Abstract
At the basis of a trophic web, coprophagous animals like dung beetles (Scarabaeoidea) utilize resources that may have advantages (easy gain and handling) as well as drawbacks (formerly processed food). Several studies have characterized the nutrients, e.g. C/N ratios and organic matter content, for specific types of dung. However, a comparative approach across dung types and feeding guilds of dung producers, and relationships between dung nutrients and preferences by coprophages, have been missing. Hence, we analyzed water content, C/N ratio, amino acid, neutral lipid fatty acid, free fatty acid and sterol composition and concentrations in dung from 23 vertebrates, including carnivore, omnivore and herbivore species. Our analyses revealed significant differences among the three vertebrate feeding guilds for most nutritional parameters. Although formerly processed, dung grants sufficient amounts of essential nutrients for insects. We tested whether nutrients can explain the dung beetles’ preferences in a field experiment, using 12 representative dung types in baits that were installed in 27 forests and 27 grasslands. Although consistent preferences for specific dung types were pronounced, the nutritional composition did not predict the variation in attractiveness of these dung diets, suggesting a primary role of dung volatiles irrespective of food quality.
Similar content being viewed by others
Introduction
Heterotrophic organisms have to consume food to generate energy, grow and maintain metabolism1, thus various strategies for detection, foraging and processing to exploit a wide range of diets have evolved2. Moreover the patchy distribution of manifold resources and its constant dynamics selected for differences in feeding behaviours; ranging from opportunistic to highly specialized feeding3. Some animals mainly use the metabolic trash of others (dung) to fulfil their energy requirements. Such coprophages (=“dung eaters”) substantially contribute to nutrient and energy flows in ecosystems4,5, since “in nature, nothing is wasted – not even waste”6.
One of the most common invertebrate coprophages are dung beetles (Scarabaeoidea); a cosmopolitan superfamily of insects which evolved a detritivorous life-style several million years ago7,8,9,10. Although the ancient detritivorous feeding behaviour (using all organic material, including dung, litter, humus and carcasses) still exists in many families of Scarabaeoidea11,12, the increased occurrence of megafauna during the lower Jurassic has provided a new exploitable resource in large quantities, facilitating the evolution of dung beetles towards coprophagy13. Dung beetles are often generalistic in their use of different types of faeces, although certain dung types are clearly more frequently utilized than others14. Such preferences may be influenced and modified by the “host” animals’ diet (carnivore, herbivore, and omnivore) which affects the nutrients, volatile organic compounds or odour intensities in their faeces11,14,15,16,17,18. Dung nutrients are of particular importance in dung beetle development, e.g. for their body size or the length of the male’s horns19,20. Dung itself consists of metabolic waste products and undigested remains of the original food. However, also other food sources like humus, fungi and carrion are used by dung beetles7, thus questioning dung as the only trophic resource for dung beetles which can supplement them with all mandatory nutrients. Furthermore, endosymbiotic bacteria associated with dung beetles may facilitate the digestion of dung and could foster a well-balanced nutritional supplementation21,22,23 as regularly found in others insects24,25,26. Several studies analyzed C/N ratios, organic matter contents, amino acids as well as further components (recently reviewed by Holter27) and addressed the high variability of composition and nutritional values among dung types and feeding guilds14. However, a comparative approach analysing the nutrients, dung type preferences and nutrient-preference-relationships in a broad variety of dung types from different species has not been conducted so far.
Hence, we address the following questions; i) does the nutritional composition differ among the dung from different feeding guilds of vertebrates; ii) can this dung potentially supply all essential macronutrients for insects like dung beetles; iii) does the dung differ in its attractiveness to dung beetles, and if; iv) do preferences correspond to nutritional composition? We analyzed C/N ratios, amino and fatty acids, sterol and water contents of dung from 23 vertebrate species (7 carnivores, 6 omnivores and 10 herbivores) using gas-chromatography (GC), gas chromatography/mass spectrometry (GC/MS) and ion-exchange chromatography (IEC). Furthermore, we used 12 (out of the 23) dung types for a field experiment to compare the attractiveness of dung from different feeding guilds to dung beetles. We show that i) the nutritional composition of dung differed among feeding guilds, although ii) almost all essential macronutrients were found in all samples; iii) dung beetles showed significant dung type preferences; but iv) preferences did not correspond to nutritional parameters.
Methods
Dung sampling and processing
We used 23 different dung types of carnivorous (otter, lynx, mink, raven, snowy owl, wolf, wild cat), omnivorous (chicken, wild boar, brown bear, fox, gerbil, raccoon) and herbivorous (cow, donkey, deer, elephant, elk, goat, horse, rabbit, sheep, wisent) species, which we collected at two organic farms, wildlife parks/zoos and private stocks around Darmstadt (detailed information on origin and diet of each species are provided in the Supplementary Information; Table S1 and Supplementary Methods S1). To better represent each dung type, most dung samples originated from several individuals, and individual droppings were mixed. We sampled dung at different times and checked for intraspecific variation between samples in all nutrients. As expected, variation among measurements was much smaller than across dung types: the coefficient of variation of amino acids, fatty acids and sterols, C/N ratios and water content was 3.4–7.6-fold higher across dung types than within each dung type (Supplementary Table S2). Moreover, cow dung from 11 different farms clustered together in a discriminant analysis of principal components (Supplementary Figure S1; for details see statistical analysis). We thus disregard the intraspecific variation in the main results.
