Abstract
Toll-like receptors (TLRs) play a key role in innate and adaptive immunity, protecting the host from viral pathogens. We studied the effect of TLR7 polymorphisms on disease susceptibility and progression of chronic hepatitis B (CHB) infection in Chinese adults. Blood samples were taken from 612 patients with confirmed CHB, hepatitis B virus (HBV)-related liver cirrhosis (LC) or hepatocellular carcinoma (HCC) and 293 controls. TLR7 polymorphisms (rs179010-C > T, rs2074109-T > C, and rs179009-A > G) were analyzed by PCR-based sequencing. A significantly higher frequency of TLR7 rs179010 C allele was found in male CHB patients than in controls (74.8% vs 59.5%, P = 0.002). The frequency of rs179009 G allele was markedly increased with disease progression when male patients with CHB, LC and HCC were compared (P = 0.012). The haplotype CTA was significantly associated with an increased susceptibility to CHB among male patients (P = 0.000). Frequency of the haplotype CTG was higher in male patients with HCC than CHB (P = 0.005). No such differences in these allele frequencies were found between female patients and controls. Our results indicated that TLR7 polymorphisms play an important role in disease susceptibility and the progression of CHB infections in Chinese adults, and may partly explain the high incidence of HBV related diseases in Chinese men.
Similar content being viewed by others
Introduction
Hepatitis B virus (HBV) infection is a global health problem1. HBV carriers are at an increased risk of liver damage and many of them suffer from progressive liver diseases, such as chronic hepatitis B infection (CHB), liver cirrhosis (LC) and hepatocellular carcinoma (HCC)2. In China, there are 97 million HBV carriers and at least 20 million of them have an active CHB infection, alone or combined with LC and/or HCC3. In the 1980s, vaccination against HBV was introduced in some regions of China4. Since 2002 vaccination against HBV has been included in the National Program of Immunizations5.
It is believed that the human immune system initiates protective mechanisms following viral infection, including the rapid release of type I interferons (IFNs)6,7. Toll-like receptors (TLRs) are the first line of defence against viruses. TLRs stimulate the innate immunity response by recognition of pathogen-associated molecular patterns and promote subsequent adaptive immune responses8. Activation of TLR-mediated signaling pathways could inhibit HBV replication9, and can also enhance HBV-specific T-cell and B-Cell responses10. Of the 10 TLRs in human, TLR3,7,8 and 9 are the important ones against viral infections11. Although studies indicated that HBV could not efficiently induce a TLR-mediated immune response, resulting in the lack of type I IFN release in its natural hosts during early phase of infection12, the virus can possibly activate TLR pathways in infected hepatocytes and nonparenchymal liver cells to limit viral replication10. Previous data indicated that HBV and HBsAg preferentially abrogated TLR9, but not TLR7, agonist-induced IFN-α production in plasmacytoid dendritic cells (pDC)13. Lee et al. reported that HBV can activate TLR7 with HBcAg packaged single-stranded RNA (ssRNA)14. Although the exact interaction between TLR7 and HBV has not been fully understood, some clinical studies have shown that TLR7 agonists can mediate the antiviral effect. Recently, GS-9620, an oral agonist of TLR7 has been found to induce prolonged suppression of HBV in chronically infected chimpanzees15.
