Abstract
Individualized assessment of hepatocellular carcinoma (HCC) risk in chronic liver disease remains challenging. Serum biomarkers including cytokines may offer helpful adjuncts to standard parameters for risk prediction. Our aim was to identify markers associated with increased HCC incidence. This was a prospective cohort study of 282 patients with both viral or non-viral chronic liver disease. Baseline serum cytokines and other markers were measured in multiplex with a commercially-available Luminex-based system. Patients were followed until death or HCC diagnosis. We performed Lasso-based survival analysis to determine parameters associated with HCC development. Cytokine mean florescence intensity (MFI) was the primary predictor and HCC development the primary outcome. 25 patients developed HCC with total follow-up of 1,363 person-years. Parameters associated with increased HCC incidence were cirrhosis, hepatic decompensation, and soluble serum intercellular adhesion molecule 1 (sICAM-1) MFI. No other molecules increased predictive power for HCC incidence. On univariate analysis, the parameters associated with HCC incidence in patients with cirrhosis were age, antiviral treatment, and high sICAM-1 MFI; on multivariate analysis, sICAM-1 remained associated with HCC development (adjusted HR = 2.75). On unbiased screening of serum cytokines and other markers in a diverse cohort, baseline sICAM-1 MFI is associated with HCC incidence.
Similar content being viewed by others
Introduction
Liver cancer, of which hepatocellular carcinoma (HCC) constitutes 70–85%, is the fifth most common cancer and second most common cause of cancer death worldwide, and was responsible for approximately 700,000 deaths in 20081, 2. HCC incidence is rising in the United States and worldwide1, 3. While HCC is known to arise in the setting of chronic liver disease, such as infection with hepatitis B virus (HBV) or hepatitis C virus (HCV)4, 5, and there have been major advances in our understanding of HCC tumorigenesis in recent years, HCC pathogenesis remains incompletely and poorly understood. This is particularly true in regards to potential differences in pathogenesis based on etiology of underlying liver disease, which may have contributed to the lack of a universal risk prediction model for HCC based on currently available clinical parameters. Minimally invasive serum biomarkers to assess HCC risk have potential utility as adjuncts to standard clinical risk assessment6, 7.
Prior studies suggest that several cytokines have important effects in the pathogenesis of and survival after HCC. Interleukin (IL)-6 is one of the best studied in this context. In murine HCC models, IL-6 produced in either an autocrine or paracrine fashion by hepatic macrophages drives HCC tumorigenesis8,9,10. Interestingly, IL-6 inhibition also eliminated the increased risk of HCC in male mice10. Clinically, serum IL-6 concentration in HCC patients correlates with larger tumor size and decreased survival11, 12; and among at-risk patients with chronic hepatitis B and C13,14,15,16. Elevated serum IL-10 is also associated with poorer survival in HCC patients17, 18, possibly related to inhibition of γδ-T cell and dendritic cell function19, 20. More recently, soluble proteins associated with angiogenesis have been found to correlate with survival after HCC diagnosis21.
Intercellular adhesion molecule (ICAM-1) is an adhesion molecule that induces leukocyte trafficking to endothelial cells22, 23. ICAM-1 is also expressed on HCC cancer stem cells24. The soluble form of ICAM-1, sICAM-1, is a counter-receptor for the integrin lymphocyte function-associated antigen 1 and inhibits leukocyte trafficking22. sICAM-1 inhibits lymphocyte adhesion to major histocompatibility complex-restricted tumor-specific T cell immunity and promotes angiogenesis25, 26. In humans, high sICAM-1 concentration is associated with larger HCC tumor size27 and increased risk of HCC recurrence following resection28. High sICAM-1 concentration may also correlate with HCC incidence, based on one small study of 99 HCV patients29. It is also associated with poor prognosis in other malignancies such as breast cancer30 and non-Hodgkin lymphomas31.
These findings indicate that specific cytokines and other serum peptides are important in HCC tumorgenesis and are also associated with disease severity, and thus may be attractive targets in biomarker development. However, data on the use of cytokines to assess risk of HCC and other consequences of chronic liver disease remain highly limited. We hypothesized that serum cytokines correlate with HCC incidence and mortality in chronic liver disease, independently of known clinical risk factors or underlying etiology of liver disease. In order to investigate this question, we performed a prospective cohort study of HCC-free patients to determine how baseline cytokine profile may influence future risk of HCC.
