Abstract
The CLAVATA pathway that regulates stem cell numbers of the shoot apical meristem has exclusively been studied in Arabidopsis; as such insight into other species is warranted. In this study, a GmCLV1A mutant (F-S562L) with altered lateral organ development, and two mutants of GmNARK, isolated from a Forrest M2 population (EMS-mutated soybean) were studied. GmCLV1A and GmNARK encode for LRR receptor kinases, and share 92% of protein sequence. While GmNARK is critical for systemic regulation of nodulation (new organ made on the root through symbiosis), we show that GmCLV1A functions locally and has no apparent function in nodulation or root development. However, a recessive, loss-of-function mutation (S562L) in a putative S-glycosylation site of GmCLV1A causes stem nodal identity alterations as well as flower and pod abnormalities (deformed flower and pod). The mutant also exhibits a homeotic phenotype, displaying abnormal leaf development/number, vein-derived leaf emergence, and a thick, faciated stem. The mutant phenotype is also temperature-sensitive. Interestingly, a novel truncated version of GmCLV1A was identified upstream of GmCLV1A that is absent from GmNARK, but is present upstream of the GmNARK orthologues, MtSUNN and PvNARK. Taken together, our findings indicate that GmCLV1A acts on shoot architecture, whereas GmNARK, functions in controlling nodule numbers.
Similar content being viewed by others
Introduction
Legumes are an important family of angiosperms as they are able to form a symbiosis with bacteria, named rhizobia that fix atmospheric nitrogen gas1. This symbiosis can be highly beneficial as it reduces the nitrogen fertiliser inputs in agriculture. Among the over 18,000 legume species, soybean (Glycine max (L) Merr.) is one of the most globally significant crops (2015 annual global yield of 319 million metric tons)2. Nearly 75% of its genome is duplicated, or homeologous, due to duplication events occurring ~59 and ~13 million years ago3. Following the duplication event, both gene diversification and gene loss have occurred.
The shoot apical meristem (SAM) of plants contains a population of undifferentiated stem cells, which give rise to aerial organs4,5,6,7. This process is best characterised by the CLAVATA (CLV) signalling network in Arabidopsis, where receptors CLV1, CLV2, Coryne and RPK2 (Receptor Protein Kinase 2) interact with the peptide ligand CLV3 to regulate the stem cell reservoir4, 8,9,10 through WUSCHEL (a homeodomain transcription factor; WUS) gene11. Furthermore, CLV1 is also involved in regulating stem cell number at the root apical meristem (RAM)12 as well as fruit development13. CLV1 and RPK2 belong to a large family of Leucine-Rich-Repeat (LRR) receptor kinase genes9, 14. CLV2 encodes a LRR receptor protein15, Coryne encodes a receptor-like kinase with a short extracellular domain16, while CLV3 encodes a prepropeptide17 that is edited and modified into a mature, short (12-13 amino acid) CLE peptide signal (reviewed in Hastwell et al.18).
Perception of CLV3 restricts the production of the transcription factor WUSCHEL11, 19, which promotes stem cell activity and increases the production of CLV320, 21. WUS may function in cytokinin signalling22 as the cytokinin response regulator gene ARR7 is suppressed by WUS. Therefore, a balance in soma and stem cell populations is maintained23. These ligand signals, dynamic receptor complexes and phytohormones, shape the plant architecture via short- and long-distance communication.
In Arabidopsis, mutations in CLV genes lead to enlarged shoot apices and abnormal floral meristems24,25,26. In soybean, two genes called GmCLV1A and GmNARK (Glycine max Nodule Autoregulation Receptor Kinase; formerly known as GmCLV1B), that belong to the LRR receptor kinase protein family, are highly similar to AtCLV1. At the amino acid level, GmCLV1A and GmNARK are 92% similar to one another27, 28, and 60% similar to AtCLV1.
Mutations in GmNARK lead to a super- or hyper-nodulation phenotype due to an inability to inhibit early nodulation events29,30,31. NARK mutant and wild type are similar in nitrogen fixation efficiency, but individual nodules in NARK mutants are smaller than the wild type ones29. GmNARK orthologues have been identified in Phaseolus vulgaris, PvNARK 32; Lotus japonicus, LjHAR1 33, 34; Medicago truncatula, MtSUNN 30; Glycine soja, GsNARK 27 and Pisum sativum, PsSYM2933. These genes act in the shoot and regulate nodule numbers via systemic Autoregulation Of Nodulation (AON) in which early nodulation events prevent further nodule development1, 35, 36. They act to perceive root-derived, rhizobia-induced, CLE peptides signals that are highly similar to CLV337,38,39. In soybean, these signals are called Rhizobium-Induced CLE peptides (RIC1 and RIC2)39. Interestingly, GmNARK also functions locally in nitrate-regulation of nodulation by perceiving a separate root-derived, nitrate-induced, CLE peptide signal, called Nitrate-Induced CLE peptide (NIC1)36, 39. In Lotus japonicus, Too Much Love (TML) (a root factor of nodulation)40 and HAR1 (a shoot factor) constitute the same long-distance signaling that control nodule formation.