The TLR7 gene is located at Xp22.2, spanning three exons16. It is expressed on intracellular compartments and involved in the regulation of innate immune response via MyD88-dependent proinflammatory signaling cascades. Associations between TLR7 polymorphisms and autoimmune and viral diseases have been recently reported17,18,19. Schott et al. first reported that a TLR7 SNP (c.1-120 T > G) protects from advanced inflammation and fibrosis in male patients with chronic hepatitis C virus (HCV) infection20. Yue et al. recently found that Chinese female patients who carry the TLR7 rs179009 GG genotype and haplotype GCG of rs179009, rs179010 and rs179012 had an increased susceptibility to HCV infection21. Wang et al. also found that the polymorphism of TLR7 rs179009 might impair immune responses during HCV infection among the Chinese population22. Moreover, the TLR7 rs179010 polymorphism was associated with an increased risk for systemic lupus erythematosus (SLE) in Japanese females23. The SNP rs2074109 of TLR7 is located in 31 nucleotide upstream of rs179009. The minor allele frequency of rs2074109 is 0.099 in Japanese and 0.069 in Chinese Han populations, highest among all other populations reported in the 1000 genomes study24. The effect of this SNP on health and disease has been not reported. Although associations between polymorphisms of TLR7 and HCV infection have been reported, there has been no reported study to investigate association between TLR7 SNPs and the susceptibility and outcome of an HBV infection. Therefore, we aimed to explore the relationship between these three TLR7 polymorphisms and HBV-related liver diseases in the Chinese Han population to gain a better understanding of the role of TLR7 in the development of HBV infection.
Results
Clinical information of study subjects
Demographic and clinical parameters of the patients are summarized in Table 1. There were significant differences in terms of gender ratio, age and clinical parameters except for ALT among three groups of patients. All of the patients were positive for HBsAg and anti-HBc antibodies. Levels of AST, AFP, TBIL and DBIL were increased, but ALB levels were decreased significantly with the progression of the disease (all P < 0.001). HBV-DNA copies were significantly decreased from CHB to LC and HCC (P < 0.001). Other indices of patients, such as white blood cell, hemoglobin, blood platelet, creatinine and prothrombin activity were also examined. No increasing/decreasing trends were observed among the three groups of patients.
Polymorphisms of TLR7 and susceptibility to CHB-related diseases
The observed genotype distributions of these three SNPs were in agreement with the Hardy-Weinberg equilibrium (all P values > 0.05). For TLR7 rs179010, the prevalence of major allele C was significantly higher in male patients with combined CHB, LC and HCC than that of controls (68.3% vs 59.5%, P = 0.029) (Table 2). After subsets stratification, a significantly higher frequency was found in male patients with CHB (P = 0.002) adjusted by age (Table 3). No differencea in distributions of genotypes and alleles were observed between female patients with HBV-related CHB, LC and HCC combined or alone and controls. No significant differences in frequencies TLR7 rs2074109 and rs179009 polymorphisms were observed when male or female patients with CHB, LC and HCC (either combined or alone) were compared with controls.
Polymorphisms of TLR7 and CHB disease progression
The genotype and allele distributions of the three SNPs of CHB, LC and HCC groups are shown in Table 4.
An increased frequency of the minor allele G of TLR7 rs179009 was found in men along with severity of CHB-related diseases, being from 12.9% in patients with CHB to 22.6% in patients with HCC (P = 0.012) adjusted by age. No difference was found in the genotype frequency of TLR7 rs2074109 and rs179010 among male patients with CHB-related diseases. In contrast, no difference in frequencies of the three studied SNPs were observed among three groups of female patients.
Furthermore, when patients who were HBeAg-positive were compared among the three groups, the frequency of allele G of TLR7 rs179009 increased from 12.3% in male patients with CHB to 31.8% in those with HCC. However, this was not statistically significant (P = 0.059).
We next performed haplotype analysis. Four major haplotypes CTA, TTA, CTG and CCA were observed (Table 5). In the male group, the frequency of haplotype CTA was markedly higher in patients with CHB (P = 0.000). In contrast, a lower frequency of haplotype TTA was found in patients with CHB than in controls (25.2% vs 40%, P < 0.0001). As for disease progression, the possible influence of TLR7 rs179009 on the development from CHB to HCC in men was confirmed by haplotype CTA and CTG distribution in patients with HCC compared to those with CHB (P < 0.0001 for haplotype CTA; P = 0.003 for haplotype CTG) (Table 6). A significant difference of haplotype CTA distribution was found in men who were HBeAg positive with HCC compared to those with CHB (P < 0.00001) (Supplementary Table 1). No significant difference of haplotype CTA distribution was found in men/women who were HBeAg negative and with HCC compared to those with CHB (Supplementary Table 2).