Patients and Methods
Study design and patient population
We performed a prospective cohort study of 282 patients with chronic liver disease who were enrolled at liver clinics at Stanford University Medical Center between 2004 and 2010. This was a convenience cohort. Only patients with HBV, HCV, alcohol, nonalcoholic fatty liver disease, or cryptogenic liver disease were included; patients with HBV-HCV or HBV-HDV or HIV coinfection were not included, since as part of another study we were interested in characterizing immune phenotypes in individual causes of liver disease. Patients with a prior history of HCC and those diagnosed with HCC within 12 months of enrollment were excluded. Patients were followed approximately every 6 months until death or HCC development or end of study follow-up. HCC diagnosis was determined by CT or MRI imaging based on the 2011 American Association for the Study of Liver Disease guidelines5. Cirrhosis was defined as having pathological evidence of fibrosis stage 4, clinical evidence of portal hypertension (platelet count <120,000/μL, otherwise-unexplained splenomegaly, ascites, or gastroesophageal varices on imaging), prior hepatic decompensation (hepatic encephalopathy, ascites, variceal gastrointestinal bleeding), or laboratory evidence of decreased synthetic function (total bilirubin >2.0 mg/dL or INR > 1.2 without alternative explanation). Hepatic decompensation included patients with cirrhosis and one of the following decompensating events: variceal bleeding, hepatic encephalopathy, or ascites. Serum specimens were collected and questionnaires were administered by research coordinators at the time of enrollment. Additional patient characteristics were also collected by individual chart review. All patients provided written informed consent. This study was approved by the Institutional Review Board at Stanford University (Stanford, CA). All experiments were performed in accordance with relevant guidelines and regulations.
Cytokine multiplex analysis
Serum specimens were submitted to the Stanford Human Immune Monitoring Core for analysis of cytokines and other serum biomarkers using an FDA-approved Luminex 200-based platform (Austin, TX). In brief, human 51-plex plates were purchased from Affymetrix and used according to the manufacturer’s recommendations with modifications as described below. Samples were mixed with antibody-linked polystyrene beads on 96-well filter-bottom plates and incubated at room temperature for 2 h followed by overnight incubation at 4 °C. Room temperature incubation steps were performed on an orbital shaker at 500–600 rpm. Plates were vacuum filtered and washed twice with wash buffer, then incubated with biotinylated detection antibody for 2 h at room temperature. Samples were then filtered and washed twice as above and re-suspended in streptavidin-phycoerythrin. After incubation for 40 minutes at room temperature, two additional vacuum washes were performed, and the samples re-suspended in Reading Buffer. Each sample was measured in duplicate. Plates were read using a Luminex 200 instrument with a lower bound of 100 beads per sample per cytokine. Custom assay Control beads by Radix Biosolutions were added to all wells. This multiplex system is based on a flow cytometer and allows detection of up to 100 cytokines in a single 96-well plate.
A total of 51 serum cytokines and other biomarkers were measured simultaneously. The cytokines were: cluster of differentiation (CD) 40 ligand, epithelial cell-derived neutrophil-activating peptide-78, eotaxin, fibroblast growth factor-basic, granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, growth-regulated oncogene-α, hepatocyte growth factor, sICAM-1, interferon-α, -β, and -γ, interleukin (IL)-10, IL-12 p40, IL-12 p70, IL-13, IL-15, IL-17A, IL-17F, IL-1α, IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, interferon-γ-induced protein-10, leptin, leukemia inhibitory factor, macrophage, monocyte chemotactic protein-1 and-3, monocyte induced by gamma interferon, macrophage inflammatory protein-1α and -1β, nerve growth factor, plasminogen activator inhibitor-1, platelet derived growth factor-BB, chemokine (C-C motif) ligand 5, resistin, stem cell factor, soluble Fas ligand, transforming growth factor-α and -β, tumor necrosis factor-α and-β, tumor necrosis factor-related apoptosis-inducing ligand, soluble vascular cell adhesion molecule-1, and vascular endothelial growth factor. Several of these proteins overlap with previously-published cancer hallmark-based gene signatures, including IL-2, IL-8, and tumor necrosis factor-α32. Each plate contained two replicates of a “control” serum (taken from a middle-aged Caucasian male), calibration samples to aid in converting median fluorescent intensity values to units of concentration, and CHEX quality control beads designed to assist in detecting experimental failures. Specific anti-cytokine antibodies linked to unique polystyrene beads were applied to each 25-μl sample. Each plate was read on a Luminex reader, which identifies and classifies individual analytes by their bead color using the red laser, and quantifies analyte levels using the excitation of the green laser. Data were imported into Masterplex software and analyzed to obtain 51 standard curves for each analyte. Data are presented as median fluorescence intensity (MFI).