The receptors CLV2 in M. truncatula 41 , L. japonicus and P. sativum 42 and KLAVIER (KLV) in L. japonicus 43 and Coryne in M. truncatula 41 may participate in receptor complexes with their respective GmNARK orthologue to perceive the nodulation-suppressing CLE peptides and subsequently trigger the production of a ‘Shoot Derived Inhibitor’ (SDI). SDI then travels to roots where it inhibits early nodule development44, 45. The SDI signal may be a shoot-derived cytokinin that acts to regulate cell divisions specifically in the nodule primordia44, 45. Recent findings indicate that this may occur through the regulation of miR172c, which down-regulates GmNNC1 transcripts, which in turn negatively regulates the expression of early nodulin ENOD40 46.
Despite being homeologs and having extremely high amino acid similarity, GmCLV1A does not complement GmNARK 27. In fact, to date the function of GmCLV1A has remained totally unclear. Here, for the first time we report our characterisation of GmCLV1A, and provide genetic and developmental analyses of the recently isolated, and unique, soybean mutant having a missense mutation in the GmCLV1A gene. The data clearly demonstrate neodiversification of gene function between GmCLV1A and its homeologous duplicate, GmNARK.
Results
Identification and characterisation of GmNARK mutants by TILLING
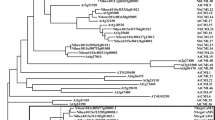
GmCLV1A and GmNARK genes and their protein structure are highly conserved28, except for the length and sequence of the intron, which is 74 bp for GmCLV1A and 467 bp for GmNARK (Fig. 1A). Dot plot analysis failed to reveal any significant similarity between the intron sequences of GmCLV1A, GmNARK and AtCLV1A (SM, BJF and PMG, unpublished).
Structure and genomic environments of CLAVATA1 and AON-related genes. (A) Intron and exon positions and sizes of AtCLV1, GmCLV1A, GmNARK, MtSUNN, LjHAR1, PsSYM29 and PvNARK. (B) TILLed regions of GmNARK and GmCLV1A. (C) Genomic environment of AtCLV1A, GmCLV1A, GmNARK, LiHAR1, MtSUNN and PvNARK; approximately 100 kb is shown. The same number and colour indicates similar genes. The CLV1 and its orthologs in legumes are in grey. The number ‘1’ represents a truncated gene. (D) Positioning and size of GmCLV1A with GmTrCLV1A, PvNARK with PvTrNARK and MtSUNN with MtRLP1.
TILLING was used to isolate mutants of GmNARK from the cultivar Forrest. Two thousand EMS mutagenised Forrest seeds formed a M1 population with 50–70% germination rate. The average mutation frequency in this population was estimated to be one mutation per 150 kb47. From this, two pooled cv. Forrest M2 multi-titre plates, consisting of total genomic DNA from 1,536 families that was divided into ‘8 family’ pools, were screened to identify mutations within a 1,406 bp region of the GmNARK gene. This region spans parts of the LRR, transmembrane and kinase encoding domains of GmNARK and represents about half of the GmNARK gene (Fig. 1B). If a DNA pool contained a mutant amplicon, mis-match pairing caused by melting and re-annealing of mixed mutant and wild type DNA, created an ENDO1 cleavage site and two instead of one DNA bands after gene-specific PCR. For a candidate mutant, the apparent mass of the corresponding highlighted bands at the 700 and 800 nm channels were required to equal the total amplicon size. Amplicon-specific primers (Supplementary Table S1) were designed and used for candidate mutant detection and further validation.
In total, twelve GmNARK mutants were predicted based on the original TILLING/LiCor output, of which two, F-W677* and F-H811Q, were confirmed (Table 1). Mutant allele nomenclature defines the parent cultivar (F for Forrest) and the predicted amino acid alteration (e.g., H to Q). A non-sense mutant allele is indicated by an asterisk (*).
Each of the mutants identified contain two closely linked mutations in their GmNARK coding sequence; thus, overall there were four mutations identified within the gene. The F-H811Q mutant contained a missense mutation (H811Q; located in the kinase domain) and an equal sense mutation (H789 = ), in which the nucleotide, but not the amino acid was changed. Three families were identified which contained these mutations. This mutant did not show any obvious phenotypic differences in root nodule compared to wild type Forrest (Table 1). The severity of the observed phenotypes (data not shown) was consistent with PyMol protein model predictions (data not shown).