Discussion
In this study, we found that genetic variation of TLR7 plays a role in susceptibility to CHB infection and affects disease progression from CHB to HCC in Chinese men. To our knowledge this is the first exploratory study to show the relationship between TLR7 polymorphisms and susceptibility and disease progression of chronic HBV infection in humans.
It has been shown that several agonists of TLRs including TLR7 can inhibit HBV replication, probably as a result of the production of cytokines induced by the TLR signaling9. Activation of the TLR7 signaling pathway can facilitate the production of antiviral cytokines, including IFNs, while activation of NF-κB induces secretion of TNFα, interleukin-6(IL-6) and IL-1225. As one of the TLRs family members, TLR7 recognizes ssRNA derived from viruses. However, no matter where it came from, whether bacterial, yeast or mammalian, the ssRNA encapsidated within HBcAg did function as a TLR7 ligand at the T and B cell levels with TLR7 knock-out mice verification14, indicating HBcAg packaged ssRNA could activate TLR7 signaling pathway in HBV natural infection.
Previous ex vivo and in vitro studies showed that HBV infection could suppress the intracellular TLR-induced antiviral activity of pDCs and liver cells26,27. TLR7 is one of such TLRs. Although pDCs display an impaired ability to secrete IFN-α following ex vivo stimulation with TLR9/TLR7 ligands during a chronic HBV infection, HBV particle internalization could inhibit TLR9- but not TLR7-mediated secretion of IFN-α by pDCs13.
In this study, we assessed the impact of three SNPs of TLR7 on susceptibility to and disease progression of CHB infection in Chinese adults. The three polymorphisms rs179010, rs2074109 and rs179009 with the respective change from C to T, T to C and A to G, do not cause alteration in amino acids. TLR7 rs2074109 is near to rs179009, and located at 31 bp upstream of rs179009. Based on SNP function prediction (http:snpinfo.niehs.nih.gov), mutations of TLR7 rs179009 and 179010 are predicted to occur at transcription factor binding sites (TFBSs), which could affect level and/or timing of TLR7 expression and result in difference in production of downstream cytokines such as IFN-α, suggesting that these SNPs are functional.
Xiao et al. reported that the risk of Grave’s Disease (GD) decreased significantly as the frequency of TLR7 rs179010 T alleles increased in Chinese females, indicating a protective effect of TLR7 rs179010 polymorphism against GD18. Moreover, this SNP rs179010 was also associated with increased risk for SLE in Japanese females23. It has been considered to be associated with autoantibody production, suggesting that TLR7 might increase B-cell sensitivity to RNA-containing autoantigens in the development of systemic autoimmunity28. Numerous antagonists targeting the TLR signaling cascade are identified as potential therapeutic targets for SLE29, including TLR7 antagonists which could inhibit Th1-type cytokines release, such as IFN-α and modulate the imbalance of Th1/Th2 cytokines environment in SLE patients. Stimulation of TLR7 could mediate an effective Th1-dependent immune response, which is known to be critical in development of a broad and effective protection against hepatitis viruses25. Nonetheless, cytokines are the primary cause of inflammation and can mediate liver injury after HBV infection30.
In this present study, Chinese men with CHB had a significantly higher frequency in major allele C of TLR7 rs179010, suggesting an increased risk of CHB infection. The finding highlighted that polymorphism of TLR7 rs179010 is functional and its effect may be partially mediated via B cell response during HBV persistent infection. However, in a recent study on the relationship of TLR7 polymorphisms with susceptibility to HCV infection, no association between TLR7 rs179010 and HCV infection was found21. It has been shown that TLR7 can recognize HCV RNAs, leading to the production of IFN and antiviral cytokines to influence HCV infection and progression31. Recently genetic variation of TLR7 was reported to influence HCV infection with gender difference32. In a study conducted in Taiwan, SNP rs179009 G allele of TLR7 was present at a higher frequency in males with HCV infection, as compared to healthy controls22.