The rationale for presenting MFI rather than concentration is as follows. Detection for sICAM1 and other biomarkers in the samples are close to the upper limit on the standard curves. Since this panel measures 51 cytokines in a multiplex setup, the dilution is not optimal for all biomarkers. Therefore, it is preferable to use MFI values to prevent any extrapolation and add distortion with use of pg/ml values.
Statistical Analysis
Analysis of clinical characteristics
Descriptive statistics were reported as proportion (%) for categorical variables, and mean ± standard deviation (SD) or median (and interquartile range) for continuous variables. Analyses of normally distributed continuous variables were performed using the Student t test. Nonparametric statistics were applied when continuous variables were not normally distributed. The chi-square test was used to evaluate categorical variables. Lasso-based survival analysis was used to determine the parameters most strongly correlated with HCC development. The primary predictor variables were serum MFI of the individual cytokines and the primary outcome was development of HCC. Subsequent analysis with these parameters was performed using Kaplan-Meier methods. The log-rank test was used to compare survival between independent subgroups. The Cox proportional hazard model was used to estimate the hazard ratio (HR) relating risk factors to HCC incidence. Statistical significance was defined as a 2-tailed P value < 0.05. Survival analysis and time-dependent receiver operating characteristics (ROC) analysis was performed in R. All other statistical analyses were performed using Stata 11.0 (Stata Corporation, College Station, TX).
Analysis of HCC incidence based on cytokine profiles
To identify the most important clinical and molecular factors that influence the incidence of HCC, we utilized a Lasso-based survival method (R package glmnet). This method generates a collection of Cox proportional hazard models with increasing number of covariables and generally better performance. A key feature of Lasso-based survival analysis is that in each iteration the complexity of parameter space is contained, and then the optimal set of covariables are determined under such constraint through efficient optimization algorithms. By varying the complexity of parameter space from small to large, we increase the number of covariables in each model gradually. Therefore, covariables that enter models in early steps are the most prominent when only a few covariables are allowed in the modeling as they fit better models by explaining a larger amount of variance in the data than those unselected or selected in later steps.
This model included all MFI status of all 51 cytokines and seven clinical characteristics, namely sex, race/ethnicity, baseline cirrhosis status, history of hepatic decompensation, and underlying liver disease etiology. For cytokine MFI status, we used a cutoff of above vs. below the median MFI for that individual cytokine and classified MFI as high or low based on that.
To further assess and confirm the robustness of the association of identified biomarker with clinical outcomes, we also conducted re-sampling based tests following previously-described algorithms32, 33.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Disclosures
Vincent Chen, Jacqueline Estevez, An Le, Philip Vutien, Yael Rosenberg-Hasson, and Ellen Chang have no disclosures. Ondrej Podlaha, Biao Li, Stefan Pflanz, Zhaoshi Jiang, Dongliang Ge, and Anuj Gaggar are employees of Gilead Sciences. Mindie Nguyen: research support: Janssen Pharmaceutical, Bristol-Myers Squibb, Gilead Sciences. Consultant and/or an advisory board member: Roche Laboratories, Gilead Sciences, Janssen Pharmaceutical, Intercept Pharmaceutical, Alnynam Pharmaceutical, and Dynavax Laboratories.
Results
Overall patient baseline characteristics
Baseline patient characteristics are shown in Table 1. A total of 282 patients were included. The etiology of liver disease was HBV in 112 patients, HCV in 119, and nonviral in 51. HBV patients were primarily Asian (92%) while most HCV and nonviral patients were not (16% and 6%; p < 0.0001 for the between-group comparison). Approximately 61% of patients had cirrhosis, and 68% of those with cirrhosis had a history of hepatic decompensation. The overall mean model of end-stage liver disease score was 12.8. HBV patients were less likely to have cirrhosis and had lower model of end-stage liver disease scores.
