Abstract
Populus euphratica Olivier, which has been considered as a tree model for the study of higher plant response to abiotic stresses, survive in the desert ecosystem characterized by extreme drought stress. To survive in the harsh environmental condition the plant species have developed some plasticity such as the development of heteromorphic leaves and well-developed roots system. We investigated the physiological and molecular mechanisms enabling this species to cope with severe stress caused by drought. The heterophylly, evolved from linear to toothed-ovate shape, showed the significant difference in cuticle thickness, stomata densities, and sizes. Physiological parameters, SOD, POD, PPO, CAT activity, free proline, soluble protein and MDA contents fluctuated in response to soil drying. Gene expression profile of roots monitored at control and 4 moisture gradients regimes showed the up-regulation of 124, 130, 126 and 162 and down-regulation of 138, 251, 314, 168 DEGs, respectively. Xyloglucan endotransglucosylase/ hydrolase gene (XET) up-regulated at different moisture gradients, was cloned and expressed in tobacco. The XET promoter sequence harbors the drought signaling responsive cis-elements. The promoter expression activity varies in different organs. Over-expression and knocked down transgenic tobacco plant analysis confirmed the role of XET gene in roots growth and drought resistance.
Similar content being viewed by others
Introduction
Abiotic stress, drought stress particularly, impacts plant productivity1, and imposes restrictions on the distribution of plant species across different types of environments. Some plants, for example trees, have evolved distinct morphological and physiological traits2, and exhibit strong tolerance to water shortage than other plants3. The desert popular, Populus euphratica (Olivier), is a unique tree that can survive in the serious desert environments (Figure S1) and exhibits remarkable tolerance to environmental stresses4. Due to its greater ability to cope with environmental stresses, P. euphratica is widely considered as an ideal model system when studying the molecular mechanisms of abiotic stress responses in woody species4,5,6,7,8,9,10.
The P. euphratica developed heteromorphic leaves to acclimatize to the desert conditions. During developmental stages, the leaf appears in several shapes, stomata structures, epidermal appendages (wax crystals and trichomes) and specialized cells (mucilage cells and crystal idioblasts)11. The structural characteristics of the heteromorphic leaf are related to its eco-adaptability12, achieved by the differential gene expression13, which might alter specific regulatory pathways according to the degree of drought stress it receives14. Recently, transcriptome analysis of Populus leaves confirmed the Stomata closure inhibition under moderate drought to maintain water transportation and a relatively high rate of carbon dioxide assimilation15. Molecular mechanisms covering the differential expression of the genes in P. euphratica under different stress levels have been described previously16,17. Different genes have been reported with altered transcript abundance including genes for the small HSP, HSP70 and HSP90 heat shock protein families, as well as members of the transcription factor families bZIP, AP2/EREPB, NF-Y, NAC, MYB and Homeobox and WRKY14. The reference genes, RPL17, HIS, EF1𝛼, TUB GII𝛼 and PeHAB1, from one-year-old seedling leaves, were found to be altered by various abiotic stresses. Genome-scale transcriptome analysis provides an extensive catalogue of the genes expressed in P. euphratica under different stress conditions i.e. PeuHsf gene expression was significantly induced by drought, salt and heat stresses18. The Populus special miRNA-target interactions19 might be involved in some biological process-related water deficit stress tolerance.
The P. euphratica have developed phreatophyte roots which deeply penetrate into the soil for moisture availability. Roots respond to a variety of below and above ground signals that modulate root system architecture20,21 by showing tropic growth responses. The root response is mainly governed by environmental stimuli22,23 as well as differential gene expression patterns and hormonal regulation24. Hypothetically, roots respond to and exploit the water gradients in the soil by the positive hydrotropism and grow towards increasing moisture, which explains the drought avoidance and precise exploitation of water patches25,26,27. The gene expression pattern changes with varying soil moisture and groundwater table28, showing a sensitive hydrotropic response. The fundamental and significant advances of the mechanism of hydrotropism and its interaction with gravitropism, at the cellular, molecular and genetic level, are not fully understood yet, as a little has been reported about the genetic players to explain how hydrotropism operates in the real world at molecular levels. Recent reports showed that MIZ1, MIZ2, AHR1 and NHR1 seem to play important roles in hydrotropism27,29. Similarly, the Ps-EXGT1 (XET) isolated from the roots of an agravitropic pea mutant, ageotropum, strongly expressed in hydrotropically responding roots30.
The PsEXT belongs to the large multi-gene family xyloglucan endotrans-glucosylases/hydrolases (XTHs)31, which mainly regulates cell wall strength and extensibility32,33. MtXET, PsXET homologous gene, from Medicago truncatula expressed significantly in root and showed the positive hydrotropic response, thereby helping in drought resistance34. Multigene families of XTHs have been reported in different plant species, including rice35,36, Arabidopsis37, wheat38,39, tomato40 and poplar trees species41. Their expression is tissue specific and is regulated by hormonal and environmental factors36,42,43, and play vital roles in various differentiation and growth processes, including: fruit ripening40,44, flower opening45, petal abscission46, vein differentiation47, wood formation48, hypocotyl growth49 and primary root elongation50.
P. euphratica developed some plasticity to adapt to the gradual environmental gradients such as the development of heteromorphic leaves and well-developed roots system. We investigated the physiological and molecular mechanisms enabling this species to cope with severe stress caused by drought. The microstructures of heteromorphic leaves were studied inside and outside greenhouse condition. The deep penetration of well-developed root system was studied in its natural habitat “Gobi desert” as well as in control condition. The root gene expression profiles of root system subjected to drought stress was monitored. The XET gene activated as hydrotropic responsive gene was cloned in accordance with DEGs information and its functions for root hydrotropism and drought resistance were confirmed in T2 transgenic tobacco.
Materials and Methods
Plant material and growth conditions
The drought tolerance characteristics, of P. euphratica, were investigated in National Natural Reserve of Populus euphratica Forest in Ejinaqi County, Inner Mongolia, China, in early spring. P. euphratica seeds and 2-year old seedlings were provided by the administration of the National Natural Reserve. Seedlings were planted in a container with 25 cm high and 12 cm diameter containing cleaned river sand, placed in a greenhouse with temperature/humidity (25 ± 5) °C/(60 ± 5%) in September, 2015. The containers were watered according to evaporation demand and supplemented with 1/2 MS medium solution once per week. After fully acclimatization to the new environment, part of the potted plants was kept out door in the natural condition, and rest of them were maintained in greenhouse.
Leaf sampling and preparation for electron microscope scanning (EMS)
Five types of heterophylly were collected for EMS (Fig. 1A). Liner Leaves were sampled from 3-year-old plant grew inside and outside of the greenhouse conditions, other types of heterophylly were taken from over 10 years old tree nursery of Beijing Forestry University. The plant tissues were prepared, scanned and imaged with KYKY-2800 EMS. Each specimen was measured 5 times and their average value was taken. The data was processed with DPS 3.01 Data processing system.
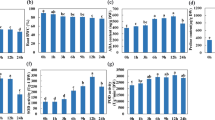
Scanning leaves with Electron Microscopic (SEM) in Populus euphratica. (A) (a) Adult plant five different heteromorphic leaves of 10-years old trees 1: Linear leaf (LL) 2: lanceolate leaf (LAL) 3: ovate leaf (OL) 4: rhombic leaf (RL) 5: toothed-ovate leaf (TOL). (b & c) LL stomata inside and outside greenhouse. (d & e) LAL upper and lower epidermis stomata outside greenhouse. (f & g) OL upper and lower epidermis stomata outside of greenhouse. (h) RL upper epidermis double stomata apparatuses (bar = 10 µm); (i & j) LL and RL upper epidermis trichome. (k) RL transection of principal vein of (bar = 100 µm) (l) LAL transection of spiral vessel in principal vein (bar = 10 µm). (B) Cuticle thickness, stomata density and stomata size (length and width) of upper and lower epidermis of P. euphratica. (C) Cuticle thickness, stomata density and stomata size (length and width) of linear leaf of 3-year-old P. euphratica inside and outside of greenhouse condition.