Interestingly, we found an increased frequency of the minor allele G of TLR7 rs179009 in men along with the severity of CHB-related diseases, indicating an increased risk for development from CHB to HCC. Haplotype analysis also supported these findings, as CTG was confirmed as a risk factor of disease progression for men. Although the pathogenesis of HCV infection differs from that of an HBV infection, TLR7 rs179009 was observed to be related to both of them, which may be due to the impaired host antiviral response. HBeAg+ represents a surrogate of viral replication and indicates an increased susceptibility to HCC33. Data showed that HBV virions or elements including HBeAg or HBsAg can suppress TLR7-induced antiviral activity of murine parenchymal and nonparenchymal liver cells27. We evaluated the influence of TLR7 rs179009 on disease development among patients of HBeAg(+) and observed that among the male individuals of HBeAg(+), the frequency of allele A was decreased from 87.7% in patients with CHB to 68.2% in those with HCC. However, this change did not reach a statistical significance.
It has been recently shown that variation in the copy number of TLR7 is linked to the disease progression of HBV infection in Chinese population34. A low copy number of TLR7 was significantly associated with an increased risk of chronic HBV infection in female patients (P < 0.001). However, no significant differences were found in the copy number of TLR7 among patients with CHB, LC or HCC.
Gender is one of the major factors that influence the outcome and severity of an HBV infection in clinical practice35. In line with this, we noticed a gender difference in association of TLR7 rs179010 and 179009 polymorphisms with CHB-related diseases among Chinese men but not in women. The finding might explain at least in part the higher incidence and more severe manifestations of HBV infection in Chinese men compared to women35,36. Mounting data has highlighted the sex-based differences in the pathogenesis of infectious and autoimmune diseases, which might attribute to the action of sex hormones and X chromosome-linked genes37. Berghöfer et al. reported a sex-dependent pathway of TLR7-induced IFN-α with high production in females unrelated to hormonal effects38. Meier et al. also showed that sex differences in TLR7-mediated activation of pDCs with enhanced activation of CD8+ T cells could account for a higher immune response in women compared to that of men when the same amount of HIV-1 RNA was used. This enhanced immune activation in women might explain the finding on why higher viral loads occur in men during early phase of HIV infection and the clinical observation on why women had a higher risk for HIV-1 disease progression during chronic infection at a given HIV-1 viral load39.
The SNP rs2074109 of TLR7 is close to rs179009. The reported frequency of minor allele C based on the 1000 genomes project was only 0.069 in Chinese Han population24. In this study, similar minor allele frequencies in both controls and patients were noticed. Moreover, no significant association was found among patients with CHB, LC and HCC, suggesting that this polymorphism may be not functional.
There are certain limitations in this study. The first was a relatively small number of subjects included in each group, especially the number of female patients with HCC. Therefore, a risk of type-2 statistical error may be present due to the low number of female patients with HCC, which means that further studies with a large number of subjects are needed. Secondly, it is known that there are multiple phases of disease in CHB: immune tolerance, immune clearance, immune control and immune escape40. These phases are divided based on viral load, ALT, HBeAg and HBeAb. In this present study, all CHB phases were aggregated and analyzed together. Therefore the results should be interpreted with caution as these above-mentioned confounding factors were not taken into analysis. Thirdly, only three intronic SNPs of TLR7 were assessed. Fourth, the function of SNP rs179010 and rs179009 on TLR7 expression and its signaling pathway was not assessed. Some important cytokines like IFN-α and IL-6 were not determined in study subjects.
In conclusion, our findings supported the role of TLR7 rs179010 in predisposition of CHB in Chinese men, while TLR7 rs179009 A allele was associated with a decreased risk of disease progression from CHB to HCC. To our knowledge, this is the first report of TLR7 SNPs and HBV infection. However, given the limited sample size, this finding requires verification by larger studies in diverse ethnicities.