Overall cumulative HCC incidence
Median follow-up was 65.3 months (IQR 21.5–88.5 months) with a total follow-up period of 1,382 person-years. During this period, there were a total of 25 cases of HCC, 8 in patients with HBV, 13 in patients with HCV, and 4 in patients with nonviral disease. The overall HCC incidence was 18.8 per 1,000 person-years. For HBV, it was 14.6 per 1,000 person-years; for HCV, 23.8 per 1,000 person-years; and for nonviral, 14.8 per 1,000 person-years. For patients with cirrhosis, the incidence was 25.6 per 1000 person-years; and for patients without cirrhosis, 0.6 per 1000 person-years.
Cytokine profiles and risk for development of HCC
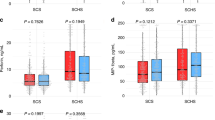
On Lasso analysis, the parameter most highly correlated with HCC development was history of hepatic decompensation, followed by sICAM-1 MFI, presence of cirrhosis, granulocyte-macrophage colony stimulating factor MFI, and soluble CD40 ligand MFI. On Kaplan-Meier analysis of sICAM-1 and granulocyte-macrophage colony stimulating factor MFI, high sICAM-1 MFI is significantly associated with increased HCC incidence (p = 0.001; Fig. 1A) but granulocyte-macrophage colony stimulating factor and CD40 ligand MFI were not (p = 0.411 and 0.871, respectively; Fig. 1B and C). Soluble vascular cell adhesion molecule-1, soluble CD95 ligand, and resistin MFI were correlated with sICAM-1 MFI (r = 0.63, 0.53, and 0.51, respectively) and the first two are associated with HCC incidence (p = 0.005, 0.014, and 0.232, respectively; Fig. 1D–F). However, sICAM-1 entered the Lasso regression model first, which indicates that it is more strongly associated with HCC incidence than these other three and that the other three do not provide additional predictive information beyond what sICAM-1 provides.
Results of Lasso-based regression to determine parameters associated with hepatocellular carcinoma incidence. (A–F) Kaplan-Meier curve of hepatocellular incidence based on baseline serum MFI of (A) soluble intercellular adhesion molecule 1, (B) granulocyte-macrophage colony stimulating factor, (C) CD40 ligand, (D) soluble vascular adhesion molecule 1, (E) CD95 ligand, and (F) resistin. In all panels, “high” denotes MFI above the median for that individual cytokine while “low” denotes MFI below the median.
We conducted re-sampling based tests to confirm the robustness of the association of ICAM1 with HCC patient survival rate. Following previously-published algorithms32, 33, we randomly selected half of the patient cohort (n = 138) while retaining the proportion of death event as the same as that of the whole cohort. We performed 100,000 times random selection and identified 17 sampling sets that showed less than 50% of overlap among them. For each of these 17 data sets, we ran the same log-rank test as on the whole data set. A total of 14 out of 17 (82%) tests showed a P-value < 0.05. Such a high replication rate from randomly generated data sets confirmed that the expression level of ICAM1 is strongly related to HCC survival probability.
The above HCC incidence analysis used a sICAM-1 cutoff of above vs. below the median (approximately 11,000 MFI). On ROC analysis, sICAM-1 MFI that was most predictive of HCC development within 10 years was at a cutoff of 11,861 MFI with an AUROC of 0.739 (Fig. 2A). At two years, the cutoff was 17,404 MFI with AUROC 0.955 (Fig. 2B), though there were only two events in this time period. Cutoffs for other time points are shown in Fig. 2C. The cutoff appears to decrease over greater length of follow-up. At the cutoff of 11,861 MFI, for the overall cohort, the HR for sICAM-1 MFI and HCC incidence was 4.42 (p = 0.0001; Fig. 3A). For patients with cirrhosis, the HR was 3.33 (p = 0.011; Fig. 3B) and for patients without cirrhosis there was no association between sICAM-1 and HCC incidence (p = 0.69; Fig. 3C); of note the total number of patients without cirrhosis who developed HCC was very low as detailed above. These results indicate that in the overall cohort and in patients with cirrhosis, sICAM-1 MFI is associated with increased HCC incidence. This association persisted with both Asian and non-Asian patients, and with the cutoffs of above vs. below the median and of 11,861 MFI (Supp. Figure 1), in the overall cohort. Supp. Table 1 shows HR for various specified subgroups among patients with baseline cirrhosis. Overall, the associations either were significant or trended toward significance. Several subgroups were not examined, such as patients with nonviral disease, due to low numbers of incident cases of HCC in this group.