P. euphratica root architecture pattern in response to moisture level
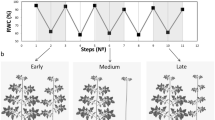
The P. euphratica root response to soil moisture was investigated in field (Fig. 2a) and lab, respectively. A field survey was conducted in P. euphratica forest National Natural Reserve in Badain Jaran Desert, Egina Qi in Inner Mongolia of China (geographic coordinates: 41°56′57.93″N; 101°04′24.04″E). The soil profile of 10-year old plant was monitored and relative soil water contents and root pattern was recorded for every 20 cm soil layer.
Root growth in natural habitat Badain Jaran Desert and in control (lab and greenhouse) condition and physiological parameters at moisture gradients. (a) Root section penetrating the hard calcareous crust at natural habitat. (b) Soil water contents of root zone at Badain Jaran Desert. (c) Root length of 30d old P. euphratica root seedling on MS media at moisture gradients. (d,e,f) P. euphratica root hydrotropism induction pot. (g) Specially designed horizontal cultivation equipment with 6 gradient moisture layer. Only the box left side had hole (Ø5mm × 8 rows × 8 columns). Down arrow followed by # % showing SWC difference in gradient soil drying. (h) Distribution of soil water (upper and deep soil water contents) and relative leaf water percentage in response to gradual soil drying. (i) Root fresh weight at soil moisture zone. (j) P. euphratica roots activity of antioxidant enzymes and the soil moisture profile. (SWC: soil water content; SOD: superoxide dismutase; POD: superoxide dismutase; CAT: peroxidase; PPO: polyphenol oxidase). (k) P. euphratica roots proline, MDA and soluble protein content.
In the lab, the seeds were surface sterilized and sewed on autoclaved solidified agar gel medium. Before allowing the media to solidify the medium container was kept slanted to achieve the varying levels of agar deposition within the same containers to achieve different level of water content. The P. euphratica aseptic seeds were cultured and germinated on the plain and slanted solidified medium. In greenhouse, a simple device was designed (Fig. 2d) to study the root growth pattern. The device consists of two cylindrical pots; the smaller one was hollow and placed on the main pot. The main pot had divided into two sub-pots A and B. Both sub-pots were filled with cleaned river sand, having embedded water pipe in side B for watering. Aseptic seedlings of Populus grown on MS media were transferred to plastic bags. After acclimatization in control condition, the plastic bags were removed and the plant was put on upper cylinder pipe and placed on the centre of the main pot (Fig. 2e).
Root hydrotropism induction with reduced geotropism effects
A special chamber (length 180 cm × width 150 cm × height 45 cm) was designed to provide the natural plant growth and to exclude the influence of gravity51, having small pores (Ø5mm × 8 rows × 8 columns) along its width to maintain the natural flow of water. By rotating the designed apparatus by 60o angle produced different moisture gradients (Fig. 2g) and reduced the interference of gravity. Chamber was filled with cleaned river sand and 3-year old Populus seedlings were grown in 6 lines (line space 30 cm) having 12 plants per line (row space 15 cm). The plant container was watered according to evaporation demand and supplemented with 1/2 MS medium solution once per week. After the growth of seedling to 15–20 cm, the growth chamber was tilted to obtain different moisture levels by withholding the water till the apex 1st leaf wilting appeared in the chamber upper part (line 6). Soil water content (SWC) gradients and leaf water content were recorded with Psypro for all lines except line 6 to minimize false positive results. The fresh root morphology and fresh weight were recorded. The root samples were wrapped in foil and frozen immediately in liquid nitrogen prior to store at −80 °C refrigerator for microarray analysis.
Leaf physiological index in response to soil different moisture levels
Leaves samples from P. euphratica plants grown in water deficit conditions were collected and physiological parameters proline content52, soluble sugars53, MDA54, polyphenol oxidase (PPO)55, plant peroxidase (POD)56, superoxide dismutase (SOD)57, hydrogen peroxide enzyme (CAT)58 and malondialdehyde (MDA)59 were analyzed.
Microarrays analysis of root transcriptome in gradient soil drying
The microarray experiment was completed by Shanghai Biochip Company (SBC), China accredited by China National Accreditation Service for Conformity Assessment (CNAS). RNA was extracted from root sample and purified using the QIAGEN RNAeasy mini kit. The experimental lines (line 1–4 plant roots subjected to different drought stress) RNA was labeled with Cy3-dUTP and was henceforth described as regime 1, 2, 3 and 4, respectively. Control group (the 5th line plant roots with sufficient moisture) RNA was labeled with Cy5-dUTP fluorescence and was henceforth described as regime 0. Further hybridization procedure was followed according to the chip manufacturer instructions (Shanghai Biochip Co., Ltd.).
Microarrays were scanned using an Axon microarray scanner (Angilent technology) and analyzed by split-tif Imagene software. Background fluorescence was subtracted from the value of each spot on the array. Normalization of the signal intensities was carried out according to Deyholos et al.60. Transcript regulation has been expressed as the ratio of intensities between stress and control root samples. Changes in signal intensity between stress and control experiments exceeding a >2.0-fold difference in repeat experiments were considered significant. GO, COG terms and KEGG pathways enrichment analysis was used for functional categorization of unigenes.
RT-qPCR validation of microarray unigenes of P. euphratica roots in response to soil drying
Total root RNA was isolated from all lines (1–5) treated with RNase-free DNase (Chang et al., 2012). The root RNA samples were reverse-transcribed with a cDNA Synthesis Kit (CWBIO Inc., Beijing, China). cDNA products were used for SYBR Green-based RT-qPCR analysis, each sample was run in triplicate. The RT-qPCR running conditions were: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 52 °C for 20 s, and 72 °C for 30 s, with a final step of 72 °C for 10 min. Using the roots of line 5 group plants as control, the expression levels of differential expression genes (DEGs) were calculated using the 2−∆∆Ct method61.
Functional analysis of PeXET gene and promoter in root response to soil drying
According to the sequenced results of the microarray analysis and that of Ps-EXGT1 (GenBank Acc. AB015428)30, a pair of primers (PeXET-722–1 and PeXET-999–3, Table S1) were designed to obtain the ORF of PeXET. The PCR running conditions were: 94 °C for 3 min, followed by 35 cycles of 94 °C for 40 s, 55 °C for 30 s, and 72 °C for 1 min with a final step of 72 °C for 3 min. After electrophoresis, the PCR product was extracted from the agarose gel using Agarose Gel Extraction Kin (TianGen, Beijing) and inserted into T-vector for sequencing (Sangon Biological Engineering Co., LTD). 3′ RACE and 5′ RACE were used to obtain the full-length cDNA of PeXET according to Molecular Cloning Manual: A Laboratory Manual (Chapter 8), the sequences of primers shown in Table S1.
The PeXET gene function was analyzed in transgenic tobacco. pBin438-PeXET and pBin438-RNAi-PeXET vectors were constructed by picking primer sequence from PeXET gene and GUS sequences (Table S1 and Figure S3). GV3101/ pBin438-GUS, pBin438-PeXET and pBin438-RNAi-PeXET were delivered into tobacco (Nicotiana tabacum) through agrobacterium mediated transformation62, respectively. Marker resistance shoots were tested by PCR and T2 generation plants were subjected to RT-PCR analysis. The RT-PCR products were sequenced and analyzed by DNAMAN sequence alignment software.