Animals involved in this study had not faced any veterinarian treatment for several weeks before dung collection. After collecting fresh samples (i.e. droppings from the collection day) in a sufficient amount, the dung was filled in a tea bag (for dung baits used in the field), subsequently transferred into a freezer bag, sealed and labelled. A part of the sampled dung was further processed for chemical analysis (see below). All samples were stored in a freezer at −20 °C until use.
Water content and dry weight
Water content of each dung was determined with a microbalance (Mettler Toledo, XS3DU, Columbus OH, USA; readability 0.1 μg and 1 μg repeatability) two times with three replicates (20 mg, 50 mg and 100 mg) per dung; (a) the initial fresh mass of the dung prior to drying and (b) its dry mass after the dung has been dried until weight constancy in an oven at 60 °C for at least 4 days. For the determination of the dry weight of the bird faeces (raven and snowy owl), which were partly absorbed in sand, the actual dry organic matter was determined by fully annealing the dung samples with a Bunsen burner and back-weighing of the ignition residues. Linear regression formulas for the calculation of each dung’s dry weight are given in the Supplementary Information (List S1).
Lipid and sterol analyses
Total neutral lipids (hereafter, neutral lipid fatty acids = NLFAs) were extracted from the fresh dung samples (40–50 mg of fresh weight) using 1 ml of a chloroform:methanol-mixture, 2:1 (V/V)28 over a period of 24 h. Afterwards two replicate extracts were purified and separated29,30 to fractionate neutral lipid fatty acids (NLFAs) and free fatty acids (FFAs), respectively (for detailed procedure see Supplementary Method S1). After the samples had been fractionated by column chromatography, they were finally measured with a QP2010 Ultra GC/MS (Shimadzu, Duisburg, Germany). The gas chromatograph (GC) was equipped with a ZB-5MS fused silica capillary column (30 m × 0.25 mm ID, df = 0.25 μm) from Phenomenex (Aschaffenburg, Germany). 1 μl sample aliquots were injected by using an AOC-20i autosampler-system (Shimadzu, Duisburg, Germany) into a PTV-split/splitless-injector (Optic 4, ATAS GL, Eindhoven, Netherlands), which operated in splitless-mode. Injection-temperature was programmed from initial 70 °C up to 300 °C and then an isothermal hold for 59 minutes. Hydrogen was used as carrier-gas with a constant flow rate of 1.5 ml/min. The temperature of the GC oven was raised from initial 60 °C for 1 min, to 150 °C with a heating-rate of 15 °C/min, to 260 °C with a heating-rate of 3 °C/min, to 320 °C with a heating-rate of 10 °C/min and then an isothermal hold at 320 °C for 10 min. Electron ionization mass spectra were recorded at 70 eV from m/z 40 to 650. The ion source of the mass spectrometer and the transfer line were kept at 250 °C. FAMEs were identified based on their retention indices31 and m/z fragmentation patters as well as by comparison with the FAME and BAME analytical standards (Sigma-Aldrich, St. Lois, USA). The configurations of the double bonds were not specifically determined. The amount of fatty acids (i.e. NLFAs and FFAs) [µg] was standardized using the dry weight [mg] calculated form the initial fresh weight of the sample.
Sterols were quantified based on the peak area of detected compounds relative to the constant amount of the internal standard (220 ng/µl nonadecanoic acid) expressed in [%] of this standard, because we did not determine the response factor of the sterols to the internal standard. Cholesterol was the only sterol that was identified based on its m/z fragmentation [as cholesteryl methyl ether: 400 (M+, 60); 385 (24); 368 (100); 353 (59), 329 (31), 301 (25), 275 (37), 213 (26), 145 (42), 107 (50), 81 (46), 69 (27), 55 (41)], for the other sterols we just checked for correct substance class assignment (as sterols) based on their mass spectra. The amounts of sterols [% Std.] and cholesterol [% Std.] were standardized using the dry weight [mg] calculated form the initial fresh weight of the sample. The cholesterol/sterol ratio [%] was calculated based on both values.
Amino acid analysis
For analysis of the amino acids (free amino acids and protein-bounded), 5 mg (±0.1 mg) dried dung was diluted in 200 μL of hydrochloric acid (6 mol/l) and boiled for four hours at 100 °C, processed (for detailed procedure see Supplementary Method S1) and finally measured (as described in32) with an ion exchange chromatograph with ninhydrin post-column derivatization (Biochrom 20+, Amino Acid Analyzer, Cambridge, UK). A standard amino acid mixture (Laborservice Onken GmbH, Gründau, Germany) was used as external standard. The amount of total amino acids [µg] was standardized using the dry weight [mg] of the initial sample. Note that this acidic chemical extraction decays asparagine, glutamine and tryptophan.