Methods
Study subjects
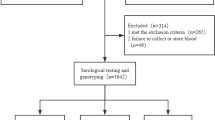
A total of 905 study subjects included 612 patients with confirmed HBV infection recruited from July 2014 to September 2015 in Beijing You’an Hospital, and 293 controls who attended annual physical examination. All study subjects were Han ethnic. The patients were divided into three groups: 250 patients with CHB, 219 patients with LC and 143 patients with HCC (Table 1). The diagnosis of CHB infection was made based on previous history of hepatitis B or positive HBsAg more than 6 months. Serum HBsAg and/or HBV DNA has been positive, and alanine aminotransferase (ALT) or aspartate aminotransferase(AST) intermittent elevated, or liver tissue examination with hepatitis lesions. LC was diagnosed on the basis of a clear previous history of chronic hepatitis B, HBsAg test positive, pathologic exams, laboratory features, and the findings of computed tomography (CT) or ultrasonography. HCC was diagnosed by at least one positive iconography examination result, including CT and magnetic resonance imaging, or positive findings on cytological or pathological examination. All patients were free of other viral infections, including human immunodeficiency virus (HIV), HCV, cytomegalovirus and Epstein–Barr virus. They did not report any other type of liver disease (for example, autoimmune hepatitis, toxic hepatitis, and so on), or other cancers. Clinical samples were collected from those who had not received antiviral treatment or immunotherapy during the past 6 months and if patients had not received any surgical treatment. According to the characteristics of specific serology, patients were divided into HBsAg-, HBeAg-and anti-HBc antibodies positive (here referring as HBeAg(+)) group and HBsAg-, anti-HBe and anti-HBc antibodies positive (here referring as HBeAg(−)) group. Demographical data of all study subjects were collected at the first time of visit to the hospital and the following laboratory parameters were obtained and were available for most of the patients, such as serum AST, ALT, Alphafetoprotein(AFP), total bilirubin(TBIL), direct bilirubin(DBIL), albumin (ALB) and HBV-DNA copies (Table 1). The median and age range of patients (N = 612) and controls (N = 293) were 45 years (12–86 years) and 39 years (21–60 years), respectively. No significant difference was found in gender ratio between patients (male vs female: 419/193) and controls (195/98).
This study was approved by the Ethics Committee of Beijing You’an Hospital, Capital Medical University. Informed consent for both study participation and publication of identifying information/images (when applicable) was obtained from each of the participants at the time of inclusion. The methods were carried out in accordance with the relevant guidelines, including any relevant details.
TLR genotyping
Genomic DNA was extracted from blood using a commercial kit according to the manufacturer’s instructions (QIAGEN DNA Blood Mini Kit, Hilden, Germany). Three SNPs (rs179010, rs2074109 and rs179009) of the TLR7 gene were identified using PCR-based sequencing. Primers for TLR7 rs179010 were forward (5′-AGCCAGTCCACGGTTAAAGC3′) and reverse (5′-AGCCCAAGGTTACCCAGTAG3′) and primers for TLR7 rs2074109 and rs179009 were forward (5′-AGCAGGCCGACATAAATTGC3′) and reverse (5′-GTCTGTGCAGTCCACGATCA3′). PCR was performed in a total reaction volume of 50 µl with 100–200 ng genomic DNA. After an initial denaturation at 95 °C for 2 min, the DNA was amplified for 38 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min 10 s, with a final elongation at 72 °C for 5 min on the PCR System 9700 (PE Applied Biosystems, Foster City, CA, USA). Both positive and negative controls were used in each PCR run. The amplicons were sequenced at the Sino Geno Max Co., Ltd, Beijing, China and SNPs were identified by the computer program of mutation surveyor V5.0.0 (SoftGenetics, USA). To validate the sequencing results obtained by the forward sequencing primer, every tenth of the PCR-amplified DNA samples were selected and re-sequenced by the reverse primer. The results obtained between the two sequencing analyses were completely concordant.