Transgenic tobacco seeds were surface sterilized (8 seeds of each transgenic pBin438-PeXET, pBin438-RNAi-PeXET, and pBin438 plants), and germinated on MS media in Petri dishes. The germinated plants were investigated for 14–20 days for the seedling growth and development. The plants from three sort T2 generations were transplanted into a box (67 cm × 47 cm × 18 cm) and care was taken to gain the height of 15 cm. The plants were uprooted and root-shoot morphology was recorded. For drought tolerance test the plants grown in separate boxes were subjected to water withholding treatment. Soil water contents, the plant apex leaf wilting and survival rates were recorded.
The PeXET gene promoter (pPeXET) was cloned to insight its gene driven activity63. The promoter region was double digested to elucidate their function with restriction enzymes HindIII and NcoI, created sub-sequences i.e. 1831bp, 1425 bp, 993 bp, 717 bp and 588 bp PeXET 5′-flanking sequences. In pCAMBIA expression vector, CaMV35S promoter was substituted with the pPeXET (1831 bp) and 5′ end deleted portion of pPeXET (588 bp), respectively. The expression vector pCAMBIA-GUS, pCAMBIA-pPeXET1831-GUS and pCAMBIA-pPeXET588-GUS were transformed into GV3101 via freeze-thaw method64. GV3101/ pPeXET1831-GUS, pPeXET588-GUS and pCAMBIA-GUS vector (control) were transformed into tobacco. Hygromycin resistance tobacco plants were confirmed by PCR using GUS upstream primer 5′-AGCGTTGAACTGCGTGAT-3′ and downstream primer 5′-GTTCTTTCGGCTTGTTGC-3′. Histochemical staining and fluorescence quantitative analysis were performed to identifying the promoter activity derived GUS expression in the transgenic tobacco plant systems65.
Data analysis
Data were subjected to analysis of variance (ANOVA) and differences between means were evaluated with Student′s t-test. The differences were considered statistically significant at P < 0.01 and P < 0.05. Statistix v. 8.1 (Analytical Software, 2005) package was used for this purpose.
Results
Heterophylly characteristics of Populus euphratica in response to environmental conditions
The adult plant leaves of P. euphratica are heteromorphic. The leaf showed the disciplinary change from lanceolate leaves to dentate broad-ovate leaves. At least five different leaf shapes were found in a tree of P. euphratica, i.e. Linear leaf (LL), lanceolate leaf (LAL), ovate leaves (OL), rhombic leaf (RL) and toothed-ovate leaf (TOL) (Fig. 1A). Attending to the different cutin structure, P. euphratica leaf cutins have been grouped into two general morphological types. (i) Compact layer, the black compact structure covers the leaf surface continuously or disconnectedly. (ii) Loose layer, the white color covers the leaf surface in a crisscross stagger network and honeycomb-like structures. Cutical thickness was fond higher in lower epidermis (LE) as compared to the upper epidermis (UE). The cutin coverage ratio increased in the order: LL<LAL<OL<RL<TOL (Fig. 1B). Cutin layers were found higher in old trees (10 year old) than younger plants (3 year old), while no significant difference was observed for the leaves growing inside and outside of greenhouse (Fig. 1C).
Stomata of the P. euphratica tree leaves are rectangular round and distribute on both UE and LE, raise or sink parasitically in the cuticle layers (Fig. 1A). Outer stomata ledge swells up outwards and forms the front chamber and communicates with the environment. Stomata density on LE and UE were found same for all the heteromorphic leaves except for OL (Fig. 1B). Whether UE or LE, the stomata density in the five heterophylly from lower to higher is in the order: LL <LAL<OL<RL<TOL, respectively. Stomata size on upper and lower epidermis showed significant difference growing inside and outside of greenhouse (Fig. 1C).
The UE stomata size was found longer than LE except for LAL. The maximum length was 5.4 µm in the LAL and the minimum one is 0.22 µm in RL. The stomata width from UE and LE were similar in length, the maximum width was 1.44 µm in TOL and the minimum was 0.04 µm in LAL. While the UE stomata sizes showed no significant difference among the five heterophylly (P < 0.05). In greenhouse condition, the stomata length and width of UE in leaves of 3-year- old plants of P. euphratica were significantly longer and wider than open field plants, while no significant difference found in the case of LE (Fig. 1C).
Leaf trichomes occurred on both epidermal side of RL and on the UE of LL in the 10-years old trees, other heterophylly growing inside or outside greenhouse were without trichomes. The maximum trichome length was recorded as 51.58 µm and base diameter of 12.67 µm.
Three vascular bundles, one smaller and two bigger, were found in RL principal vein, smaller one was located between two bigger ones. However, only one vascular bundle appeared in other heterophylly from 10 years-old plants and the 3-year-old P. euphratica plants growing both inside and outside greenhouse. The vascular bundle sheath was composed of 2–5 layers of stereid cells arranged closely. 4–8 layers of parenchyma and sclerenchyma cells developed between the vascular bundle sheathes and epidermis, meanwhile, the parenchyma cells or the sclerenchyma cells were different in sizes. Vessels of vascular bundles in the principal vein were mainly spiral vessel with 11.04 ± 6.31 µm in diameter.
Root growth in response to Badan Jaran Desert soil moisture in P. euphratica
Root distribution, relative soil water content of the soil profile and other features were studied as shown in Fig. 2a and b. In the Badain Jaran desert, soil water content increase gradually from up to down, Populus root were found to penetrate the hard calcareous crust (50–60 cm below from the soil surface) as the moisture level found double below the crust (salt deposits).
In the lab, the seedlings were grown on water potential gradient media with low water content at the top as compared to the lower part. Accordingly, 30 days old seedling roots were significantly longer at the top zone as compared to the root length at low zone (Fig. 2c). In green house, Populus seedlings were tested in specially designed apparatus as shown in Fig. 2d and after five months of regular irrigation to one side, the plant roots were found bent deeply in the soil chamber with high moisture gradients (Fig. 2e,f). Results showed the strong hydrotropic response of Populus roots toward moisture.
Physiological responses in leaves to soil water stress in the water-withholding regimes
The P. euphratica plants showed distinct physiological features when subjected to different moisture gradients. At different soil moistures zones (lines 1–6) in the same cabinet (Fig. 2g), P. euphratica roots were differed in the terms of growth and fresh weight. The highest fresh weight of roots was achieved in line 3 (average SWC 2.71%), the soil moisture higher or lower than this, such as line 4 (average 2.79%) and top line (SWC less than 2.50%), reduced the fresh root growth (Fig. 2i).
Physiological parameters of leaves subjected to water withholding are shown in Fig. 2h–k. Several antioxidant enzymes, SOD, PPO and POD showed a relatively stable expression at earlier drought stress but further reduction in soil water content caused the decline activity. When the soil moisture levels reduced to a certain level the enzymatic activity showed a transition stage and gain high activity. CAT activity showed a rapid decline as SWC fall down but further reduction of soil water moisture caused the up-regulation. In contrast, SOD showed a slight increase activity to deficit water condition but after that further reduction in moisture level caused decline and then gain high activity. Soluble protein, MDA and proline concentration showed rising trend against to reduced SWC but further decline trend followed the reduction in SWC, while a sharp decline trend was in MDA content as shown in Fig. 2k.
Microarrays (chip) analysis of gene expression profile in roots of P. euphratica in response to soil drying
Data analysis of the four regimes (i.e. the individual drought treated regimes compared to regime 0) revealed that the DEGs showed differentially expressed transcripts profiles in response to the moisture gradients. A total of 1394 transcripts were significantly up and down-regulated in all four regimes compared to regime 0. Regime 2 and regime 3 showed the highest number of specific, differentially expressed transcripts (124 and 128 up-regulated, 312 and 249 down regulated, respectively), in comparison to regime 1 (160 up and 166 down regulated) and regime 4 (122 up and 136 down-regulated genes).
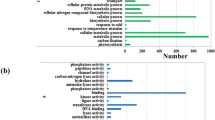
The transcriptional information was obtained by BLAST software and the unigenes were assigned to NR, Swiss-Prot, GO, COG, and KEGG database (Table 1). A total of 835 unigenes were identified, in which531 unigenes were analyzed by COG (Fig. 3A), 515 unigenes were enriched in GO database (Fig. 3B) and 287 unigenes were mapped to KEGG pathways (Table S4).