Receiver operating characteristic (ROC) analysis for soluble intercellular adhesion molecule 1 (sICAM-1) and hepatocellular carcinoma (HCC) incidence. (A and B) ROC curves for association between sICAM-1 and HCC incidence at (A) 10 years with sICAM-1 cutoff of 11,861 mean fluorescence intensity (MFI) and (B) 2 years with sICAM-1 cutoff of 17,404 MFI. (C) Area under the ROC curve and sICAM-1 cutoff based on duration of follow-up.
Association of elevated sICAM-1 MFI with patient baseline clinical characteristics
Because of the very low number of new HCC diagnoses among the patients without baseline cirrhosis, we focused for the remainder of our analysis on the patients with baseline cirrhosis. Having identified an association between sICAM-1 MFI and HCC incidence, we next sought to characterize the baseline clinical characteristics of these patients, stratified by sICAM-1 status, among patients with cirrhosis (Table 2). Patients with high sICAM-1 MFI had similar age but were more often male and non-Asian. They also had a greater prevalence of prior hepatic decompensation, higher transaminases, and more advanced liver disease based on platelet count, international normalized ratio, albumin, Child-Pugh score, and model of end-stage liver disease score.
Elevated sICAM-1 MFI: univariate and multivariate analysis
Univariate analysis of parameters associated with HCC incidence was shown in Table 3 for patients with cirrhosis and Supp. Table 2 for all patients. Among the overall cohort, factors associated with increased HCC incidence were advancing age, presence of cirrhosis, high Child-Pugh score, low platelet count, low albumin, successful treatment with antiviral medications, and high sICAM-1. Most of these parameters are associated with cirrhosis, and among patients with cirrhosis the only factors associated with increased HCC incidence were age, treatment with antiviral agents, and sICAM-1. We constructed a multivariate model that included these three variables, and on multivariate analysis, sICAM-1 remained significantly associated with increased HCC incidence (HR 2.75; p = 0.041). Of note, many of the variables that were associated with higher sICAM-1 MFI, as detailed in the previous section, were not themselves associated with increased HCC incidence in patients with cirrhosis.
Discussion
In summary, our comprehensive profiling of 51 cytokines in 282 patients with various chronic liver diseases who were free of HCC at baseline identified sICAM-1 to highly associate with development of future HCC (AUROC 0.74 at year 10 and 0.955 at year 2). These findings held across diverse etiologies of liver disease and in patients with and without cirrhosis, and were independent of established clinical risk factors. To our knowledge, this study is the largest and best-characterized prospective cohort study investigating the cytokine profiles of HCC-free patients with chronic liver disease with respect to hepatic complications including liver cancer development. In addition, it investigated multiple different cytokines in an unbiased way.
Biomarkers to assess survival after HCC development have been comparatively well-established. A seminal paper from 2008 examined gene expression in tumor and peritumor liver tissue in patients treated with partial hepatectomy, and found that peritumor gene signatures were associated with disease recurrence34. This research has been extended to biomarkers in serum, which is more easily accessible, especially in patients with advanced-stage HCC who would generally not undergo surgery or liver biopsy. For example, higher serum MFI of macrophage migratory inhibitory factor35 and IL-1036 is associated with greater likelihood of recurrence in patients treated with partial hepatic resection. A recent analysis of the EVOLVE-1 trial investigating everolimus in HCC patients with tumor progression on sorafenib found that the angiogenesis markers vascular endothelial growth factor and soluble vascular endothelial growth factor receptor 1 were associated with decreased survival21. A molecular classification of HCC based on tissue specimens is emerging based on genomic analysis of resected tumor specimens, with three molecularly-defined subclasses of HCC with implications for patient survival37. Applying this research to circulating tumor cells raises the possibility of defining molecular subclasses in advanced disease, rather than only early-stage disease. This type of research has important implications in assessing prognosis, identifying biomarkers that might be used to stratify patients in future clinical trials, and discovering potential therapeutic targets for drug development.