F-W677* contained a non-sense mutation (W677*), causing a premature stop codon, and also a mis-sense mutation (L829V). The root nodulation phenotype of this mutant showed a dramatic supernodulation phenotype (Fig. 2, left column; here shown at three months after inoculation). The nodule number of the mutant F-W677* was approximately 13 times greater than that of wild type Forrest; the roots were approximately 30% shorter and had a dry weight of 60% of parallel-grown wild type (consistent with previously characterised alleles at the GmNARK locus)48.
Identification and characterisation of a GmCLV1A mutant by TILLING
From the 1,536 M2 families screened, one GmCLV1A mutant, F-S562L, was confirmed (Fig. 2; right column). This mutant contained a mis-sense mutation (S562L) disrupting a putative serine phosphorylation site in the LRR extracellular receptor domain (Fig. 3A and B). Serine is a polar amino acid and usually contributes to protein phosphorylation, while leucine is a hydrophobic amino acid49. The missense mutation interrupts a predicted glycosylation site (Fig. 3C), which would extend hydrophobicity of the protein.
Structural aspects of GmCLV1A and GmTrCLV1A. (A) Predicted model of the extracellular LRR domain of GmCLV1A, including the site of the S562L mis-sense mutation. The amino acid highlighted in red represent the serine of the predicted glycosylation site that is mutated to a leucine in S562L (B) Predicted protein domains. SP = signal peptide; LRRNT_2 = Leucine-rich repeat N-terminal; TM = Transmembrane domain. (C) Protein alignment of the mutated region of S562L compared with that of AtCLV1, GmCLV1A, GmTrCLV1A, GmNARK, MtSUNN, and MtRLP1. The red box highlights the predicted glycosylation site.
F-S562L did not show any obvious differences in its root nodule phenotype compared to wild type Forrest grown under identical conditions (Table 1); however, severe shoot phenotypic differences were observed. The mutant exhibits fewer nodes per plant and increased branching and leaves in the basal nodes (cotyledon, unifoliate and first trifoliate leaf) compared to its wild type, Forrest (Table 2; and Fig. 2; right column). This phenotype was highly related to the fasciated phenotypes of Arabidopsis CLV1 mutants. No additional mutants of GmCLV1A have been found in any mutant population, despite an extensive search, suggesting potential fitness factors and the possibility that some mutations in this gene are lethal, as compared with GmNARK which has many available mutants32.
GmCLV1A genomic environment
To determine whether GmCLV1A and GmNARK arose from a common ancestor through divergent evolution, or are the result of genome duplications in soybean3, the genomic environments within about 50 kb of GmCLV1A and GmNARK were determined relative to their orthologues, AtCLV1A, LjHAR1 and MtSUNN. In all investigated species, the genes reside within highly similar genomic regions (Fig. 1C). GmCLV1A and GmNARK are located on soybean chromosomes 11 and 12 respectively, proposed to be segmentally duplicated regions of the palaeopolyploid genome3 (Fig. 1C).
Of interest, a truncated GmCLV1A-like gene was identified 4.7 kb upstream of GmCLV1A, and designated here as GmTruncatedCLV1A (GmTrCLV1A). A similarly truncated gene called MtRLP1 30 is located 6.2 kb upstream of MtSUNN in Medicago truncatula, and PvTrNARK is a truncated gene located 4.9 kb upstream of PvNARK in common bean (Fig. 1D)32. All of these truncated versions of the genes has high nucleotide sequence identity to the receptor and transmembrane portions of their genes and lose the kinase domain. It seems that they lead to similar proteins in different legumes are involved in similar biological pathways. However, investigations into GmNARK and LjHAR1 indicated that these genes do not have a truncated copy located upstream, suggesting evolutionary deletion, or independent origins of the truncation. Either way, the function of the truncated copies, if any, remains unknown.
GmCLV1A and GmTrCLV1A expression
Steady-state mRNA levels of GmCLV1A and GmTrCLV1A were investigated in root, leaf and shoot tissues of un-inoculated soybean plants. GmCLV1A and GmTrCLV1A were expressed in all tissues analysed, including the shoot tip (Fig. 4). GmTrCLV1A was expressed at a much lower level compared with GmCLV1A (Fig. 4), which is consistent with MtRLP1 expression30. Moreover, GmTrCLV1A expression was consistent with transcriptome data available in the soybean gene atlas (http://soybase.org/soyseq) and soybean eFP browser (http://www.bar.utoronto.ca/efpsoybean/cgi-bin/efpWeb.cgi). GmNARK, was also expressed throughout the plant, but not at a high level in the shoot tip (consistent with Nontachalyapoom, S. et al.50).