Statistical analysis
For each SNP, allele and genotype frequencies were descriptively summarized. Statistical analysis was carried out using SPSS statistical software version 17.0 (SPSS Inc., Chicago, USA). Because the TLR7 gene is located on X chromosome, the allele frequency of each SNP was separately analyzed for male and female patients. Each SNP was tested for deviation from Hardy-Weinberg equilibrium (HWE). Differences of demographic data, genotype, allele frequencies between cases and controls or among three groups of patients (CHB, LC and HCC) were evaluated by Student’s t-test, chi-square test, one-way analysis of variance (ANOVA) or nonparametric test and Kruskal-Wallis test where appropriate. Logistic regression was used for the multivariate analyses throughout the paper, adjusted for age. P values, odds ratios (ORs), and 95% confidence intervals (95% CIs) were used to evaluate the association between polymorphisms and the risk of disease. SHESIS on line (http://analysis.bio-x.cn/myAnalysis.php) were used for haplotype analysis. A two-sided P value of less than 0.05 was considered significant.
References
Ott, J. J., Stevens, G. A., Groeger, J. & Wiersma, S. T. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30, 2212–2219 (2012).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
Cui, Y. & Jia, J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 28(Suppl 1), 7–10 (2013).
CB, L. & CA, S. Hepatitis B vaccine and Problems. Chin J Epidemiol 25, 377–378 (2004).
Wang, F. S., Fan, J. G., Zhang, Z., Gao, B. & Wang, H. Y. The global burden of liver disease: the major impact of China. Hepatology 60, 2099–2108 (2014).
Garcia-Sastre, A. & Biron, C. A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312, 879–882 (2006).
Woltman, A. M., Op den Brouw, M. L., Biesta, P. J., Shi, C. C. & Janssen, H. L. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One 6, e15324 (2011).
Lee, C. C., Avalos, A. M. & Ploegh, H. L. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol 12, 168–179 (2012).
Isogawa, M., Robek, M. D., Furuichi, Y. & Chisari Francis, V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol 79, 7269–7272 (2005).
Ma, Z., Zhang, E., Yang, D. & Lu, M. Contribution of Toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses. Cell Mol Immunol 12, 273–282 (2015).
Skevaki, C., Pararas, M., Kostelidou, K., Tsakris, A. & Routsias, J. G. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious diseases. Clin Exp Immunol 180, 165–177 (2015).
Dunn, C. et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology 137, 1289–1300 (2009).
Vincent, I. E. et al. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS One 6, e26315 (2011).
Lee, B. O. et al. Interaction of the hepatitis B core antigen and the innate immune system. J Immunol 182, 6670–6681 (2009).
Lanford R. E. et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 144, 1508–1517, 17 e1–10 (2013).
Du, X., Poltorak, A., Wei, Y. & Beutler, B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw 11, 362–371 (2000).
Shen, N. et al. Sex-specific association of X-linked Toll-like receptor 7 (TLR7) with male systemic lupus erythematosus. Proc Natl Acad Sci USA 107, 15838–15843 (2010).
Xiao, W. et al. Association of Toll-like receptor 7 and 8 gene polymorphisms with Graves’ disease in Chinese Cantonese population. Tissue antigens 85, 29–34 (2015).
Said, E. A. et al. Association of single-nucleotide polymorphisms in TLR7 (Gln11Leu) and TLR9 (1635A/G) with a higher CD4T cell count during HIVinfection. Immunol let 160, 58–64 (2014).
Schott, E. S. et al. A toll-like receptor 7 single nucleotide polymorphism protects from advanced inflammation and fibrosis in male patients with chronic HCV-infection. J Hepatol 47, 203–211 (2007).
Yue, M. et al. Toll-like receptor 7 variations are associated with the susceptibility to HCV infection among Chinese females. Infec Genet Evol 27, 264–270 (2014).