Transcriptome analysis of P. eupheritica roots at moisture gradients. (A) COG classification of differentially expressed genes (DEGs). Total of 531 DEGs were classified into different groups; (B) Functional classifications of GO terms of all DEGs. Total unigenes were classified into three main groups namely: cellular component, molecular function and biological process.
The functions of the differentially expressed genes (DEGs) were searched by GO database for plotting annotation results. A total of 515 DEGs were categorized into three main categories (cellular component, molecular function and biological process) and 42 functional groups (Fig. 3B). Annotations of DEGs assigned into the GO database showed different moisture levels changed the expressions of the transcripts in stressed roots (Table S3). Overall results of GO database annotation showed that 198 DEGs involved in the catalytic reaction pathways (catalytic activity), 251 DEGs belong to protein binding ways (Binding), 331 DEGs involved in metabolic pathways of exogenous stimuli (response to stimulus), 138 DEGs involved in the biological regulatory pathways (biological regulation). The highest numbers of transcript changes were observed for regime 3 followed by regime 4. In regime 3, highest number of transcripts 95 and 69 involved in “response to stimulus” and “cellular process” were up-regulated.
Based on COG analysis, total of 531 DEGs were classified into 26 groups (Fig. 3A). According to COG annotation, 225 DEGs involved in post-translational modifications, protein folding, chaperones metabolic pathway, 173 DEGs involved in carbohydrate transport and metabolism pathways, 40 DEGs involved in lipid transport and metabolism, 28 DEGs involved in the inorganic ion transport and metabolic pathways.
Expression profiles of stress-inducible transcriptomes in response to soil drying
In microarray data, unigenes belonged to the same family were in different expression patterns. Unigene for inositol-3-phosphate synthase was up-regulated 6.557 fold in regime 3, while 2.885 fold increased regime 4. The same unigene was down-regulated in regime 2 as its expression was 0.218 (Table S2). A total of 24 unigenes for inositol-3-phosphate synthase were up and down-regulated in the roots sample subjected to different soil moisture levels. Phosphatase 2C as a regulator of ABA signaling was down-regulated in regime 1 by 0.373 fold expression and up-regulated in regime 3 by 2.052 fold. Stress responsive signaling molecule “mitogen-activated protein kinase kinase 2 (MAPKK)” in regime 3 P. euphratica plant roots increased 5.412 folds, while in regime 2 it was down regulated 0.299. NAC TF was up regulated 2.282 and 3.036 times in regime 4 and regime 3, respectively.
Unigenes for histidine-containing phosphotransfer proteins (HPts) which act as cytokinin signaling receptor was up-regulated by 2.532 folds only in regime 3. Similarly, homeobox-leucine zipper protein was up-regulated by 2.116 folds and 3.193 folds in regime 4 and regime 3, respectively. In the Populus root regime 1 and regime 2, the auxin-responsive protein was up-regulated by 2.5 fold.
Unigenes for stress responsive transcription factor was up-regulated in regime 3 and regime 4 by 3.036 and 2.282 folds, respectively. High water content levels up-regulated the expression of SKP1-like protein while below water content levels up-regulated the expression trend of BON1-associated protein 2 which act as cell death regulator. Details of up and down regulated unigenes are given in Table S3.
The expression pattern of XET gene involved in root hydrotropic growth
In microarray data, several XET transcripts were found to be up-regulated at different moisture levels. Six transcripts of XET were up-regulated specifically according to the level of drought, such as four transcripts were up-regulated (2.556, 2.854, 3.199 and 3.471) in regime 4. Similarly, three transcripts of XET were up and down regulated (2.445, 0.429 and 0.128) in regime 3. For XET transcripts in regime 2, same trend was observed with up and down regulated of 3 transcripts (2.355, 0.446 and 0.374). The XET activities remained highest for regime 3 and were selected for the further analysis.
Validated the DEGs from microarray analyses by RT-qPCR analysis
For validating the data from microarray, 26 DEGs were randomly selected for RT-qPCR analysis at different moisture levels. The primers of selected genes are listed in Table S1. PeActin was used as reference gene for data normalization according to Hu et al. 201566. The RT-qPCR results showed a strong correlation with the RNA-seq-generated data with few exceptions. Only two genes encoding protein phosphatase 2 C and suspected new gene displayed a higher expression in the microarray data as compared to RT-qPCR analysis.
Functional analysis of PeXET promoter and its spatial expression profile
Analysis of the promoter sequence (1831 bp) showed different cis-elements mainly involved in growth regulation, growth and environment responsive element. Most of growth response cis-elements and growth regulator cis- elements fall in 588 bp sub-sequence of the pPeXET promoter region (Table 2).
1831 bp and 588 bp fragments pPeXET were analyzed for their GUS driven expression activity in transgenic tobacco. The Hygromycin resistance transgenic tobacco shoots of pPeXET1831-GUS, pPeXET588-GUS and control pCaMV35S-GUS were confirmed by PCR and sequence analysis63. The GUS gene expression in the transgenic tobaccos is given in Fig. 4, GUS staining showed blue colors in roots, stems and leaves in the transgenic tobacco but the color intensity was different from dark to light (Fig. 4b). Quantification of Fluorescence of 4-MU (Fig. 4c) showed that the GUS expressions activities vary for the different used promoters and transgenic plant organs (root, stem and leaves). pPeXET1831 driven GUS expression levels was similar in roots, highest in stems while diminished in leaves as compared to CaMV35S. Lowest GUS expression level was recorded for pPeXET588 fragment which showed a decreasing trend of expression in roots stems and leaves, respectively.
PeXET gene function in root growth and drought resistance in transgenic tobaccos
PeXET gene sequence was 2167 bp in length, included four exons and three introns (Figure S2) with length of 132 bp, 143 bp and 892 bp, respectively. The four exons were spliced together according to the rule of GT-AG as that of most plants. PeXET ORF sequence was cloned and submitted to GenBank (Accession No EF612703). The ORF sequence was 1224 bp, included the start codon (ATG) and stop codon (TAA), and encoded a 293-amino-acid residue with predicted molecular weight of 33.8 kDa. The protein sequence blast analysis subjected to NCBI TBLASTN homology alignment showed more than 90% similarities to the Populus XTH (more than 90%), and 83% to the trigged gene Ps-EXGT1.
The transgenic tobaccos were identified by PCR and T2 transgenic tobaccos were analyzed by RT-PCR (Fig. 5a). Total of nine vector controls, nine pBin438-PeXET transgenic tobacco plants and 10 pBin438-RNAi-PeXET transgenic plants were confirmed through RT-PCR and analyzed by DNAMAN software sequence alignment (data not shown).
PeXET transgenic and RNAi tobacco plant root growth and drought tolerance. (a) T2 transgenic tobacco RT-PCR, A: M: Marker; 1: Vector control; 2: GV3101/ pBin438-35S-peXET; 3–11: transgenic pBin438- PeXET tobacco; B: M: Marker; 1: Vector control; 2: GV3101/ pBin438–35S-peXET; 3–12: pBin438-RNAi-PeXET tobacco; C: M: Marker; 1–2: Negative control; 3–11: transgenic pBin438 tobacco. (b–c) Tobacco roots of WT, RNAi and over-expressed PeXET gene plant on medium and pot soil, respectively. (d) T2 transgenic tobacco plant survival rate of WT, RNAi and over-expressed PeXET gene at different SWC.