Transcript levels of GmCLV1A and GmTrCLV1A in various tissues of 14 day-old, uninoculated soybean plants. Values were measured using qRT-PCR; n = 3 biological replicates per tissue; error bars indicate SE. TR1 = first 2 cm from taproot tip; TR2 = second 2 cm from taproot tip; LR1 = first 2 cm from lateral root tip; LR2 = second 2 cm from lateral root tip; UF = unifoliate leaf; TF = trifoliate leaf; Vein = vein of trifoliate leaf; Hypo = hypocotyls; Stem = stem above hypocotyl; STip = shoot tip. Note the 10-fold difference in scale.
GmCLV1A controls shoot nodal identity patterns
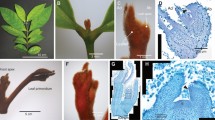
The S562L mutant exhibits an environmentally influenced phenotype with abnormal leaf development and number (Fig. 5), similar to the homeotic mutant of pea, Pscochleata 51, 52. Unlike Pscochleata, S562L also displays a fasciated phenotype, with thick, woody stems, and bifurcated pods that often abort, especially at basal nodes (Fig. 5A,B and D–F). In addition, S562L exhibits significantly increased lateral shoot branching (Figs 2 and 5B, Table 2). This is consistent with the differential expression of many genes in the apical meristem of the mutant compared with its wild-type parent, which may cause altered phenotypes53. Strikingly, the underside of some leaves of more mature (8-12 week old) mutant S562L plants produced small leaf-like structures emanating from their veins (vein-bladed; Fig. 5C). These unique leaf-like structures emerge after flowering and are exclusively located at the junction of the main and lateral veins, sometimes occurring in clusters.
Macro- and microscopic phenotypes of the soybean wild type Forrest, and its mutant S562L. (A) Stem thickness of 5 month-old plants (plants were intentionally defoliated to enhance visibility of stem architecture); (B) First trifoliate node showing fasciation and excessive flowering in the mutant; (C) Vein-bladed leaf structures on the underside of Gmclv1a mutant leaves. (D) Young pod morphology (dashed line indicates the position of the cross-section seen in (F). (E) Stem section at node 4 of Forrest and S562L mutant plants (4 month-old). (F) Young pod cross-sections. Note the bifurcated, deformed pod of the S562L mutant. VB = Vascular bundle; Ep = Epidermis; IS = Inner sclerenchyma.
Segregation of GmCLV1A in a S562L x Forrest population
Crossing S562L × Forrest (wild type), and segregation was determined in the F2 generation. The GmCLV1A phenotype was complemented in all F1 plants. Fifty F2 plants were subsequently derived from self-pollination of a single F1 plant. These exhibited the predicted Mendelian ratio for a single gene: 1:2:1 (Fig. 6; χ2 = 0.39, p > 0.05). In the F2 generation, Gmclv1a homozygous mutants produced significantly more branches at the first 3 nodes (juvenile phase) compared to wild-type Forrest and wild-type homozygous segregants. Branching at later nodes was similar among all lines (Fig. 6). This confirms that the phenotype segregates with the genotype, demonstrating that the Gmclv1a mutation could be the cause of the observed developmental changes.
Branching phenotype of 4 week-old soybean cv. Forrest, its mutant Gmclv1a (S562L), and F2 segregants from a cross between them. CC = wild-type segregants; cc = Gmclv1a segregants. Forrest n = 10, S562L n = 9, CC n = 11 and cc n = 14. Error bars indicate SE. Different letters above bars represent statistically significant differences (Student’s t test; P ≤ 0.05).
Local and systemic function of GmCLV1A
Hetero-grafting the S562L mutant and parent Forrest plants, or the Gmnark W677* supernodulating mutant, showed that S562L has no root- or shoot-effect on nodule number, lateral root number, nodule size, and nodulation index (nodulated portion of the root; Fig. 7). In contrast, W677* strongly controls the nodule number (Fig. 7A) and nodulation index through the shoot, consistent with previous reports for GmNARK mutants35. Moreover, W677* had a pronounced root-controlled effect on lateral root formation, with an additive effect when shoots were W677*, which is also consistent with other GmNARK mutants54 (Fig. 7B). All combinations having W677* in either the root or shoot exhibited smaller nodules; however, this effect was pleiotropically stronger when W677* was in the shoot (Fig. 7C).
Phenotypes of reciprocally grafted (scion/rootstock) plants between wild-type soybean cv. Forrest and its mutants Gmclv1a (S562L) Gmnark (W677*). Plants were grafted 12 days after sowing. Data were collected 45 days later. (A) Nodule number per plant; (B) lateral root number per plant (in the 5–15 cm region below the crown); (C) average nodule weight; and (D) nodulation index (i.e., % of root nodulated). Different letters above the bar represent statistically significant differences (Duncan test; P ≤ 0.05). Error bars indicate SE.