In contrast, the field of serum biomarkers to predict clinical outcomes in liver disease remains underdeveloped, though it too has important implications in guiding surveillance frequency: higher-risk patients may warrant more intensive surveillance, while lower-risk patients might safely be surveyed less frequently or with less costly modalities which could decrease healthcare expenditures. The largest study published on serum biomarkers to predict HCC risk is the European Prospective Investigation into Cancer and Nutrition, which prospectively followed over 500,000 healthy individuals and investigated risk factors for liver cancer6. This study identified C-reactive protein, IL-6, C-peptide, and non-high weight adiponectin as risk factors for liver cancer. In contrast to ours, this study focused on a healthy population, with a low prevalence of diabetes (under 4%) and no known viral hepatitis or cirrhosis. The most likely risk factor for HCC in this study was non-alcoholic fatty liver disease and HCC incidence was extremely low ( <0.01% per person-year). Other studies investigating biomarkers to predict liver-related outcomes have generally been smaller and limited to a single etiology of disease. For example, IL-6 has been associated with increased HCC risk in chronic hepatitis B38, and adiponectin and IL-6 in chronic hepatitis C39. There are multiple well-established models for predicting HCC risk in chronic hepatitis B as well as chronic hepatitis C albeit to a lesser extent for the latter14,15,16, but these models have mostly been developed in restricted geographic regions and are not universally applicable across regions or ethnicities. In addition, risk calculators for nonviral liver disease are much less developed. Therefore, further research to enhance current risk prediction for HCC in diverse patient population is needed and novel biomarker with high predictive accuracy and precision can improve the current diagnostic strategies for HCC as well as therapeutic and surveillance strategies for high-risk patients.
In regards to sICAM-1, there is biologic rationale underlying its association with clinical outcomes such as HCC. sICAM-1 is correlated with more advanced tumor stage in humans though mechanisms for this finding are not well-established28. Potential explanations include that ICAM-1 expressed by tumor cells may help cancer cells adhere to leukocytes and enter the blood stream; sICAM-1 inhibits interactions between tumor-specific T cells and cancer cells; and sICAM-1 shed by tumor cells promotes angiogenesis23, 25,26,27. Surface ICAM-1 helps regulate leukocyte extravasation, It may also inhibit the host immune response to malignant cells23. However, the mechanism behind the association between sICAM-1 MFI in cancer-free patients and HCC incidence is even more speculative and may relate to sICAM-1 production by pre-malignant HCC stem cells24.
Our study has several strengths. First, it is relatively large and includes patients of diverse racial/ethnic backgrounds, with different etiologies of disease, and with different severities of underlying liver disease. In addition, the method detailed here is practical since it is based on readily-available serum rather than tissue. The selection of cytokines and other serum markers was unbiased in that it was not selected for specific proteins known to be involved in the pathogenesis of liver disease. There are also several limitations of this study. It included only cytokines and other proteins related to immune function, while there are a number of other pathways known to be implicated in HCC development, including oxidative stress and numerous other modulators of metabolism40, 41. Finally, this cohort was too small to investigate how cytokines affected HCC risk in patients without cirrhosis, who had a low overall incidence of HCC, so future larger studies will be required to assess biomarkers as risk factors for HCC in this lower-risk population.
In conclusion, this is to our knowledge the largest and most diverse prospective study of patients with chronic liver disease examining associations between longitudinal clinical outcomes and the baseline levels of serum cytokines and other biomarkers. We have identified sICAM-1 as a biomarker associated with HCC incidence with chronic liver disease. Use of these biomarkers could potentially guide decisions surrounding HCC surveillance frequency and therapeutic decision for antiviral therapies in higher-risk patients with viral hepatitis. Future studies will be required to validate these findings, extend them to patients without liver cirrhosis, and create a predictive model for HCC that includes sICAM-1.
References
American Cancer Society. Global Cancer Facts & Figures 2nd Edition. Atlanta: American Cancer Society, 2011.
Cancer Incidence in Five Continents. Volume IX. (International Agency for Research on Cancer, 2007).
American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014.
Bruix, J. & Sherman, M. Management of hepatocellular carcinoma. Hepatology 42, 1208–1236, doi:10.1002/hep.20933 (2005).