Seedling roots architecture of WT, RNAi and PeXET over expressed transgenic tobaccos lines on in-vitro MS medium were shown in Fig. 5b. The longest root length, high fresh weight and rapid growth were recorded for pBin438-PeXET transgenic plants, while significantly inhibited root numbers and growth were recorded for pBin438-RNAi-PeXET plants. The upper parts of the WT, vector control and pBin438-PeXET transgenic plants showed no significant difference. The three transgenic lines grown in soil and cultured in greenhouse suggested statistically significant differences in root length, root fresh weight and lateral root numbers (Figure S4b and S4c). As compared to WT, pBin438-PeXET transgenic plant root number and length was high with more number of lateral roots. Short roots and few of lateral roots was recorded for pBin438-RNAi-PeXET plants, even main taproot was absent in the plants (Fig. 5c).
The PeXET T2 transgenic plants were subjected to drought stress by withholding water and soil drying naturally. RNAi transgenic T2 plants (pBin438-RNAi-PeXET) were found drought sensitive in response to the soil drying. Leaves of the plants wilted earliest (Fig. 5d) at the 22nd day of withholding water with SWC 20.3%. WT tobacco plants appeared wilting at 26th day of drought stress (SWC 16.8%), at this time stomata conductance was found higher in transgenic plants as compared to WT (Figure S4a). The pBin438-PeXET transgenic plants were found healthy at 10.5% SWC. The PeXET over-expressed plants did not appear wilting at apex first leaves until 38 days of drought stress (SWC 7.2%). The drought resistance tests suggested that PeXET gene increased the transgenic tobacco root growth and enhanced the plant drought tolerance.
Discussion
In this study, the drought tolerant characteristics, the heteromorphic leaf and hydrotropically responding root system, of the P. euphratica were studied in soil water deficit conditions. The leaf covers, stomata architecture, physiological and biochemical changes, and characteristic gene expression of the spatial heterophylly from below Linear leaf (LL) to upper toothed-ovate leaf (TOL) showed the adaption pattern to the dramatic alteration in environmental air conditions. The extremely well-developed root system, characteristic microarray DEGs and individual PeXET gene expression driven by its root special PeXET promoter suggested that the plant root hydrotropism plays an essential role in adaption to soil moisture from the soil surface to deep groundwater.
The changes in the molecular sizes of heteromorphic leaves, especially stomata size and density and vascular bundles of the P. euphratica (Fig. 1), are considered to have a functional significance in response to fluctuating air condition. Morphological variation in leaf traits occurs from linear leaf (LL) to toothed-ovate leaf (TOL) according to the canopy position as the plant grows from young plant to big tree. The leaf cuticular thickness (Fig. 1B) was statistically classified into two groups (LL, LAL and LO; RL and TOL) on the upper epidermis and three groups (LL, LAL; LO; RL and TOL) on the lower ones. The epicuticular wax (hydrophobic layer) coverage increased from LL to TOL in the heteromorphic leaves (Fig. 1B). This cuticular protector is considered as a self-defense mechanism to reduce water loss through the trichomes and epidermis transpiration67 in water deficit condition.
Euphrates poplar showed difference in the stomata density, length and width on upper and lower epidermis as well as in the different heterophyll (Fig. 1B). Environmental conditions inside and outside greenhouse (mainly the humidity difference) affected the stomata densities of upper and lower epidermis and the stomata length and width of upper epidermis but not of the lower epidermis (Fig. 1B). Data suggested that the plant spatial air conditions control the stomata apparatus (open and close state) by affecting the stomata density, size length and width in upper and lower epidermis. This complex stomata apparatus and deep sunken stomata control the water balance by managing the transpiration rate according to the plant spatial air conditions68. The heteromorphic leaf of P. euphratica showed the close resemblance of xeromorphic characters which maintain normal growth and development even under undesired conditions11.
Mechanisms involved in the plant drought resistance were elucidated in physiological analysis at different moisture gradients. When the plant were subjected to soil drying, SOD, POD, PPO and CAT activity shows varying trends (Fig. 2j), offers improved protection against oxidative injury induced by drought. The fluctuating activities of MDA, free proline and soluble protein (Fig. 2k), enhance the plant drought stress resistance. The similar effects were studied in some model plants69,70,71. Antioxidant molecules and enzymes that are situated in various cell sections can scavenge ROS. Depends on the plant species, organ, developmental stage and the severity of the stress expression of the antioxidant capacity fluctuate. To keep the ROS concentration relatively low and acclimatized to water deficit condition is generally linked with improved activity of the antioxidant enzymes72. Beside this, ROS also utilized as secondary messengers in the signaling for the activation of defense responses. Tolerance to water stress circumstances is a complex feature accomplished by plants through coordinated action of biochemical, physiological and molecular adaptations. The bio-synthesis of osmotically-active substances, non-enzymatic and enzymatic antioxidants, contributes a key role in tolerance development against water deficit condition in Poplar73. Thus the varying expression trends of physiological parameter might be important for the P. euphratica to minimize the injury caused by drought stress and to fully activate the defense responses. Similar results for the same species were also reported by Song et al., 201474.
The root system of P. euphratica was characterized with hydrotropism in response to natural growth condition (Fig. 2a), confirmed in lab (Fig. 2c) and greenhouse (Fig. 2f). At organ level, the dynamic responses to limited water availability result in an intricate pattern of roots within the soil, and this emerging characteristic finally specifies the extent of water accessibility in the soil. To date, in the field of plant breeding, rather static, idealized root phenotypes, namely ideotypes, have been targeted to optimize plant growth under a particular environment or stress condition. To understand the actual molecular mechanism of root phenotypes, tree physiologists focused on gene expression profile of plants across a wide range of environmental conditions75,76 to unhide the basic theme of root development of the woody plants in different environmental conditions.
Microarray data showed that unigenes belonging to the same family have different expression patterns (Fig. 6a). Recent studies have reported that plant root elongation and lateral root growth dependent on the regulation of growth hormone24. Auxin biosynthesis, signaling and responding are required for lateral root formation, but the high concentration of auxin inhibit the lateral growth. In the popolus root of regime 1 and regime 2 the Auxin-responsive protein was up-regulated by 2.5 fold, indicated that the Populus roots experienced drought stress and retarded the root growth in the stress environment. Class phosphatidylinositol phosphate protein is an important component that promotes auxin signal and affecting cell membrane tightness77. Maximum number (24 unigenes) of phosphatidylinositol phosphate protein was up and down-regulated in the different moisture level roots sample, suggesting that the P. euphratica cell membrane is extremely sensitive to moisture gradients. Unigenes for inositol-3-phosphate synthase was up-regulated 6.557 fold in regime 3, while 2.885 fold increases was studied for regime 4. The Same unigene was down regulated in regime 2 as its expression was 0.218. In higher plants, myo-inositol becomes incorporated into myo-inositol phosphate (InsP), certain sphingolipid signaling molecules and phosphatidylinositol phosphate (PtdInsP), that participate in many biological processes, such as gene expression regulation78 and stress tolerance in particular79. Unigenes for histidine-containing phosphotransferproteins (AHP) which act as cytokinin signaling receptor was up-regulated by 2.532 fold only in regime 3 Populus root. AHP take part in cytokinine signal transduction in higher plants80 and play important roles in the development of several shoot and root organs through maintenance of cell proliferation activity81. Salicylic acid responsive protein (Transcription factor TGA1) was up regulated in regime 4 and regime 3, previous studies showed the direct involvement of TGA (salicylic acid responsive protein) in lateral root development. These results showed that hormonal regulation has the direct influence on root growth and their regulations alter with soil water gradients.