Gmclv1a induced phenotypes are temperature regulated
To determine whether GmCLV1A-controlled phenotypes were affected by temperature, wild type, S256L and W677* plants were grown at 28/25 °C or 20/17 °C (sub-optimal temperature) over a day-length regime of 13–15 h. At 28/25 °C, S562L and W677* mutant plants exhibited a similar height (Fig. 8A), but developed significantly fewer nodes (Fig. 8B), and had a significantly reduced shoot and root biomasses, compared with wild type plants (Fig. 8G and H).
Temperature influence on phenotypes of wild-type soybean cv. Forrest, and its mutants Gmclv1a (S562L), Gmnark (W677*) and the Gmclv1a Gmnark double mutant (DM). Plants were grown at 28/25 °C or 20/17 °C. (A) Plant height. (B) Node number. (C) Leaf number at node 3. (D) Percentage of plants having at least one vein-bladed leaf; Vein-bladed phenotype were scored 4 weeks after flowering. (E) Pod number (including both developing and mature pods). (F) Nodule number per plant. (G) Shoot dry weight. (H) Root dry weight. Plant height, node number and leaf number at node 3 were measured 4 weeks after sowing; n = 9–13. Nodule number, shoot and root dry weight were measured 3 weeks after sowing; n = 6. Error bars indicate SE. Nd = ‘not detected’. Different letters above the bar represent statistically significant differences (Duncan test; P ≤ 0.05).
At 20/17 °C, S562L plants were significantly taller than wild type and W677* plants (Fig. 8A). However, they produced a similar number of nodes compared with the wild type (Fig. 8B) and had a similar shoot and root biomass (Fig. 8G and H). Sub-optimal temperature delayed the flowering onset similarly in all genotypes, but caused S562L mutant plants to form significantly fewer pods (Fig. 8E).
Regardless of temperature, S562L plants formed significantly more leaves at node 3 than both wild type and W677* plants (Fig. 8C). S562L was also the only genotype tested to exhibit the novel vein-bladed phenotype (compare to Fig. 5C), with 70 and 80% of S562L mutant plants having at least one leaf displaying the phenotype at 28/25 °C and 20/17 °C, respectively (Fig. 8D). Mutant S562L plants also displayed more branching at the basal node compared with both wild type and W677* plants at both temperatures investigated (data not shown). S562L plants exhibited a normal nodulation-response to nitrogen, with no nodules detected on the well-fertilized plants at either temperature tested (Fig. 8F), whereas W677* plants exhibited the classical nitrate-tolerant supernodulation phenotype of Gmnark mutant plants29, 55. Besides temperature, day length (16 h/8 h vs. 10 h/14 h day/night) also affected the intensity of the phenotype, with short day length significantly increasing the frequency and severity of the phenotype (data not shown).
A double mutant of Gmclv1a (S562L) and Gmnark (W677*)
To determine whether GmCLV1A and GmNARK function in the same pathway, a cross between the Gmclv1a and Gmnark mutants (W677* and S562L) was conducted and a verified double mutant was isolated.
In the F2 generation, 102 plants from a single F1 parent showed the predicted Mendelian ratio for two unlinked genes (9:3:3:1). The F1 plant was genotyped and both mutant SNPs were detected. Twenty-three F2 plants showed a supernodulating phenotype and were homozygous (by SNP determination and phenotyping) for Gmnark and 79 plants exhibited normal nodulation (χ2 = 0.33, p > 0.05). Six of the 23 supernodulating plants were homozygous for the Gmclv1a mutation (χ2 = 0.014, p > 0.05).
Gmclv1a Gmnark double mutant plants maintained the supernodulation phenotype characteristic of their Gmnark single-mutant parent (Fig. 8F). They developed fewer nodes, with a similar growth rate to wild type and the Gmnark mutant, but slower than the Gmclv1a mutant, at 20/17 °C (Fig. 8A and B). Double mutant plants produced similar leaf numbers as wild type on node 3 at both temperatures, but they had fewer leaves compared to the Gmclv1a mutant and more than the Gmnark mutant at 28/25 °C (Fig. 8C). All mutants investigated produced significantly fewer pods compared to wild type at 20/17 °C, whereas only the double mutant produced significantly fewer pods at 28/25 °C (Fig. 8E). The vein-bladed phenotype was only observed in double mutants grown at 20/17 °C, with more than 20% of the double mutant plants having this abnormal phenotype (Fig. 8D).
Shoot biomass at 28/25 °C of Gmclv1a Gmnark mutants was significantly lower compared to wild type and all single mutants, while it was not significantly different at 20/17 °C. Double mutant root biomass was similar to all single mutants and significantly lower than wild type, whereas it did not change among the lines at 20/17 °C (Fig. 8G and H). At 28/25 °C, the Gmclv1a Gmnark mutant did not produce any branches at any nodes. However, at 20/17 °C, they had some branches at the cotyledonary node, which was similar to the Gmnark mutant, but significantly lower than wild type and the Gmclv1a single mutant (data not shown).
