 single crystal, the main diffraction peak is amplified, as shown in Fig. 1(b). In general, the RE3+ ions with larger ionic radius (rHo 3+ = 0.89 Å, rYb 3+ = 0.86 Å, rTm 3+ = 0.87 Å, rLi + = 0.68 Å, rNb 5+ = 0.69 Å) will enter into the LiNbO3 crystals in the form of lattice substitution. So the host lattice is expanding, which could lead to the shift of the main diffraction peak towards smaller angle, as shown in Fig. 1(b). However, Mg2+ ions with smaller ionic radius (rMg 2+ = 0.66 Å) may enter into the crystals in the form of lattice substitution or interstitial. For lattice substitution, the host lattice shrinking is induced since the ionic radius of Mg2+ ion is smaller than that of Li+ or Nb5+ ions, corresponding to the shift of the main diffraction peak towards larger angle. By contrast, for the interstitial, the host lattice expanding occurs, resulting in the shift of the main diffraction peak towards smaller angle. Based on the above mentioned, the site occupancy of Mg2+ ion is mainly indentified by the shift of the main diffraction peak. It can be seen from Fig. 1(b) that with increasing Mg2+ ion concentrations, the main diffraction peak shifts gradually towards smaller angles, which means the lattice is expanding and the Mg2+ ions enter into the crystals in the form of interstitial. As a result, the occupation of interstitial site for Mg2+ ion can tailor the local crystal field around RE3+ ions in the host lattice, which will affect its luminescence properties.
(a) XRD patterns of pure LiNbO3 and Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with different Mg2+ ion concentrations. (b) Amplified the main diffraction peaks of pure LiNbO3 and Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with different Mg2+ ion concentrations. (c) Upconversion emission spectra of Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with different Mg2+ ion concentrations under 980 nm excitation at room temperature. (d) Calculated color coordinates (x, y) of the upconversion emissions for Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with different Mg2+ ion concentrations under 980 nm excitation. The S point is the standard white-light coordinate.
The upconversion emission spectra of Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with various Mg2+ ion concentrations under 980 nm excitation are shown in Fig. 1(c). As shown, the blue emission has a luminescence peak at 477 nm that corresponds to Tm3+: 1G4 → 3H6 transition; the green emission band centered around 549 nm is contributed to Ho3+: 5S2, 5F4 → 5I8 transition; and the red emission has luminescence peaks at 652 nm and 665 nm originating from Tm3+: 1G4 → 3F4 transition and Ho3+: 5F5 → 5I8 transition, respectively. From Fig. 1(c), it is observed that the intensities of blue, green and red emissions increase first and then decrease with increasing the Mg2+ ion concentrations. The optimum Mg2+ ion concentration is 0.5 mol% in the melt, corresponding to 0.66 mol% in the single crystal. In our case, we argue that Mg2+ ion with small ionic radius can be doped into the host lattice easily in the form of interstitial according to the XRD results, and this will break the symmetry of the crystal field around the rare earth ions. If the rare earth ions are placed at a low symmetry site, the forbidden transitions will be weakened, leading to the enhancement of upconversion emission. The similar phenomena are also found in the research reports, such as refs22,23. But when the Mg2+ ion concentration is above optimum concentration, the doped ions may cause the lattice distortion around the rare earth ions, resulting in the quenching of the upconversion emission. To investigate the color tunability, Fig. 1(d) shows the CIE coordinates of Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with various Mg2+ ion concentrations. It can be seen that the CIE coordinates of samples undoped and doped Mg2+ ion are located in the white-light region basically. Moreover, the CIE coordinates have the trend of shift towards green/red region first and then tend to shift towards blue region with increasing the Mg2+ ion concentrations. But it should be noted that it shows little color tunability under Mg2+ ion doping. So the Mg2+ ion doped Ho3+/Yb3+/Tm3+/LiNbO3 single crystals may be suitable for making the non-tunable white-light display devices.
To analyze the possible white-light upconversion mechanism in Mg2+ ions doped Ho3+/Yb3+/Tm3+/LiNbO3 single crystal, the dependences of upconversion emission intensities on pump powers are measured under 980 nm excitation, as shown in Fig. 2. Without Mg2+ ion doping, the slopes of blue, green, and red emissions for Ho3+/Yb3+/Tm3+/LiNbO3 single crystal are 2.30, 1.78, and 1.63, respectively. It can be obtained that the blue emission is a three-photon process, the green and red emission are two-photon processes. When the Mg2+ ion concentration in the melt is 0.5 mol%, the slopes of blue, green, and red emissions are 1.52, 1.02, and 0.96, respectively. And when the Mg2+ ion concentration in the melt reaches up to 4.0 mol%, the above values are 1.65, 1.14, and 1.01, respectively. As known, the slopes deviating from the integer values (3 or 2 or 1) are attributed to the competition between the linear decay and the upconversion processes for the depletion of the intermediate excited states and the local thermal effect24,25. These results indicate that the blue emission is a two-photon process, the green and red emission are one-photon processes with Mg2+ ion doping. As a supplement, the luminescence decay behaviors of Ho3+: 5I6 → 5I8 (λem = 1150 nm), Ho3+: 5I7 → 5I8 (λem = 2000 nm), and Tm3+: 3F4 → 3H6 (λem = 1800 nm) are investigated, as shown in Fig. 3. It can be easily seen that all the emission intensities decay exponentially. The double-exponential is adopted to fit the experiment data using the equation (1)26.