Wang, C. H. et al. TLR7 and TLR8 gene variations and susceptibility to hepatitis C virus infection. PLoS One 6, e26235 (2011).
Kawasaki, A. et al. TLR7 single-nucleotide polymorphisms in the 3′ untranslated region and intron 2 independently contribute to systemic lupus erythematosus in Japanese women: a case-control association study. Arthritis Res Ther 13, R41 (2011).
1000 Genomes Project Consortium et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Funk, E., Kottilil, S., Gilliam, B. & Talwani, R. Tickling the TLR7 to cure viral hepatitis. J Transl Med 12, 129 (2014).
Xu, N., Yao, H. P., Lv, G. C. & Chen, Z. Downregulation of TLR7/9 leads to deficient production of IFN-alpha from plasmacytoid dendritic cells in chronic hepatitis B. Inflamm Res 61, 997–1004 (2012).
Wu, J. et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 49, 1132–1140 (2009).
Wang, C. M. et al. Genetic variations in Toll-like receptors (TLRs 3/7/8) are associated with systemic lupus erythematosus in a Taiwanese population. Sci Rep 4, 3792 (2014).
Wu, Y. W., Tang, W. & Zuo, J. P. Toll-like receptors: potential targets for lupus treatment. Acta Pharmacol Sin 36, 1395–1407 (2015).
Chang, J. J. & Lewin, S. R. Immunopathogenesis of hepatitis B virus infection. Immuno Cell Bio l85, 16–23 (2007).
Zhang, Y. L., Guo, Y. J., Bin, L. & Sun, S. H. Hepatitis C virus single-stranded RNA induces innate immunity via Toll-like receptor 7. J Hepatol 51, 29–38 (2009).
Yue, M. et al. Sex-specific association between X-linked Toll-like receptor 7 with the outcomes of hepatitis C virus infection. Gene 548, 244–250 (2014).
Geier A., Gartung C. & Dietrich C. G. Hepatitis B e Antigen and the Risk of Hepatocellular Carcinoma. N Engl J Med 347, 1721–1722; author reply 1721–1722 (2002).
Li, F. et al. Association between TLR7 copy number variations and hepatitis virus infection outcome in Chinese. World J Gastroenterol 23, 1602–1607 (2017).
Chen, C. J., Yang, H. I. & Iloeje, U. H. & REVEAL-HBV Study Group. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 49, S72–84 (2009).
Zhang, H. et al. Seroprevalence and risk factors for hepatitis B infection in an adult population in Northeast China. Int J Med Sci 8, 321–331 (2011).
Fish, E. N. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8, 737–744 (2008).
Berghofer, B. et al. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 177, 2088–2096 (2006).
Meier, A. et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15, 955–959 (2009).
Croagh, C. M. & Lubel, J. S. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol 20, 10395–10404 (2014).
Acknowledgements
We thank those who helped collect patients’ samples and extract DNAs from the samples. We also thank Mr. Tom Hamilton for polishing the language of this manuscript. This study was supported by the Collaborative Innovation Center of Infectious Diseases, Capital Medical University (PXM 2015_014226_000058), by the Beijing Key Laboratory (No: BZ0089), by the National Natural Science Foundation of China (No: 81571973), by the Scientific Research Project of Beijing Educational Committee (KM201410025005) and by the Beijing Municipal of Science and Technology Major Project(No: D141100000314005).
Author information
Authors and Affiliations
Contributions
Q.H., H.W., J.Z. and T.Z. conceived, designed and supervised the study. J.Z., L.C., A.L., K.Z., N.Z., B.S., Z.C. and N.C. collected samples and performed the experiments. J.Z. and Q.H. analyzed the data and wrote the paper. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, J., Zhang, T., Cao, L. et al. Toll like receptor7 polymorphisms in relation to disease susceptibility and progression in Chinese patients with chronic HBV infection. Sci Rep 7, 12417 (2017). https://doi.org/10.1038/s41598-017-12698-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12698-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.