Discussion
The soybean genome is duplicated and segmented3, resulting in the loss, inactivation and rearrangement of homeologous gene pairs. In the case of the GmCLV1A and GmNARK homeologues, neofunctionalization appears to have taken place, as both genes segregate as Mendelian recessives, but the presence of one is not sufficient to compensate for mutation in the other27. Whereas GmNARK has a distinct regulatory role in AON, and associated inhibition of nodule formation by nitrate, GmCLV1A functions in shoot development with no apparent role in AON.
A GmCLV1A mutant, induced by EMS and screened by TILLING, showed an environmentally susceptible phenotype characterized by stem fasciation, increased stem branching, pod abnormalities, abnormal leaf development and vein-leaf development. Furthermore, expression of Glyma06g01940 (WUSCHEL related homeobox gene) in the shoot tip of Gmclv1a mutant was increased53, which is reminiscent of the finding in Arabidopsis where the WUS expression domain expanded in the clv SAM10. This indicates that GmCLV1A of soybean is acting through a similar component as the CLV network in Arabidopsis.
Despite the large size of the GmCLV1A gene, and the fact that numerous mutants have been isolated in its paralogous partner, GmNARK, no other mutants of GmCLV1A are known. This may indicate that severe mutations in GmCLV1A are lethal and that the S562L mis-sense mutation identified here, though clearly disruptive to CLV1A function, is somewhat leaky, enabling plant growth and development. Consistent with this hypothesis is the location of the mutation, which is in a putative glycosylation site situated towards the end of the LRR domain, and not in the central-cleft that represents the predicted binding site for its ligand partner36.
The phenotype of the Gmclv1a mutant (S562L) is influenced by temperature and day length; these environmental conditions would represent stress for the soybean cv. Forrest. It is possible that the degree of plasticity is also influenced by the truncated version of GmCLV1A (GmTrCLV1A) or even GmNARK.
The truncated-GmCLV1A encoding gene (GmTrCLV1A) was expressed throughout the plant and is similar to that reported upstream of MtSUNN in M. truncatula 30 and PvTrNARK 32. Interestingly, a comparable truncated gene was not found upstream of GmNARK, LjHAR1 or AtCLV1. In Arabidopsis, AtCLV1 dimerises with other proteins to perceive the AtCLV3 peptide ligand. Similarly, a model has been proposed for L. japonicus where LjHAR1 might form a heterodimer with LjCLV2 or KLV to perceive a CLE peptide ligand to control nodulation42, 43. It is possible that GmTrCLV1 also forms complexes with other receptor proteins in signal transduction mechanisms.
Gmclv1a phenotypes were fully complemented in the F1 generation of a cross between S562L × Forrest. In the F2 generation, Gmclv1a homozygous mutants produced significantly more branches at juvenile nodes (Node 1 to 3) compared with both the wild-type parent and wild-type segregants. Collectively, these findings demonstrate that the Gmclv1a mutation is the most likely cause of the developmental abnormalities. That some phenotypes of the S562L mutant were stronger in cold conditions is consistent with the function of CLV1 in Arabidopsis, where the clv1–4 mutant exhibits a stronger phenotype when grown at cold temperatures (16 °C)24. Furthermore, phenotypes of S562L mutants were intensified under short-day, which is consistent with Atclv2 mutants, particularly in regards to shoot fasciation26.
The S562L mutation did not affect nodulation, indicating that GmCLV1A does not function in nodulation control like GmNARK 35, 39. Some GmNARK mutations, including W677* reported here, also affect lateral root formation, reminiscent of the severe root effects seen in Ljhar1 31, 56 and Mtsunn mutants30. Interestingly, both GmCLV1A and GmNARK function in the control of cell division in nascent meristems, which is reminiscent to the function of Arabidopsis CLV1 in shoot ontogeny14, 57. AtCLV1 is reported to be expressed in cells across the centre of the shoot meristem, in addition to floral meristems14 and roots12, 58, and has a function in regulating stem cell population of the root apical meristem12, further demonstrating that these receptors often function in more than one process.
The Gmclv1a Gmnark double mutant displayed the classical Gmnark supernodulation phenotype. Double mutant plants were small, with fewer nodes and a reduced pod number when grown at an optimal temperature (28/25 °C) for soybean cv. Forrest. They also had a higher number of leaves on node 3 than Forrest and W677*, but less than S562L. This demonstrated that GmNARK has a distinct function in the regulation of nodule number, which is not complemented by GmCLV1A, consistent with previous reports27. Moreover, GmCLV1A has a function in regulating nodal identity that is distinct from GmNARK. However, GmCLV1A does share a function with GmNARK in plant growth, as all double mutant plants were significantly reduced in stature compared with the wild type and single mutant parents. This suggests that GmNARK influences plant architecture, previously undetected in single mutants due to the presence of a functional GmCLV1A. This is reminiscent of Ljhar1 mutants of Lotus japonicus, which lack a GmCLV1A orthologue, and not only hypernodulate but are also drastically reduced in size31, 59.