In addition to plant hormone pathways, several transcriptional factors and vital genes were found to be regulated by water availability. MAPKK in regime 3 Populus root showed 5.412 fold increases, while in regime 2 it was down regulated 0.299. Mitogen-activated protein kinase (MAPKs) cascades are signal transduction modules regulating various aspects of plant biology, including stress responses and root growth82. Lopez-Bucio et al.83, described the direct involvement of MAPKKs in root formation and architecture. NAC transcription factors, was up-regulated and members of the NAC gene family have been suggested to play important roles in the regulation of the transcriptional reprogramming associated with plant stress responses84. Different moisture level may accelerate cell wall stiffening possibly mediated by peroxidase. Data showed that 8 peroxidases transcripts were found to be differentially expressed with 7 down-regulated and 1 up-regulated in different root transcripts. The down-regulation of those peroxidases transcript may favor root elongation by the reduction of apoplastic H2O2, and the enhancement of oxygen radicals to break the cell wall polymers and as a result, accelerates cell wall loosening and therefore roots growth85. Unigene for Stress responsive transcription factor was up-regulated in regime 3 and regime 4 as a result the self-defense system of the plant became fully operative in optimum environmental condition. On the other hand, plants grown at drought stress condition, BON1-associated protein 2 was found up-regulated, which act as Cell death regulator. Results showed that each key gene plays an important role in root elongation under fluctuating moisture environment.
Transcriptome analyses of different moisture level indicate that the expression of genes related to cell wall stiffening and loosening is differentially expressed. Several XET transcripts were found to be up-regulated in different moisture levels (Fig. 6a) and confirmed by RT-PCT (Fig. 6b). XET homologous gene from P. euphratica was selected for further analysis as XET gene regulated root growth was reported in pea30 and Medicago truncatula 34. Very recently similar results with different expression of XTH gene family was found in Arabidopsis plants roots subjected to drought stress by microarray determination86. A particularly tissue specific regulation of XTH genes contribute to loosening or strengthening the cell wall in well-defined topological organs/ regions of the plant contributing to tolerance/ susceptibility to water availability. The balance among cell wall hardening and loosening activities explains the parts of accelerated and decelerated root growth in the elongation zone87. Root tips in elongation zone are the main regions that produce specialized sorts of cells and tissues in a particularly pre-defined pattern to assist development during different moisture level88. In the case of high moisture level the expression was recorded high which showed a decrease expression trend by decreased moisture level, and the resulting XET activity was proposed to be involved in cell wall adaptation processes during root cell elongation, which might be stimulated by water gradients and thus shows hydrotropic growth. The previous studies showed that XET activity was boosted in the apical area of roots grown under low water potential49,66,89. These results indicated that the increased expression level of XET transcript in the root tips of Populus roots under various moisture levels might be necessary for maintaining root elongation under these conditions. The moderate water level provide better environment for high expression of Stress responsive signaling molecule and XET gene expression which showed positive result for root growth during drought stress. The differentially gene expression profile of PeXET gene of P. euphratica roots subjected to soil drying suggested that PeXET gene might be involve in the hydrotropic positive development of roots to absorb underground water. PeXET over-expression confirmed the increased drought tolerance and in this study and confirmed the involvement of PeXET gene in root development (Fig. 5). This data is supported by the work of Osata et al.50, by sorting out the Arabidopsis thaliana XTH genes are dominantly expressed in the roots. However, the expression of Arabidopsis thaliana XTH genes showed different trends and respond to hormonal signals differently. To investigate the PeXET gene functions in primary root growth, we examined phenotypes of loss-of-function mutants for these genes RNAi plants. These functional analyses disclosed a principal role for the PeXET gene in primary root elongation. Similar results for the four Arabidopsis thaliana XTH genes were confirmed by previous work50. The corresponding promoters region of PeXET was isolated and the bioinformatics analysis exposed the presence of growth regulation, growth and environment responsive element. Due to the presence of several cis-elements the expression of GUS gene was found higher in lower part as compared to leaves. The expression trend of PeXET promoter not only organ specific but also induced by external stimuli63.
Several other genes along with PeXET involved in the root response to soil drying (Fig. 6a). Further study should broaden our understanding of the molecular networks of the plant drought tolerance in a desert environment.
References
Calanca, P. P. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability (eds Mukhtar Ahmed & O. Claudio Stockle) 165–180 (Springer International Publishing, 2017).
Smith, S. D., Monson, R. K. & Anderson, J. E. Physiological ecology of North American desert plants. (Springer, 1997).
Lawlor, D. W. & Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, cell & environment 25, 275–294 (2002).
Lv, F. L., Zhang, H. C., Xia, X. L. & Yin, W. L. Expression profiling and functional characterization of a CBL-interacting protein kinase gene from Populus euphratica. Plant Cell Rep 33, 807–818, https://doi.org/10.1007/s00299-013-1557-4 (2014).
Chen, S. L. et al. Effects of NaCl on shoot growth, transpiration, ion compartmentation, and transport in regenerated plants of Populus euphratica and Populus tomentosa. Can J Forest Res 33, 967–975, https://doi.org/10.1139/X03-066 (2003).
Ottow, E. A. et al. Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139, 1762–1772, https://doi.org/10.1104/pp.105.069971 (2005).
Junghans, U. et al. Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant, cell & environment 29, 1519–1531 (2006).
Sun, J. et al. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 149, 1141–1153, https://doi.org/10.1104/pp.108.129494 (2009).
Ding, M. et al. Salt-induced expression of genes related to Na(+)/K(+) and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol Biol 73, 251–269, https://doi.org/10.1007/s11103-010-9612-9 (2010).
Chen, S. & Polle, A. Salinity tolerance of Populus. Plant Biology 12, 317–333, https://doi.org/10.1111/j.1438-8677.2009.00301.x (2010).
Liu, Y. B. et al. Epidermal Micromorphology and Mesophyll Structure of Populus euphratica Heteromorphic Leaves at Different Development Stages. Plos One 10, https://doi.org/10.1371/journal.pone.0137701 (2015).
Li, Z.-X. & Zheng, C.-X. Structural characteristics and eco-adaptability of heteromorphic leaves of Populus euphratica. Forestry Studies in China 7, 11–15, https://doi.org/10.1007/s11632-005-0050-8 (2005).
Ma, X. D. et al. Genome-wide Identification of TCP Family Transcription Factors from Populus euphratica and Their Involvement in Leaf Shape Regulation. Sci Rep-Uk 6, https://doi.org/10.1038/Srep32795 (2016).
Yan, D. H., Fenning, T., Tang, S., Xia, X. & Yin, W. Genome-wide transcriptional response of Populus euphratica to long-term drought stress. Plant science: an international journal of experimental plant biology 195, 24–35, https://doi.org/10.1016/j.plantsci.2012.06.005 (2012).
Tang, S. et al. Populus euphratica: the transcriptomic response to drought stress. Plant Mol Biol 83, 539–557, https://doi.org/10.1007/s11103-013-0107-3 (2013).
Chen, J. H., Yin, W. L. & Xia, X. L. Transcriptome Profiles of Populus euphratica upon Heat Shock stress. Curr Genomics 15, 326–340 (2014).
Zhang, J. et al. Molecular evolution and expression divergence of the Populus euphratica Hsf genes provide insight into the stress acclimation of desert poplar. Sci Rep 6, 30050, https://doi.org/10.1038/srep30050 (2016).
Qiu, Q. et al. Genome-scale transcriptome analysis of the desert poplar, Populus euphratica. Tree physiology 31, 452–461, https://doi.org/10.1093/treephys/tpr015 (2011).
Duan, H. et al. Genome-Wide Analysis of MicroRNA Responses to the Phytohormone Abscisic Acid in Populus euphratica. Frontiers in plant science 7, 1184, https://doi.org/10.3389/fpls.2016.01184 (2016).
Hodge, A. In Progress in Botany 71 (eds Ulrich Lüttge, Wolfram Beyschlag, Burkhard Büdel, & Dennis Francis) 307-337 (Springer Berlin Heidelberg, 2010).
Sassi, M. et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development (Cambridge, England) 139, 3402–3412, https://doi.org/10.1242/dev.078212 (2012).
Kiss, J. Z. Where’s the water? Hydrotropism in plants. Proceedings of the National Academy of Sciences of the United States of America 104, 4247–4248, https://doi.org/10.1073/pnas.0700846104 (2007).