C/N analysis
Dried dung samples were mixed with hydrochloric acid (HCl; approx. 0.05 mol/l) to remove the inorganic carbonate as CO2. Samples were subsequently dried again and weighed into tin capsules (6 ± 1 mg dry weight). Total organic carbon and nitrogen contents were measured by an elemental analyser (EA 1108 Elemental Analyser, Carlo Erba, Milan, Italy). Acetanilide (Merck, Darmstadt, Germany) was used for internal calibration.
Field sampling and study site
For dung beetle field samplings we used pitfall traps equipped with the dung baits of 12 different representative subsamples (i.e. dung available in sufficient amounts) collected from mammal species, namely: wolf, lynx, fox, brown bear, wild boar, cow, horse, sheep, deer, elephant, elk and wisent (2 carnivores, 3 omnivores and 7 herbivores). Six dung types commonly occur in the sampled regions (game in forests and grasslands: fox, deer, wild boar, livestock on pastures: cow, horse, sheep). However, previous studies showed there is no effect of conditioning for dung beetles, when raised on a certain dung type33. The traps were set up randomized on a transect, in a total of 54 experimental sites (27 in forests, 27 in grasslands) in three regions of Germany within the Biodiversity Exploratories’ framework (see Supplementary Method S1). All dung baits have been taken out of the freezer approx. one hour before placing them in the field, which ensures an equal defrosting time and temperature for the samples (mean temperature during field sampling: 19.5 °C). All field samplings were performed between 29th June and 17th July 2015. Pitfall traps were collected after 48 hours, trapped beetles were labelled (date, site-ID, dung type) and stored in a freezer at −20 °C. Dung beetles were identified to species level using the keys of Freude, et al.34, Bunalski35 and Rössner36, and confirmed by taxonomic experts (see Acknowledgements). All methods were carried out in accordance with relevant guidelines and regulations, and sampling of dung and beetles was approved by local authorities, see Acknowledgements.
Data processing and statistical analyses
Preferences of dung beetles across different dung were analyzed on three different levels: i) the number of dung beetles collected per plot (dependent variable) on a certain dung type (independent variable) was analysed using Friedman test with plot ID as blocking factor. ii) The number of dung beetles collected on each plot/number of traps (dependent variable) on dung of the three different feeding guilds (independent variable) was analyzed using Friedman test with plot ID as blocking factor, as well. iii) The proportional abundance [%] of different beetle species on the dung from each of the three different feeding guilds was visualized as bipartite network and analyzed using H2'37, representing a measurement for the overall specialization of the compositional dung type – beetle species network. The network analysis was performed with the R package “bipartite”38.
We statistically analyzed the C/N ratio, amount of amino/fatty acids [µg/mg], the water content [%] and the amounts of sterols [% Std./mg] of dung types (carnivore, omnivore, herbivore) using ANOVA and TukeyHSD post-hoc test. We checked for the normal distribution of the residuals and the homogeneity of variance prior to the analyses and transformed the data if necessary (C/N ratio, the amount of NLFAs s [µg/mg] were log-transformed, the amounts of sterols [% Std./mg] were log(x + 1)-transformed, the ratio of FFA/total lipids [%] was arcsine square root transformed whereas the amount of free fatty acids [µg/mg] and amino acids [µg/mg] were square root transformed and 4th-square root transformed, respectively). To compare the cholesterol/ sterol ratio [%] and the amount of FFAs [µg/mg] across dung types we used Kruskal-Wallis tests with subsequent pairwise U-tests corrected for false discovery rate39 in multiple comparisons.
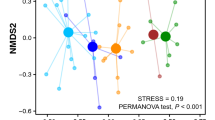
Compositional data of amino- and NLFA profiles were analyzed using discriminant analysis of principal components (DAPC) and PERMANOVA/PERMDISP as implemented in the R packages “adegenet”40 and “vegan”41, respectively. DAPC is a powerful method to discriminate a priori assigned groups in a multivariate ordination of chemical compositional data42. It transforms the original data by PCA prior to the discriminant analysis (DA) and therefore values become uncorrelated. We retained 7 (for amino acids) and 5 (for NLFAs) PC-axes based on their Eigenvalues and the explained variance. We further used PERMANOVA43 with Bray-Curtis similarities44 to test if overall composition of either amino acids (all amino acids and essential amino acids) or NLFAs differed across dung types. In case of significant PERMANOVA, we used PERMDISP45 to distinguish between location/dispersion effects (see46 and Brückner and Heethoff42 for details) and to test whether the compositional stability of nutrients differed among dung types. All statistical analyses were performed with R version 3.3.1 “Bug in Your Hair”47. Finally, we correlated the total number of dung beetles (i.e. pooled from all plots) trapped on the respective dung type to different nutritional parameters of the dungs (means of C/N ratio, amounts of all/essential amino acids, NLFAs, FFAs and sterols as well as cholesterol/sterol ratio and water content) using Spearman’s rank correlation in PAST 348.