Taken together, we demonstrated that the homeologous soybean genes, GmCLV1A and GmNARK, have neodiversified and are involved in two distinct developmental pathways, yet might also act together to maintain plant growth. One controls shoot structure locally, in an environmentally-influenced fashion, while the other acts both locally and systemically to regulate nodulation and lateral root numbers. Based on the phenotypes of the S562L mutant, and by analogy to CLV1 of Arabidopsis, we propose that GmCLV1A functions in the control of shoot meristem activity. GmCLV1A appears to have maintained more of the ancestral function of CLV1, whereas GmNARK has evolved, possibly when nodulation first emerged in legumes roughly 60 million years ago.
Methods
Mutant isolation and protein sequence analysis
A soybean mutant population was generated by chemical mutagenesis (50 mM Ethyl methane-sulfonate for 16 h) of wild type cv. Forrest and screened for mutations in GmCLV1A and GmNARK through TILLING60. Genomic DNA of candidate GmCLV1A and GmNARK mutant plants was isolated from leaf tissue using the QIAGEN DNeasy plant mini-kit according to manufacturer’s instructions. (QIAGEN, Hilden, Germany). A central segment of 1,594 bp of GmCLV1A and 1,904 bp of GmNARK were amplified with specific primers: GmCLV1A primers; 5′-AATAACTACCTTAACGGCGCA-3′ and 5′-TCCACCACTGCCAACACTACT-3′, GmNARK primers; 5′-TGAGATTTCCGGCGAATCCCTG-3′ and 5′-TCCACCACTGCCAACACCAAC-3′ using the expand high fidelity PCR system (Roche Applied System, Germany). PCR products were purified using the QIAquick PCR purification kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol. The sequences were then confirmed by sequencing. A mis-sense mutation was identified in GmCLV1A at amino acid position 562 and a non-sense mutation was identified in GmNARK at amino acid 677.
Protein domains were identified through the SMART website (http://smart.embl.de;61, 62. Molecular modelling of external domain of GmCLV1A was conducted by Phyre version 0.263 available at www.sbg.bio.ic.ac.uk/phyre/ and visualised by PyMOL64. It also was analysed with the motif scan tool available at http://myhits.isb-sib.ch (e.g. for glycosylation site prediction). Phylogenetic trees were constructed using Geneious 5.665, with distances between proteins calculated by neighbor joining with the Geneious tree builder program.
Phenotypic studies
Seeds of the wild-type cv. Forrest, and mutants S562L and W677*, were sown in 4 L, 250 mm pots filled with a grade 2 vermiculite:sand mixture (2:1) and maintained in a glasshouse under natural illumination, approximately 11/13 h standard daylight, at 28/25 °C. All plants were inoculated with Bradyrhizobium japonicum strain CB1809 at sowing. After germination, the plants were given 300 ml of modified Herridge’s nutrient solution every two days66: KNO3 2 mM; KH2PO4 0.13 mM; K2HPO4 0.13 mM; MgSO4.7H2O 0.5 mM; KCl 0.25 mM; CaCl2.2H2O 0.25 mM; Fe-EDTA 23.5 μM; H3BO3 11.5 μM; MnCl2.4H2O 2.3 μM; ZnCl2 0.2 μM; CuCl2.2H2O 0.08 μM; Na2MoO4.2H2O 0.025 μM. All plants were grown for 15 weeks, and then scored for leaf and branch number per node, and internode length.
Grafting studies
For grafting studies, seeds were sterilized by soaking in 70% ethanol for 1 min then rinsed five times with sterile water. They were sown in sterilized pots containing vermiculite (grade 2) and kept in a glasshouse as previously described. After emergence they received a modified Herridge’s nutrient solution, with the KNO3 concentration reduced to 0.5 mM. Grafting was carried out 12 d after sowing using a wedge-shaped graft. The plants were then covered with clear plastic bags as described in Delves, et al.67, Lin, et al.68. Five days after grafting, the bags were removed and the grafted plants were inoculated with B. japonicum strain CB1809. Four weeks after inoculation, nodule number, nodulation index (nodulated portion of root), nodule dry weight and lateral root number were determined.