Kobayashi, A. et al. A gene essential for hydrotropism in roots. Proceedings of the National Academy of Sciences of the United States of America 104, 4724–4729, https://doi.org/10.1073/pnas.0609929104 (2007).
Xiu, Y. et al. Improvement and transcriptome analysis of root architecture by overexpression of Fraxinus pennsylvanica DREB2A transcription factor in Robinia pseudoacacia L. ‘Idaho’. Plant biotechnology journal 14, 1456–1469, https://doi.org/10.1111/pbi.12509 (2016).
Takahashi, H., Miyazawa, Y. & Fujii, N. Hormonal interactions during root tropic growth: hydrotropism versus gravitropism. Plant Mol Biol 69, 489–502, https://doi.org/10.1007/s11103-008-9438-x (2009).
Moriwaki, T., Miyazawa, Y., Kobayashi, A. & Takahashi, H. Molecular Mechanisms of Hydrotropism in Seedling Roots of Arabidopsis Thaliana (Brassicaceae). American journal of botany 100, 25–34, https://doi.org/10.3732/ajb.1200419 (2013).
Iwata, S., Miyazawa, Y., Fujii, N. & Takahashi, H. MIZ1-regulated hydrotropism functions in the growth and survival of Arabidopsis thaliana under natural conditions. Ann Bot-London 112, 103–114, https://doi.org/10.1093/aob/mct098 (2013).
Bogeat-Triboulot, M. B. et al. Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol 143, 876–892, https://doi.org/10.1104/pp.106.088708 (2007).
Cassab, G. I., Eapen, D. & Campos, M. E. Root hydrotropism: an update. American journal of botany 100, 14–24, https://doi.org/10.3732/ajb.1200306 (2013).
Takano, M. et al. Endoxyloglucan transferase cDNA isolated from pea roots and its fluctuating expression in hydrotropically responding roots. Plant & cell physiology 40, 135–142 (1999).
Fry, S. C. et al. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. The Biochemical journal 282(Pt 3), 821–828 (1992).
Rose, J. K., Braam, J., Fry, S. C. & Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant & cell physiology 43, 1421–1435 (2002).
Vissenberg, K., Fry, S. C., Pauly, M., Hofte, H. & Verbelen, J. P. XTH acts at the microfibril-matrix interface during cell elongation. J Exp Bot 56, 673–683, https://doi.org/10.1093/jxb/eri048 (2005).
Chen, J. R. et al. Rapid Construction of a Plant RNA Interference Expression Vector for Hairpin RNA-Mediated Targeting Using a PCR-Based Method. DNA Cell Biol 28, 605–613, https://doi.org/10.1089/dna.2009.0897 (2009).
Uozu, S., Tanaka-Ueguchi, M., Kitano, H., Hattori, K. & Matsuoka, M. Characterization of XET-related genes of rice. Plant Physiol 122, 853–859 (2000).
Yokoyama, R., Rose, J. K. C. & Nishitani, K. A Surprising Diversity and Abundance of Xyloglucan Endotransglucosylase/Hydrolases in Rice. Classification and Expression Analysis. Plant Physiol 134, 1088–1099, https://doi.org/10.1104/pp.103.035261 (2004).
Yokoyama, R. & Nishitani, K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant & cell physiology 42, 1025–1033 (2001).
Okazawa, K. et al. Molecular cloning and cDNA sequencing of endoxyloglucan transferase, a novel class of glycosyltransferase that mediates molecular grafting between matrix polysaccharides in plant cell walls. The Journal of biological chemistry 268, 25364–25368 (1993).
Liu, Y. et al. The alpha- and beta-expansin and xyloglucan endotransglucosylase/hydrolase gene families of wheat: molecular cloning, gene expression, and EST data mining. Genomics 90, 516–529, https://doi.org/10.1016/j.ygeno.2007.06.012 (2007).
Saladie, M., Rose, J. K., Cosgrove, D. J. & Catala, C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J 47, 282–295, https://doi.org/10.1111/j.1365-313X.2006.02784.x (2006).
Geisler-Lee, J. et al. Poplar carbohydrate-active enzymes. Gene identification and expression analyses. Plant Physiol 140, 946–962, https://doi.org/10.1104/pp.105.072652 (2006).
Catala, C. et al. Characterization of a tomato xyloglucan endotransglycosylase gene that is down-regulated by auxin in etiolated hypocotyls. Plant Physiol 127, 1180–1192 (2001).
Becnel, J., Natarajan, M., Kipp, A. & Braam, J. Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Mol Biol 61, 451–467, https://doi.org/10.1007/s11103-006-0021-z (2006).
Miedes, E. & Lorences, E. P. Xyloglucan endotransglucosylase/hydrolases (XTHs) during tomato fruit growth and ripening. J Plant Physiol 166, 489–498, https://doi.org/10.1016/j.jplph.2008.07.003 (2009).
Harada, T. et al. Cloning, characterization, and expression of xyloglucan endotransglucosylase/hydrolase and expansin genes associated with petal growth and development during carnation flower opening. J Exp Bot 62, 815–823, https://doi.org/10.1093/jxb/erq319 (2011).
Singh, A. P., Tripathi, S. K., Nath, P. & Sane, A. P. Petal abscission in rose is associated with the differential expression of two ethylene-responsive xyloglucan endotransglucosylase/hydrolase genes, RbXTH1 and RbXTH2. J Exp Bot 62, 5091–5103, https://doi.org/10.1093/jxb/err209 (2011).
Matsui, A. et al. AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. Plant J 42, 525–534, https://doi.org/10.1111/j.1365-313X.2005.02395.x (2005).
Nishikubo, N. et al. Xyloglucan endo-transglycosylase-mediated xyloglucan rearrangements in developing wood of hybrid aspen. Plant Physiol 155, 399–413, https://doi.org/10.1104/pp.110.166934 (2011).
Wu, Y., Jeong, B.-R., Fry, S. C. & Boyer, J. S. Change in XET activities, cell wall extensibility and hypocotyl elongation of soybean seedlings at low water potential. Planta 220, 593–601, https://doi.org/10.1007/s00425-004-1369-4 (2005).
Osato, Y., Yokoyama, R. & Nishitani, K. A principal role for AtXTH18 in Arabidopsis thaliana root growth: a functional analysis using RNAi plants. Journal of plant research 119, 153–162, https://doi.org/10.1007/s10265-006-0262-6 (2006).
Takahashi, H. Gravimorphogenesis: gravity-regulated formation of the peg in cucumber seedlings. Planta 203, S164–169 (1997).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant and Soil 39, 205–207, https://doi.org/10.1007/BF00018060 (1973).
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry 28, 350–356, https://doi.org/10.1021/ac60111a017 (1956).
Bailly, C., Benamar, A., Corbineau, F. & Come, D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiologia Plantarum 97, 104–110, https://doi.org/10.1111/j.1399-3054.1996.tb00485.x (1996).
Chi, M. et al. Reduced polyphenol oxidase gene expression and enzymatic browning in potato (Solanum tuberosum L.) with artificial microRNAs. Bmc Plant Biol 14, https://doi.org/10.1186/1471-2229-14-62 (2014).
Kochhar, S., Kochhar, V. K. & Khanduja, S. D. Changes in the Pattern of Isoperoxidases during Maturation of Grape Berries CV Gulabi as Affected by Ethephon (2-Chloroethyl) Phosphonic Acid. American Journal of Enology and Viticulture 30, 275–277 (1979).
Banowetz, G. M., Dierksen, K. P., Azevedo, M. D. & Stout, R. Microplate quantification of plant leaf superoxide dismutases. Analytical biochemistry 332, 314–320, https://doi.org/10.1016/j.ab.2004.06.015 (2004).
Aebi, H. Catalase in vitro. Methods in enzymology 105, 121–126 (1984).
Kuk, Y. I. et al. Antioxidative enzymes offer protection from chilling damage in rice plants. Crop Sci 43, 2109–2117 (2003).