Results
Nutritional analyses
Nutrients in vertebrate dung samples (see summarized in Table 1) differed significantly across the three feeding guilds (ANOVA; C/N ratio: F2,18 = 26.5, p < 0.001, Fig. 1A; amino acids: F2,20 = 3.7, p = 0.044, Fig. 1B; NLFAs: F2,20 = 4.3, p = 0.028, Fig. 1C), except for water content (F2,20 = 3.1, p = 0.065, Fig. 1D) and FFAs (Kruskal-Wallis test; χ2 = 2.6, df = 2, n = 21, p = 0.267, Fig. 2A). However, the total lipid content (NLFAs and FFAs combined) showed differences among the feeding guilds (ANOVA; F2,18 = 3.9, p = 0.039, Fig. 2B) and the amounts of FFAs and NLFAs were not correlated (Spearman´s rank: ρs = 0.14, p = 0.55). Also for the FFA/total lipid ratio and cholesterol/sterol ratio we found significant differences among the feeding guilds (FFAs/total lipid ratio: ANOVA; F2,18 = 4.4, p = 0.028, Fig. 2C; cholesterol/sterol ratio: Kruskal-Wallis test; χ2 = 8.2, df = 2, n = 23, p = 0.017, Fig. 2E), while sterol amounts were similar (ANOVA; F2,20 = 0.9, p = 0.44, Fig. 2D). The amino acids differed in relative composition between the feeding guilds (PERMANOVA; all amino acids: pseudoF2,20 = 2.7, R2 = 0.22, p = 0.013, Fig. 3A; only essential amino acids: pseudoF2,20 = 2.2, R2 = 0.18, p = 0.039) but not in overall dispersion across species within each guild (PERMDISP; all amino acids: F2,20 = 1.5, p = 0.25, Figs 3A, S2 II, Table 2; only essential amino acids: F2,20 = 1.1, p = 0.36), whereas NLFAs differed both in composition and dispersion (PERMANOVA; pseudoF2,20 = 4.8, R2 = 0.32, p < 0.001; PERMDISP: F2,20 = 5.0; p = 0.017, pairwise PERMDISP herbivore vs. omnivore p = 0.008; Figs 3B, S2 I, Table 3). In summary (see Table 1), carnivore dung types provided higher nutritional values (highest mean values for amino and fatty acids, sterols and cholesterol, lowest C/N ratio). Omnivore dung types provided intermediate nutritional value throughout the analyses.
Nutritional values for carnivore, omnivore and herbivore dung (left to right): C/N ratio (A), amino acids (B), neutral lipid fatty acids - NLFAs (C) and water content (D). Each point represents a different vertebrate species, and lines within each feeding guild represent the median. Significant differences among groups are marked by different letters for each panel. (Animal drawings in Figs 1–4 by Adrian Brückner).
Nutritional values for carnivore, omnivore and herbivore dung (left to right): free fatty acids (A), total lipids (B), free fatty acids/total lipids ratio (C), sterols (D) and cholesterol/sterol ratio (E). Each point represents a different vertebrate species, and lines within each feeding guild represent the median. Significant differences among groups are marked by different letters for each panel. (F) Shows a pitfall trap with dung bait for dung beetle sampling.
Dung type preference
We sampled a total of 1191 individuals from 23 dung beetle species in 40 sites; in 14 out of 54 sites no dung beetles were trapped. Overall, dung beetles were attracted to all 12 dung types offered (Fig. 4C). Species-specific preferences of the beetles among dung types were significant (Friedman test: χ2 = 62.1, df = 11; n = 648, p < 0.0001, Fig. 4A), but not consistent across feeding guilds (Friedman test: χ2 = 2.8, df = 2, n = 162, p = 0.25, Fig. 4B). Different beetle species had relatively similar preferences and showed no clear species partitioning across dung types, hence there was only a relatively low degree of complementary specialization in the dung type – beetle network (H2′ = 0.30, 23 beetle species across dung from 3 feeding guilds).
Overview of trapped beetles for a subset of 12 representative dung types offered in 27 forests and 27 grasslands. (A) shows the mean number of trapped beetles for each dung type (red = carnivore, blue = omnivore, green = herbivore), while (B) shows the total number of trapped beetles per feeding guild. (C) A resource - beetle trophic network shows the proportional distribution of dung beetle species across dung from three feeding guilds (red = carnivore, blue = omnivore, green = herbivore dung).
Correlation between dung nutrients and attractiveness for dung beetles
Overall, there was no correlation between dung beetle abundances and any of the nutrient parameters for all 12 dung types (Spearman’s rank: C/N ratio: ρs = −0.48, p = 0.11; all amino acids: ρs = 0.46, p = 0.14; essential amino acids: ρs = 0.42, p = 0.17; NLFAs: ρs = 0.40, p = 0.20; FFAs: ρs = 0.16, p = 0.62; water content: ρs = −0.36, p = 0.24; sterols: ρs = 0.15, p = 0.55, cholesterol/sterol ratio: ρs = 0.26, p = 0.41).