Bruix, J. & Sherman, M. Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022, doi:10.1002/hep.24199 (2011).
Aleksandrova, K. et al. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology 60, 858–871, doi:10.1002/hep.27016 (2014).
Costantini, S. et al. Cancer biomarker profiling in patients with chronic hepatitis C virus, liver cirrhosis and hepatocellular carcinoma. Oncol Rep 29, 2163–2168, doi:10.3892/or.2013.2378 (2013).
He, Y. et al. IkappaBalpha gene promoter polymorphisms are associated with hepatocarcinogenesis in patients infected with hepatitis B virus genotype C. Carcinogenesis 30, 1916–1922, doi:10.1093/carcin/bgp226 (2009).
Wan, S. et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 147, 1393–1404, doi:10.1053/j.gastro.2014.08.039 (2014).
Naugler, W. E. et al. Gender Disparity in Liver Cancer Due to Sex Differences in MyD88-Dependent IL-6 Production. Science 317, 121–124 (2007).
Jang, J. W. et al. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine 60, 686–693, doi:10.1016/j.cyto.2012.07.017 (2012).
Ohishi, W. et al. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer 134, 154–163, doi:10.1002/ijc.28337 (2014).
Nakagawa, H. et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. Int J Cancer 125, 2264–2269, doi:10.1002/ijc.24720 (2009).
Yang, H.-I. et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. The Lancet Oncology 12, 568–574, doi:10.1016/s1470-2045(11)70077-8 (2011).
Papatheodoridis, G. V., Chan, H. L., Hansen, B. E., Janssen, H. L. & Lampertico, P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 62, 956–967, doi:10.1016/j.jhep.2015.01.002 (2015).
Lok, A. S. et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 136, 138–148, doi:10.1053/j.gastro.2008.09.014 (2009).
Hattori, E. Possible contribution of circulating interleukin-10 (IL-10) to anti-tumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatology Research 27, 309–314, doi:10.1016/j.hepres.2003.07.002 (2003).
Chan, S. L. et al. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer 118, 3984–3992, doi:10.1002/cncr.26726 (2012).
Yi, Y. et al. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J Hepatol 58, 977–983, doi:10.1016/j.jhep.2012.12.015 (2013).
Beckebaum, S. et al. Increased Levels of Interleukin-10 in Serum from Patients with Hepatocellular Carcinoma Correlate with Profound Numerical Deficiencies and Immature Phenotype of Circulating Dendritic Cell Subsets. Clin Cancer Res 10, 7260–7269 (2004).
Zhu, A. X. et al. Integrative biomarker analyses indicate etiological variations in hepatocellular carcinoma. J Hepatol 65, 296–304, doi:10.1016/j.jhep.2016.04.015 (2016).
Rieckmann, P. et al. Soluble forms of intercellular lymphocyte adhesion molecule-l (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol 60, 9–15 (1995).
Yoong, K. F., McNab, G., Hübscher, S. G. & Adams, D. H. Vascular Adhesion Protein-1 and ICAM-1 Support the Adhesion of Tumor-Infiltrating Hepatocellular Carcinoma Lymphocytes to Tumor Endothelium in Human Hepatocellular Carcinoma. J Immunol 160, 3978–3988 (1998).
Liu, S. et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology 144, 1031–1041 e1010, doi:10.1053/j.gastro.2013.01.046 (2013).
Becker, J. C., Termeer, C., Schmidt, R. E. & Bröcker, E. B. Soluble intercellular adhesion molecule-1 inhibits MHC-restricted specific T cell/tumor interaction. J Immunol 151, 7224–7232 (1993).
Gho, Y. S., Kim, P. N., Li, H.-C., Elkin, M. & Kleinman, H. K. Stimulation of Tumor Growth by Human Soluble Intercellular Adhesion Molecule-1. Cancer Res 61, 4253–4257 (2001).
Sun, J.-J. et al. Invasion and metastasis of liver cancer: expression of intercellular adhesion molecule 1. J Cancer Res Clin Oncol 125, 28–34 (1999).
Zhu, P. P., Yuan, S. G., Liao, Y., Qin, L. L. & Liao, W. J. High level of intercellular adhesion molecule-1 affects prognosis of patients with hepatocellular carcinoma. World J Gastroenterol 21, 7254–7263, doi:10.3748/wjg.v21.i23.7254 (2015).