where I(t) is the fluorescence intensity at time t, I 0 stands for the background fluorescence intensity, τ s and τ f represent slow fluorescence lifetime and fast fluorescence lifetime, A s and A f are the weight factor of slow fluorescence lifetime and fast fluorescence lifetime, respectively. The lifetime of fluorescence level τ can be calculated according to the fitting results by equation (2).
The obtained lifetime values are shown in Table 2. It can be observed that the lifetime values of intermediate levels are increased with Mg2+ ion doping, resulting in the decrease of upconversion photon numbers. We believe that the longer lifetime of intermediate level is beneficial to the upconversion process, leading to the stronger upconversion emission intensity. But when the Mg2+ ion concentration in the melt is too high (4.0 mol% in this article), the lattice distortion plays an important role in upconversion process, so the upconversion luminescence is not emitted effectively though the lifetime of its intermediate level is long, the upconversion emission intensity is decreased consequently. Furthermore, the decay curve analysis of the excited levels involved in the following upconversion emission processes Tm3+: 1G4 → 3H6 (λem = 477 nm), Ho3+: 5F4, 5S2 → 5I8 (λem = 550 nm), and Ho3+: 5F5 → 5I8 (λem = 665 nm) are performed and calculated, the obtained lifetime values are shown in Table 1. It is suggested that the proper Mg2+ ion incorporation modifies the crystal field and results in the fast emitting of upconversion luminescence, and hence the upconversion emission intensity is enhanced. But the excessive Mg2+ ion concentration is detrimental to the enhancement of upconversion emission intensity.
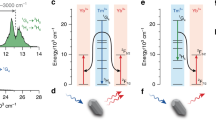
The Schematics of populating and upconversion luminescence processes for the blue, green and red emissions in the Mg2+ ions doped Ho3+/Yb3+/Tm3+/LiNbO3 system under 980 nm excitation have been described in Fig. 4. From Fig. 4, it can be seen that Yb3+ ions act as sensitizers to absorb laser photons and transfer their energy to Ho3+ and Tm3+ ions effectively. Through two successive energy transfer processes from Yb3+ ions to Ho3+ ions, the 5F4, 5S2 levels and 5F5 levels of Ho3+ ions are populated, which generate the upconversion green and red emissions, respectively. Similarly, through three successive energy transfer processes from Yb3+ ions to Tm3+ ions, the upconversion blue emissions and weak red emissions are obtained originating from the 1G4 → 3H6 and 1G4 → 3F4 transitions of Tm3+ ions. In the upconversion processes, Mg2+ ion is not the luminescent ion. The doping Mg2+ ions can impact the lifetimes of excited levels, and further influence the upconversion emission intensity.
Conclusion
Mg2+-doped Ho3+/Yb3+/Tm3+/LiNbO3 single crystals have been successfully prepared by Czochralski method. Bright upconversion white-light emission is achieved under 980 nm excitation at room temperature. It is found that Mg2+ and RE3+ ions could not alter the phase structure of host material, Mg2+ ions enter into the single crystals in the form of interstitial. The intensities of upconversion emissions are increased firstly and decreased subsequently with increasing Mg2+ ion concentrations. The optimum Mg2+ ion concentration is 0.5 mol% in the melt. The red, green and blue emissions in this system can be ascribed to Ho3+: 5F5 → 5I8, Tm3+: 1G4 → 3F4; Ho3+: 4S2, 5F4 → 5I8 and Tm3+: 1G4 → 3H6 transitions, respectively. The research results indicate that Mg2+ ion doping could not change the upconversion processes of Ho3+, Yb3+, and Tm3+ ions in LiNbO3 single crystals. The enhancement of upconversion emission intensity is mainly attributed to the increase of lifetimes for intermediate levels and the decrease of lifetimes for luminescent levels. Besides, it can be obtained that the CIE coordinates of upconversion emissions are almost unchanged with Mg2+ ion doping, which show ideal non-tunable white-light emissions. Such excellent white-light in Mg2+ ion doped Ho3+/Yb3+/Tm3+/LiNbO3 single crystals make it have potential applications in stable white-light devices and photoelectric instruments. This method would stimulate the further discovery to enhance the upconversion white-light intensity and fabricate other stable white-light materials.