Discussion
Dung beetles strongly depend on a resource that is scarce and patchy in occurrence. Yet, dung is immobile as well as chemically and mechanically undefended, which makes it an easily acquirable and valuable source of energy. Adult dung beetles are attracted to many different dung types, regardless of the type of dung on which they have fed and grown as a larva7,14,33. Furthermore, especially in the tropics, some of these beetles became highly specialized in resource usage49.
Our field experiment demonstrated a generalized usage of all offered dung types, but also a significant difference in the quantity of beetles attracted across dung types (Fig. 4A–C). Generally, higher amounts of dung attract more dung beetles50, but since we used equal amounts of dung for the baits (approx. 35 g each) there must be alternative explanations for their preferences, for which the amount and composition of volatile organic compounds must play a key role16,51 given that the beetles had no contact to the dung in our experiment (see Fig. 2F). For instance, indole and skatole, two weak/moderate attractors51 are molecules derived from the decomposition of the amino acid tryptophan, whereas phenolic compounds (e.g., phenol, p-cresol, p-ethyl phenol) are derivatives of phenylalanine and tyrosine. Also, fatty acids and fatty acid derived compounds like butyric acid, unspecific butanones and butanoles or ethyl-/butyl-esters are present in various dungs33,52. Hence, dung odour bouquets as proximate cues may potentially include intrinsic information on their ultimate cause, i.e. dung nutrients (especially amino- and fatty acids) which are converted and rearranged to volatile organic compounds.
Accordingly, besides the attractive function of dung volatiles33,51,52, these compounds may also serve as “nutritional cues” for dung beetles. Therefore we asked whether the beetles’ preference matched the differences in nutrient quality of dung. Although beetles used all dung types, some were preferred over others, and the most preferred ones occurred across the feeding guilds (e.g. lynx, wild boar and sheep). Nutritional composition, however, was no significant predictor to explain the beetle’s preference. Hence, volatiles are most probably no nutritional cues. Yet, volatile organic compounds only recently receive growing attention in dung beetle research16,17,33,51,53 and more in-depth analyses may help to unravel the beetles’ resource choices linked to dung nutrients52.
In general, animal droppings vary in nutrient amounts, even within a species or feeding guild54. Whereas higher nutrient concentrations are generally beneficial, dung beetles may face trade-offs that constrain a higher preference of nutrient-rich dung. Carnivore dung, for example, is more nutrient rich compared to herbivore dung (Figs 1–3 14), but could contain pathogenic bacteria, which are perceived by the dung beetles via olfactory cues53.
The C/N ratio is frequently used as an index for quality descriptions of organic substrates including dung54. In general C/N ratios increased over ten-fold from carnivore dung (lowest value for mink, 5.0) to herbivore dung (highest value for donkey, 59.2); omnivore dung has intermediate levels (Fig. 1, Table 1). Corresponding to a higher nitrogen (N) content (i.e. the reverse of the C/N ratio), the amount of amino acids and thus a higher nutritional value55 increased from carnivore to herbivore dung, associated with a change in proportional composition of amino acid (Fig. 3). Still, all dung types contained nearly all amino acids that are essential for insects and thus for dung beetles56 (except for methionine in mink, otter and raccoon dung). Amino acids play key roles in insect development such as the emergence from the pupal skin, they are precursors of pigments or promote growth in larvae and adults57. Therefore our results highlight that dung is able to supply the beetles’ need for amino acids in general – for further synthesis as well as for direct use.
Similar to the trend in nitrogen and amino acids, the amount of NLFAs increased over ten-fold from herbivores (goat and rabbit, 8 µg/mg) via omnivores to carnivores (lynx, 105 µg/mg). Additionally, feeding guilds had specific fatty acids corresponding to their food such as unsaturated fatty acids (e.g. oleic and linoleic acid) for carnivores and omnivores, while herbivores showed higher amounts of saturated fatty acids (Table 3). Interestingly, there was no analytical indication for α-linolenic acid (C18:3Δ9, 12, 15) in NLFAs and only in trace amounts for FFAs. α-linolenic acid is thought to be one of the essential fatty acids for insects58 and hence, needs to be consumed or supplemented by symbiotic bacteria. Although our method allows for detecting FFAs from C6:0 (caprylic acid) to C28:0 (montanic acid), we only found C16:0/C18:0 in notable amounts. These amounts were similar among all feeding guilds and also the overall lipids [µg/mg] showed a similar pattern to NLFA amounts. However, the FFAs/total lipid ratio (Fig. 2C) indicated more free fatty acids in herbivore than in carnivore dung, suggesting different performance in fat digestion in these vertebrates but also different palatability for dung beetles and other coprophagous animals. Overall, the high concentrations of fatty acids and other nutrients supports the view that dung is not waste, but a valuable resource for coprophagous beetles6, which particularly utilize fatty acids during growth and larval emergence59,60,61.