Deyholos, M. K. & Galbraith, D. W. High-density microarrays for gene expression analysis. Cytometry 43, 229–238 (2001).
Livak, K. J. & Schmittgen, T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
Horsch, R. B. et al. A simple and general method for transferring genes into plants. Science 227, 1229–1231, https://doi.org/10.1126/science.227.4691.1229 (1985).
Iqbal, A., Tang, W., Wang, T. & Wang, H. Identification and functional characterization of the promoter driving “xyloglucan endotransglucosylase/hydrolase gene (XET)” gene for root growth in the desert Populus euphratica. South African Journal of Botany 112, 437–446, https://doi.org/10.1016/j.sajb.2017.06.031 (2017).
Holsters, M. et al. Transfection and transformation of Agrobacterium tumefaciens. Molecular & general genetics: MGG 163, 181–187 (1978).
Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. Vol. 6 (1987).
Hu, L. et al. RNA-seq for gene identification and transcript profiling in relation to root growth of bermudagrass (Cynodon dactylon) under salinity stress. BMC genomics 16, 575, https://doi.org/10.1186/s12864-015-1799-3 (2015).
Bernard, A. & Joubes, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog Lipid Res 52, 110–129, https://doi.org/10.1016/j.plipres.2012.10.002 (2013).
Cunningham, S. C. Stomatal sensitivity to vapour pressure deficit of temperate and tropical evergreen rainforest trees of Australia. Trees-Struct Funct 18, 399–407, https://doi.org/10.1007/s00468-004-0318-y (2004).
Lima, A. L. S., DaMatta, F. M., Pinheiro, H. A., Totola, M. R. & Loureiro, M. E. Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environmental and Experimental Botany 47, 239–247, https://doi.org/10.1016/S0098-8472(01)00130-7 (2002).
Sajedi, N. A., Ferasat, M., Mirzakhani, M. & Boojar, M. M. Impact of water deficit stress on biochemical characteristics of safflower cultivars. Physiology and molecular biology of plants: an international journal of functional plant biology 18, 323–329, https://doi.org/10.1007/s12298-012-0129-3 (2012).
Kuang, L. & Ge, H. Effects of progressive soil drought on membrane protective enzyme in Pinus pungens. Journal of Northeast Forestry University 38, 57–59 (2010).
Dat, J. et al. Dual action of the active oxygen species during plant stress responses. Cellular and molecular life sciences: CMLS 57, 779–795, https://doi.org/10.1007/s000180050041 (2000).
Regier, N. et al. Drought tolerance of two black poplar (Populus nigra L.) clones: contribution of carbohydrates and oxidative stress defence. Plant, cell & environment 32, 1724–1736, https://doi.org/10.1111/j.1365-3040.2009.02030.x (2009).
Song, Y., Chen, Q., Ci, D., Shao, X. & Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. Bmc Plant Biol 14, 111, https://doi.org/10.1186/1471-2229-14-111 (2014).
Weston, D. J. et al. Comparative physiology and transcriptional networks underlying the heat shock response in Populus trichocarpa, Arabidopsis thaliana and Glycine max. Plant, cell & environment 34, 1488–1506, https://doi.org/10.1111/j.1365-3040.2011.02347.x (2011).
Rodgers-Melnick, E. et al. Contrasting patterns of evolution following whole genome versus tandem duplication events in Populus. Genome research 22, 95–105, https://doi.org/10.1101/gr.125146.111 (2012).
Luo, Y. et al. D-myo-Inositol-3-Phosphate Affects Phosphatidylinositol-Mediated Endomembrane Function in Arabidopsis and Is Essential for Auxin-Regulated Embryogenesis. Plant Cell 23, 1352–1372, https://doi.org/10.1105/tpc.111.083337 (2011).
Alcazar-Roman, A. R. & Wente, S. R. Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117, 1–13, https://doi.org/10.1007/s00412-007-0126-4 (2008).
Taji, T., Takahashi, S. & Shinozaki, K. Inositols and their metabolites in abiotic and biotic stress responses. Sub-cellular biochemistry 39, 239–264 (2006).
Ruszkowski, M., Szpotkowski, K., Sikorski, M. & Jaskolski, M. The landscape of cytokinin binding by a plant nodulin. Acta crystallographica. Section D, Biological crystallography 69, 2365–2380, https://doi.org/10.1107/S0907444913021975 (2013).
Perilli, S., Moubayidin, L. & Sabatini, S. The molecular basis of cytokinin function. Current opinion in plant biology 13, 21–26, https://doi.org/10.1016/j.pbi.2009.09.018 (2010).
Bergonci, T. et al. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J Exp Bot 65, 2219–2230, https://doi.org/10.1093/jxb/eru099 (2014).
Lopez-Bucio, J. S. et al. Arabidopsis thaliana mitogen-activated protein kinase 6 is involved in seed formation and modulation of primary and lateral root development. J Exp Bot 65, 169–183, https://doi.org/10.1093/jxb/ert368 (2014).
Nuruzzaman, M., Sharoni, A. M. & Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Frontiers in microbiology 4, 248, https://doi.org/10.3389/fmicb.2013.00248 (2013).
Lu, D., Wang, T., Persson, S., Mueller-Roeber, B. & Schippers, J. H. M. Transcriptional control of ROS homeostasis by KUODA1 regulates cell expansion during leaf development. Nat Commun 5, https://doi.org/10.1038/Ncomms4767 (2014).
Tenhaken, R. Cell wall remodeling under abiotic stress. Frontiers in plant science 5, 771, https://doi.org/10.3389/fpls.2014.00771 (2014).
Muller, B., Reymond, M. & Tardieu, F. The elongation rate at the base of a maize leaf shows an invariant pattern during both the steady-state elongation and the establishment of the elongation zone. J Exp Bot 52, 1259–1268 (2001).
Brady, S. M. et al. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318, 801–806, https://doi.org/10.1126/science.1146265 (2007).
Iurlaro, A. et al. Drought and Heat Differentially Affect XTH Expression and XET Activity and Action in 3-Day-Old Seedlings of Durum Wheat Cultivars with Different Stress Susceptibility. Frontiers in plant science 7, https://doi.org/10.3389/Fpls.2016.01686 (2016).
Acknowledgements
This research was supported by National Natural Science foundation of China (NSFC, grant No. 31370598, 30371148, J1103516), Scientific Research Project for National Forestry Public Welfare Industry (grant no. 200704017), National High Technology and Development Program of China (grant no. 2006AA10Z182), and China National 948 Programme (grant no. 2007-4-02). These partners participated in the sampling in the Badain Jaran Desert Populus euphratica forests, Professor Zhanyuan Dong from Inner Mongolia Agricultural University, Ms. Aizhi Liu from Agriculture and Animal Husbandry Bureau in Alashan, Inner Mongolia Autonomous Region, Mr. Ming Li from Beijing Forestry University.
Author information
Authors and Affiliations
Contributions
A.I. wrote the first draft of manuscript, conceived the experiment(s), T.W., G.W., C.Z., W.T. and D.W. conducted the experiment(s), H.W. conceived the idea, designed the study and contributed to the writing of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iqbal, A., Wang, T., Wu, G. et al. Physiological and transcriptome analysis of heteromorphic leaves and hydrophilic roots in response to soil drying in desert Populus euphratica . Sci Rep 7, 12188 (2017). https://doi.org/10.1038/s41598-017-12091-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12091-2
This article is cited by
-
Short-term transcriptomic responses of Populus euphratica roots and leaves to drought stress
Journal of Forestry Research (2021)
-
Genome-wide transcriptomic analysis of a desert willow, Salix psammophila, reveals the function of hub genes SpMDP1 and SpWRKY33 in drought tolerance
BMC Plant Biology (2019)
-
Morpho-physiological and proteomic responses to water stress in two contrasting tobacco varieties
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.