Methods
Sample preparation
The Ho3+/Yb3+/Tm3+/LiNbO3 single crystals doped with different Mg2+ ion concentrations were grown by the Czochralski method. The concentrations of Ho3+, Yb3+ and Tm3+ ions in the melts were 0.025 mol%, 2.0 mol% and 0.2 mol%, respectively. And the Mg2+ ion concentrations in the melts are 0.0 mol%, 0.2 mol%, 0.5 mol%, 2.0 mol% and 4.0 mol%. The single crystals doped with different Mg2+ ion concentrations in the melt are denoted as 0.0 Mg, 0.2 Mg, 0.5 Mg, 2.0 Mg and 4.0 Mg in this article. The raw materials were Li2CO3, Nb2O5, Ho2O3, Yb2O3, Tm2O3 and MgO with 4 N purity. Firstly, the doped LiNbO3 polycrystals were prepared by high temperature solid state method. The raw materials were weighed and thoroughly mixed for 48 h, underwent a heat treatment of 750 °C for 2 h to resolve Li2CO3 into Li2O and CO2, and then sintered at 1150 °C for 10 h to form polycrystals. Secondly, the doped LiNbO3 single crystals were grown along the [0 0 1] direction using a diametercontrolled Czochralski apparatus. To grow crystals with good quality, the following optimum growth conditions were selected: the temperature gradient above the melt was 25 °C/mm, the pulling rate was 0.2 mm/h, and the seed rotation rate was 28 rpm. After growth, the crystals were cooled down to room temperature at a speed of 30 °C/h. For phase structure analyses, the samples were grinded into powder using an agate mortar. And for optical tests, Y-cut plates of the samples were cut and polished.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Characterization
The Inductively Coupled Plasma Mass Spectrometry (ICP-MS) with Optima-7500 type was used to measure the mass fractions of Mg2+ ions and rare earth ions (RE3+) in the single crystals. To identify the crystallization phase, X-ray diffraction spectra of Mg2+ ion doped Ho3+/Yb3+/Tm3+/LiNbO3 single crystals were measured by an XRD-6000 diffractometer using a copper Kα radiation source. The upconversion luminescence spectra were recorded by Zolix-SBP300 grating spectrometer equipped with CR131 photomultiplier under 980 nm excitation. In the measurement of luminescence decay dynamics, the continuous wave from 980 nm laser diode was tuned into pulsing by a signal generator, and the luminescence decay curves were measured by a digital phosphor oscilloscope (Tektronix DPO 4140). The CIE chromaticity coordinate for the upconversion fluorescence of Mg2+-doped Ho3+/Yb3+/Tm3+/LiNbO3 single crystal was calculated based on the 1931 CIE standard and marked in the CIE standard chromaticity diagram.
References
Teshima, K. et al. Flux growth of highly crystalline NaYF4:Ln (Ln = Yb, Er, Tm) crystals with upconversion fluorescence. Cryst. Growth Des. 11, 995–999 (2011).
Mukhopadhyay, L. & Rai, V. K. Upconversion based near white light emission, intrinsic optical bistability and temperature sensing in Er3+/Tm3+/Yb3+/Li+:NaZnPO4 phosphors. New J. Chem. 41, 7650–7661 (2017).
Heer, S., Kömpe, K., Güdel, H. U. & Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 16, 2102–2105 (2004).
Zou, W. Q., Visser, C., Maduro, J. A., Pshenichnikov, M. S. & Hummelen, J. C. Broadband dye-sensitized upconversion of near-infrared light. Nat. Photonics 6, 560–564 (2012).
Lu, H. Y. et al. Optical sensing of temperature based on non-thermally coupled levels and upconverted white light emission of a Gd2(WO4)3 phosphor co-doped with in Ho(III), Tm(III), and Yb(III). Microchim. Acta 184, 641–646 (2017).
Zhang, C. M. et al. Controllable and white upconversion luminescence in BaYF5:Ln3+ (Ln = Yb, Er, Tm) nanocrystals. J. Mater. Chem. 21, 717–723 (2011).
Hu, M. et al. Intense white emission from a single-upconversion nanoparticle and tunable emission colour with laser power. J. Mater. Chem. C 4, 6975–6981 (2016).
Wang, J. W. & Tanner, P. A. Upconversion for white light generation by a single compound. J. Am. Chem. Soc. 132, 947–949 (2010).
Tang, J. F. et al. Power driven tunable white upconversion luminescence from Lu2TeO6 tri-doped with Yb3+, Tm3+ and Ho3+. Cryst. Eng. Comm. 17, 9048–9054 (2015).
Rodrigues, E. M., Gálico, D. A., Mazali, I. O. & Sigoli, F. A. Temperature probing and emission color tuning by morphology and size control of upconverting β-NaYb0.67Gd0.30F4:Tm0.015:Ho0.015 nanoparticles. Methods Appl. Fluoresc. 5, 024012 (2017).