Water contents of different dung types were similar (except for gerbil dung that contained only 7% water) (Fig. 1, Table 1). Still, water content plays an important role, as adult dung beetles mainly use the liquid phase and its nutrients/particles to feed on27, it affects the occurrence of species15 and the handling for brood balls62.
Insects, unlike other animals, lack the ability to synthesize sterols, and they must obtain such compounds via food or bacterial symbionts56. Sterols have several key functions, since they serve as components of the cell-membrane (especially cholesterol), as regulators of developmental genes and as precursors of different hormones63. Our analysis confirmed sterols and cholesterol in all dung types, yet some amounts were extremely low, especially in herbivores. Carnivore dung seems to be a valuable resource regarding cholesterol (Fig. 2D–E), which typically needs to be metabolized from certain phytosterols by herbivores64,65,66,67. Dung beetles may thus either obtain cholesterol directly from dung (especially carnivore dung) or synthesize it from sterols in herbivore/omnivore dung. The amount of sterols in dung may, however, be too low to fully supplement dung beetles, thus consumption of other food (e.g. plant material12) or bacterial symbiosis might help to acquire all mandatory sterols.
Conclusion
All nutrient parameters, C/N ratio, amino acids, fatty acids, cholesterol/sterol ratio and the composition of amino acids varied across dung types and feeding guilds. Although dung represents an already-digested, but still valuable resource it grants sufficient amounts of most (essential) nutrients for insects. Hence, symbiotic bacteria may not be mandatorily needed for nutritional upgrading. Regarding C/N, protein (=amino acid content) and fatty acids (=NLFAs and free fatty acids), dung showed similar values to resources available for other terrestrial beetles, such as litter, fruits, fungi and carcasses (see Supplementary Information; Table S3). We did not confirm that nutritional composition drives the beetles’ food selection, suggesting that the beetles’ attraction to specific blends of volatiles are uncoupled from nutrient values, and thus volatiles may not serve as nutritional cues.
Ethics statement
Field work and animal collection permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg (according to § 72 BbgNatSchG).
Data, code and materials
All datasets supporting this article have been uploaded as part of the Supplementary Material.
References
Lotka, A. J. Contribution to the energetics of evolution. P Natl Acad Sci USA 8, 147–151 (1922).
Schmidt-Nielsen, K. Animal physiology: adaptation and environment. (Cambridge University Press, 1997).
Begon, M., Townsend, C. R. & Harper, J. L. Ecology: from individuals to ecosystems. (Wiley, 2009).
Nichols, E. et al. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141, 1461–1474 (2008).
Wu, X. W., Duffy, J. E., Reich, P. B. & Sun, S. C. A brown-world cascade in the dung decomposer food web of an alpine meadow: effects of predator interactions and warming. Ecol Monogr 81, 313–328 (2011).
Jones, R. Call of Nature: The secret life of dung. (Pelagic Publishing Ltd, 2017).
Hanski, I. & Cambefort, Y. Dung beetle ecology. (Princeton University Press, 1991).
Chin, K. & Gill, B. D. Dinosaurs, dung beetles, and conifers: participants in a Cretaceous food web. Palaios 11, 280-285 (1996).
Davis, A. L. V., Scholtz, C. H. & Philips, T. K. Historical biogeography of scarabaeine dung beetles. J Biogeogr 39, 1217–1256 (2002).
Nikolajev, G. V. & Dong, R. New genus of the subfamily Geotrupinae (Coleoptera: Scarabaeoidea: Geotrupindae) from the Jehol Biota. Acta Geol Sin-Engl 84, 673–675 (2010).
Halffter, G. & Matthews, E. G. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). A monograph. Folia Entomol Mex 12/14, 1–312 (1966).
Rembialkowska, E. Energy-balance of the developmental period of Geotrupes stercorosus (Scriba) (Scarabaeidae, Coleoptera). Ekol Pol-Pol J Ecol 30, 393–427 (1982).
Cambefort, Y. in Dung beetle ecology (eds I. Hanski & Y. Cambefort) Ch. 2, 22–35 (Princeton University Press, 1991).
Whipple, S. D. & Hoback, W. W. A comparison of dung beetle (Coleoptera: Scarabaeidae) attraction to native and exotic mammal dung. Environ Entomol 41, 238–244 (2012).
Gittings, T. & Giller, P. S. Resource quality and the colonisation and succession of coprophagous dung beetles. Ecography 21, 581–592 (1998).
Schmitt, T., Krell, F. T. & Linsenmair, K. E. Quinone mixture as attractant for necrophagous dung beetles specialized on dead millipedes. J Chem Ecol 30, 731–740 (2004).
Dormont, L., Rapior, S., McKey, D. B. & Lumaret, J. P. Influence of dung volatiles on the process of resource selection by coprophagous beetles. Chemoecology 17, 23–30 (2007).