Moriyama, M. et al. Measurement of human intercellular adhesion molecule 1 in the blood is useful for predicting the occurrence of hepatocellular carcinomas from chronic hepatitis C and liver cirrhosis. Intervirology 49, 327–338, doi:10.1159/000095152 (2006).
Schroder, C. et al. Prognostic value of intercellular adhesion molecule (ICAM)-1 expression in breast cancer. J Cancer Res Clin Oncol 137, 1193–1201, doi:10.1007/s00432-011-0984-2 (2011).
Christiansen, I. et al. Elevated serum levels of soluble ICAM-1 in non-Hodgkin’s lymphomas correlate with tumour burden, disease activity and other prognostic markers. Brit J Haematol 92, 639–646 (1996).
Gao, S. et al. Identification and Construction of Combinatory Cancer Hallmark-Based Gene Signature Sets to Predict Recurrence and Chemotherapy Benefit in Stage II Colorectal Cancer. JAMA Oncol 2, 37–45, doi:10.1001/jamaoncol.2015.3413 (2016).
Li, J. et al. Corrigendum: Identification of high-quality cancer prognostic markers and metastasis network modules. Nature Communications 3, doi:10.1038/ncomms1400 (2012).
Hoshida, Y. et al. Gene Expression in Fixed Tissues and Outcome in Hepatocellular Carcinoma. N Engl J Med 359, 1995–2004 (2008).
Hira, E. et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer 103, 588–598, doi:10.1002/cncr.20818 (2005).
Chau, G.-Y. et al. Serum Interleukin-10 But Not Interleukin-6 Is Related to Clinical Outcome in Patients With Resectable Hepatocellular Carcinoma. Ann Surg 231, 552–558 (2000).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 69, 7385–7392, doi:10.1158/0008-5472.CAN-09-1089 (2009).
Wong, V. W. et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer 124, 2766–2770, doi:10.1002/ijc.24281 (2009).
Nakagawa, H. et al. Impact of serum levels of interleukin-6 and adiponectin on all-cause, liver-related, and liver-unrelated mortality in chronic hepatitis C patients. J Gastroenterol Hepatol 30, 379–388, doi:10.1111/jgh.12719 (2015).
Koike, K. Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways. J Gastroenterol Hepatol 22(Suppl 1), S108–111, doi:10.1111/j.1440-1746.2006.04669.x (2007).
Bechmann, L. P. et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56, 952–964, doi:10.1016/j.jhep.2011.08.025 (2012).
Acknowledgements
We would like to acknowledge Drs Hwai-I Yang and Mei-Hsuan Lee for insightful discussion. This study was supported in part by a Translational Grant from the Stanford Cancer Institute (MHN).
Author information
Authors and Affiliations
Contributions
Guarantor of article: Dr. Mindie H. Nguyen. Vincent Chen: study design, data collection, data analysis and interpretation, drafting of the manuscript. An Le: data collection, data analysis and interpretation, and critical review of the manuscript. Ondrej Podlaha: data analysis and interpretation and critical review of the manuscript. Jacqueline Estevez: data collection, data analysis and interpretation, and critical review of the manuscript. Biao Li: data analysis and interpretation and critical review of the manuscript. Philip Vutien: data collection, data analysis and interpretation, and critical review of the manuscript. Ellen Chang: study concept, data collection, data analysis and interpretation, and critical review of the manuscript. Yael Rosenberg-Hasson: performance of multiplex, data analysis and interpretation, and critical review of the manuscript. Stefan Pflanz: data analysis and interpretation and critical review of the manuscript. Zhaoshi Jiang: data analysis and interpretation and critical review of the manuscript. Dongliang Ge: data analysis and interpretation and critical review of the manuscript. Anuj Gaggar: data analysis and interpretation and critical review of the manuscript. Mindie Nguyen: concept development, study design, data collection, data analysis and interpretation, and critical revision of the manuscript. All authors identified above have critically reviewed the paper and approve the final version of this paper, including the authorship statement.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, V.L., Le, A.K., Podlaha, O. et al. Soluble intercellular adhesion molecule-1 is associated with hepatocellular carcinoma risk: multiplex analysis of serum markers. Sci Rep 7, 11169 (2017). https://doi.org/10.1038/s41598-017-10498-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10498-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.