Kshetri, Y. K., Joshi, B., Kim, T. & Lee, S. W. Blue, green, red and near-infrared emissions via upconversion in α-sialon. Mater. Lett. 199, 147–150 (2017).
Tang, J. F. et al. Tunable upconversion luminescence from the phosphors of Yb3+, Tm3+ and Ho3+ tridoped Re2TeO6 (Re = La, Gd, and Lu). J. Alloys Compd. 672, 1–6 (2016).
Teng, X. et al. Lanthanide-doped NaxScF3+x nanocrystals: crystal structure evolution and multicolor tuning. J. Am. Chem. Soc. 134, 8340–8343 (2012).
Sobral, G. A. et al. Tailoring red-green-blue emission from Er3+, Eu3+ and Tb3+ doped Y2O3 nanocrystals produced via PVA-assisted sol-gel route. J. Phys. Chem. Solids 98, 81–90 (2016).
Zhang, C. et al. White-light emission from an integrated upconversion nanostructure: toward multicolor displays modulated by laser power. Angew. Chem. Int. Ed. 54, 11531–11535 (2015).
Dubey, A., Soni, A. K., Kumari, A., Dey, R. & Rai, V. K. Enhanced green upconversion emission in NaYF4:Er3+/Yb3+/Li+ phosphors for optical thermometry. J. Alloys Compd. 693, 194–200 (2017).
Li, Y. et al. Unusually enhancing high-order photon avalanche upconversion of layered BiOCl:Er3+ semiconductor poly-crystal via Li+ ion intercalation doping. Mater. Design 105, 290–295 (2016).
Dey, R., Kumari, A., Soni, A. K. & Rai, V. K. CaMoO4:Ho3+-Yb3+-Mg2+ upconverting phosphor for application in lighting devices and optical temperature sensing. Sensor. Actuat. B-Chem. 210, 581–588 (2015).
He, E. J., Chen, S. F. & Zhang, M. L. Simultaneous morphology evolution and upconversion emission tuning of single Y-based fluoride microcrystal induced by Sc3+ co-doping. Mater. Res. Bull. 87, 61–71 (2017).
Luitel, H. N., Chand, R. & Watari, T. CaMoO4:RE3+,Yb3+,M+ phosphor with controlled morphology and color tunable upconversion. Displays 42, 1–8 (2016).
Guo, L., Zheng, X., Zhang, S., Zhao, B. & Wang, Y. Upconversion- and cathodo-luminescence properties of Yb3+/Ho3+/Li+ tridoped Lu6O5F8 nanoparticles. Mater. Res. Bull. 86, 1–4 (2017).
Ding, M. et al. Li+ ions doping core-shell nanostructures: An approach to significantly enhance upconversion luminescence of lanthanide-doped nanocrystals. J. Alloys Compd. 623, 42–48 (2015).
Guo, L. et al. Structure, enhancement and white luminescence of multifunctional Lu6O5F8:20%Yb3+,1%Er3+(Tm3+) nanoparticles via further doping with Li+ under different excitation sources. Nanoscale 5, 2491–2504 (2013).
Pollnau, M., Gamelin, D. R., Lüthi, S. R., Güdel, H. U. & Hehlen, M. P. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B 61, 3337–3346 (2000).
Li, D., Dong, B., Bai, X., Wang, Y. & Song, H. W. Influence of TGA modification on upconversion luminescence of hexagonal-phase NaYF4:Yb3+,Er3+ nanoparticles. J. Phys. Chem. C 114, 8219–8226 (2010).
Zhang, H. X. et al. Green Upconversion Luminescence in Er3+:BaTiO3 Films. Appl. Phys. Lett. 77, 609–611 (2000).
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (11504438, 51605499, 11374080 and 81601347), Natural Science Foundation of Guangdong Province (2015A030310348).
Author information
Authors and Affiliations
Contributions
W.Y. proposed and guided the overall project. L.X. performed the experiments, analyzed the results, and wrote the main manuscript text. J.L. and M.H. prepared the sample materials and measured the experimental data. Y.X. revised the manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xing, L., Yang, W., Lin, J. et al. Enhanced and Stable Upconverted White-light Emission in Ho3+/Yb3+/Tm3+-doped LiNbO3 Single Crystal via Mg2+ Ion Doping. Sci Rep 7, 14725 (2017). https://doi.org/10.1038/s41598-017-15367-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15367-9
This article is cited by
-
Optical, thermal and nonlinear optical properties of amaranth dye-doped sulphamic acid single crystals
Journal of Materials Science: Materials in Electronics (2020)
-
Near infrared emission properties of Er doped cubic sesquioxides in the second/third biological windows
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.