Scholtz, C. H., Davis, L. V. & Kryger, U. Evolutionary biology and conservation ecology of dung beetles. (Pensoft Publishers, 2009).
Emlen, D. J. Diet alters male horn allometry in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc Roy Soc B-Biol Sci 264, 567–574 (1997).
Moczek, A. P. & Emlen, D. J. Proximate determination of male horn dimorphism in the beetle Onthophagus taurus (Coleoptera: Scarabaeidae). J Evol Biol 12, 27–37 (1999).
Halffter, G. & Matthews, E. G. The natural history of dung beetles. A supplement on associated biota. Rev Latinoam Microbiol 13, 147–163 (1971).
Rougon, D., Rougon, C., Levieux, J. & Trichet, J. Variations in the amino-acid content in Zebu dung in the Sahel during nesting by dung beetles (Coleoptera, Scarabaeidae). Soil Biol Biochem 22, 217–223 (1990).
Estes, A. M. et al. Brood ball-mediated transmission of microbiome members in the dung beetle, Onthophagus taurus (Coleoptera: Scarabaeidae). Plos One 8, e79061 (2013).
Douglas, A. E. The microbial dimension in insect nutritional ecology. Funct Ecol 23, 38–47 (2009).
Kaltenpoth, M. Actinobacteria as mutualists: general healthcare for insects? Trends Microbiol 17, 529–535 (2009).
Gibson, C. M. & Hunter, M. S. Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol Lett 13, 223–234 (2010).
Holter, P. Herbivore dung as food for dung beetles: elementary coprology for entomologists. Ecol Entomol 41, 367–377 (2016).
Folch, J., Lees, M. & Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226, 497–509 (1957).
Frostegård, A., Tunlid, A. & Bååth, E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Meth 14, 151–163 (1991).
Tserng, K. Y. & Griffin, R. Quantitation and molecular species determination of diacylglycerols, phosphatidylcholines, ceramides, and sphingomyelins with gas chromatography. Anal Biochem 323, 84–93 (2003).
Stein, S. E. Mass Spectra by NIST Mass Spec Data Center. NIST Chemistry WebBook, NIST Standard (2015).
Leonhardt, S. D. & Blüthgen, N. The same, but different: pollen foraging in honeybee and bumblebee colonies. Apidologie 43, 449–464 (2012).
Dormont, L., Jay-Robert, P., Bessiere, J. M., Rapior, S. & Lumaret, J. P. Innate olfactory preferences in dung beetles. J Exp Biol 213, 3177–3186 (2010).
Freude, H., Harde, K. W., Lohse, G. A. & Klausnitzer, B. Die Käfer Mitteleuropas. 8. Teredilia, Heteromera, Lamellicornia. (Spektrum Akad. Verlag, 1969).
Bunalski, M. Die Blatthornkäfer Mitteleuropas (Coleoptera, Scarabaeoidea): Bestimmung, Verbreitung, Ökologie. (František Slamka, 1999).
Rössner, E. Die Hirschkäfer und Blatthornkäfer Ostdeutschlands (Coleoptera: Scarabaeoidea). (Verein der Freunde und Förderer des Naturkundemuseums Erfurt, 2012).
Blüthgen, N., Menzel, F. & Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol 6, 9 (2006).
Dormann, C. F., Fründ, J., Blüthgen, N. & Gruber, B. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J 2, 7-24 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57, 289-300 (1995).
Jombart, T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403–1405 (2008).
Oksanen, J. et al. The vegan package. Community ecology package 10, 631–637 (2007).
Brückner, A. & Heethoff, M. A chemo-ecologists’ practical guide to compositional data analysis. Chemoecology 27, 33-46 (2017).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol 26, 32–46 (2001).
Bray, J. R. & Curtis, J. T. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27, 325–349 (1957).
Anderson, M. J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62, 245–253 (2006).
Anderson, M., Gorley, R. & Clarke, K. PERMANOVA+ for PRIMER: guide to software and statistical methods (PRIMER-E Limited, 2008).
R Core Team: R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2016).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4, 9 (2001).
Larsen, T. H., Lopera, A. & Forsyth, A. Extreme trophic and habitat specialization by peruvian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopts Bull 60, 315–324 (2006).
Errouissi, F., Haloti, S., Jay-Robert, P., Janati-Idrissi, A. & Lumaret, J. P. Effects of the attractiveness for dung beetles of dung pat origin and size along a climatic gradient. Environ Entomol 33, 45–53 (2004).
Wurmitzer, C. et al. Attraction of dung beetles to herbivore dung and synthetic compounds in a comparative field study. Chemoecology 27, 75–84 (2017).
Stavert, J. R., Drayton, B. A., Beggs, J. R. & Gaskett, A. C. The volatile organic compounds of introduced and native dung and carrion and their role in dung beetle foraging behaviour. Ecol Entomol 39, 556–565 (2014).
Mansourian, S. et al. Fecal-derived phenol induces egg-laying aversion in Drosophila. Curr Biol 26, 2762–2769 (2016).
Holter, P. & Scholtz, C. H. What do dung beetles eat? Ecol Entomol 32, 690–697 (2007).
Elser, J. J. et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580 (2000).
Nation, J. Insect physiology and biochemistry. (CRC press, 2002).
Gilmour, D. The biochemistry of insects. (Academic Press, 1961).
Prosser, C. L. Environmental and metabolic animal physiology. (Wiley, 1991).
Gilbert, L. I. & O’Connor, J. D. Lipid metabolism and transport in arthropods. Chemical Zoology 5, 229–253 (2012).
Verdu, J. R., Casas, J. L., Lobo, J. M. & Numa, C. Dung beetles eat acorns to increase their ovarian development and thermal tolerance. Plos One 5, e10114 (2010).
Arrese, E. L. & Soulages, J. L. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55, 207–225 (2010).
Hanski, I. in Nutritional ecology of insects, mites, spiders and related invertebrates (eds F. Slansky, Jr., and J.G. Rodriguez) pp. 837–84 (Wiley, 1987).
Behmer, S. T. & Nes, W. D. Insect sterol nutrition and physiology: a global overview. Advances Insect Phys 31, 1–72 (2003).
Behmer, S. T. & Elias, D. O. The nutritional significance of sterol metabolic constraints in the generalist grasshopper Schistocerca americana. J Insect Physiol 45, 339–348 (1999).
Behmer, S. & Elias, D. Phytosterol structure as a basis of food aversion learning in the grass hopper Schistocerca americana (Orthoptera: Acrididae). Physiol Entomol 24, 18–27 (1999).
Behmer, S. T. & Elias, D. O. Sterol metabolic constraints as a factor contributing to the maintenance of diet mixing in grasshoppers (Orthoptera: Acrididae). Physiol Biochem Zool 73, 219–230 (2000).
Jing, X. F., Grebenok, R. J. & Behmer, S. T. Sterol/steroid metabolism and absorption in a generalist and specialist caterpillar: Effects of dietary sterol/steroid structure, mixture and ratio. Insect Biochem Molec 43, 580–587 (2013).
Acknowledgements
We would like to thank Phillipp Bauer for help during fieldwork and Ursula Lebong for help with the C/N analysis. We thank Katrin Emmerich, Marietta Hülsmann and Eckehard Rößner for their great support in species identification. We also thank the Fasanerie Wiesbaden, the farm Oberfeld/Darmstadt, the Vivarium Darmstadt, Katja Wehner, Melanie N. Chisté, Marion Ebel (Fasanerie Hanau), Jörg Beckmann (Opel-Zoo) and their staff for help in dung acquisition.We thank the managers of the three Exploratories, Kirsten Reichel-Jung, Katrin Lorenzen, Martin Gorke and all former managers for their work in maintaining the plot and project infrastructure; Christiane Fischer and Jule Mangels for giving support through the central office, Andreas Ostrowski for managing the central data base, and Markus Fischer, Eduard Linsenmair, Dominik Hessenmöller, Daniel Prati, Ingo Schöning, François Buscot, Ernst-Detlef Schulze, Wolfgang W. Weisser and the late Elisabeth Kalko for their role in setting up the Biodiversity Exploratories project. Field work permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg (according to § 72 BbgNatSchG). KF and NB were funded by the German Research Foundation (DFG) Priority Program 1374 “Infrastructure-Biodiversity-Exploratories” (DFG, BL 960/3–1). AB was supported by a PhD scholarship from the German National Academic Foundation (Studienstiftung des deutschen Volkes). We acknowledge support by the German Research Foundation and the Open Access Publishing Fund of Technische Universität Darmstadt.
Author information
Authors and Affiliations
Contributions
K.F. conceived the initial idea, carried out the field and dung sampling, identified species, assisted the chemical analyses, participated in the design and formal analyses of the study and drafted the manuscript; A.B. conceived the initial idea, performed chemical analyses, carried out the statistical analyses and drafted the manuscript; A.H. collected dung and performed amino acid analyses; M.H. designed chemical analyses and discussed results; N.B. acquired funding, designed and coordinated the overall study, and drafted the manuscript. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frank, K., Brückner, A., Hilpert, A. et al. Nutrient quality of vertebrate dung as a diet for dung beetles. Sci Rep 7, 12141 (2017). https://doi.org/10.1038/s41598-017-12265-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12265-y
This article is cited by
-
Phylogenetic and ecological reevaluation of the order Onygenales
Fungal Diversity (2022)
-
Red Panda feces from Eastern Himalaya as a modern analogue for palaeodietary and palaeoecological analyses
Scientific Reports (2021)
-
Animals connect plant species and resources in a meta-ecosystem
Landscape Ecology (2021)
-
(My Microbiome) Would Walk 10,000 miles: Maintenance and Turnover of Microbial Communities in Introduced Dung Beetles
Microbial Ecology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.