F2 segregation of S562L × Forrest
Seeds of Forrest, S562L and the F2 of a Forrest × S562L cross were sown in 200 mm pots filled with potting mix supplemented with Osmocote (Scotts, Baulkam Hill, Australia). The plants were inoculated with B. japonicum strain CB1809 at the time of planting. They were kept in the glasshouse under 28/25 °C standard Brisbane daylight in February, and watered daily. Four weeks after planting, they were scored for number of branches per node. To distinguish between the presence of a dormant bud and an actively-growing branch only buds longer than 0.5 cm were counted as a ‘growing branch’.
Temperature studies
For temperature studies, seeds were sown in 200 mm pots filled with potting mix supplemented with Osmocote (Scotts, Baulkam Hill, Australia). The plants were inoculated with B. japonicum strain CB1809 at the time of planting. They were kept in the glasshouse under 28/25 °C (normal temperature) or 20/17 °C (sub-optimal temperature) for 13–15 h day length and watered daily. Three weeks after planting six plants of each line were harvested to score their nodule number, shoot and root dry weight. The remaining plants were grown for one month following which they were scored for number of leaf and branches per node, total plant length, branch length per node and number of nodes. Only branches longer than 0.5 cm were counted as a ‘growing branch’. Four weeks after flowering, all plants were screened for leaf-like structures on the underside of their leaves. The number of pods per plant was scored at full maturity, with both mature and developing pods counted.
Pod sectioning and microscopy
Pods were fixed in 0.5% (w/v) paraformaldehyde in 100 mM sodium phosphate buffer (pH 7) for 45 min on ice and under vacuum. They were then washed three times in sodium phosphate buffer (pH 7) at room temperature. The fixed pods were embedded in 3% (w/v) agarose and sectioned to 40 µm using a Leica VT1200S vibrating microtome (Leica Microsystems, Germany). Pod sections were stained for 30 to 60 s at room temperature in 0.05% Toluidine Blue (pH 4.5) and then rinsed two times with distilled H2O. Sections were viewed on a Nikon Eclipse E600 compound microscope (Nikon Instruments Inc., Melville, USA).
Stem sectioning and microscopy
Stem samples from 4 month-old plants were placed in fixative solution (formaldehyde, glacial acetic acid and 95% ethanol, 2:1:10 v/v) under vacuum, infiltrated on ice for 10 min to enhance penetration of fixative, and then kept at 4 °C for 24 h. The samples were then dehydrated for 2 h at 4 °C using 70 and 95% ethanol and at room temperature using 100% ethanol69. After dehydration, the samples were placed in chloroform for 5 min and then were imbedded in paraffin wax. To soften the samples, the paraffin blocks were trimmed to exposure one side of the tissue and were placed in softening solution (1% sodium lauryl sulphate and 10% glycerol) and kept for two to three days at room temperature70. The samples were sectioned by hand with a razor blade. Sections were stained for 30 to 60 s at room temperature in 0.05% Toluidine Blue (pH 4.5) and then rinsed two times with distilled H2O. Sections were viewed on a Nikon SMZ800 compound binocular microscope (Nikon Instruments Inc., USA).
RNA extraction and cDNA synthesis
Ten different tissues including root, shoot and leaf were collected from un-inoculated 14 day-old plants. RNA extraction was performed using the TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. DNA contamination was removed using DNaseI (Fermentase, Burlington, Canada). Approximately 1 μg of RNA was subjected to 1 unit of DNaseI at 37 °C for 40 min. The reactions were inactivated by adding 1 μl of 25 mM EDTA (Invitrogen) and incubating at 65 °C for 10 min. RNA was converted to cDNA in a 20 μl reaction mixture containing 0.5 mM deoxynucleoside triphosphates (dNTPs), 1 μl of 50 μm oligo(dT) primers, 40 units of RNaseOUT (Invitrogen), 0.5 μg of DNA-free RNA, 1x first-strand buffer (Invitrogen), 5 mM dithiothreitol (DTT) and 100 units of SuperScript III reverse transcriptase (Invitrogen) at 50 °C for 60 min. Finally, cDNA was confirmed using GmATP synthase (Glyma20g25920) primers with PCR.
Quantitative real time PCR
Primers used for quantitative real-time PCR (qRT-PCR) were designed using the online primer design program Primer 3 0.4.0 (available at http://frodo.wi.mit.edu). Sequences from the soybean genome (Phytozome version 4.0; available at http://www.phytozome.net) were used to design the primers. The sequences for forward and reverse primer for each gene were 5′-TTTGGCGTGGTGCTGTTG-3′ and 5′-CCAACACTACTGCTGCATCCG-3′ for GmCLV1A and 5′-ACAGGCAAGGTCCCCAAC-3′ and 5′-GCATCCGTGAATGGAACAGAG-3′ for GmTrCLV1A. To distinguish between them, qRT-PCR primers for GmCLV1A were designed on the second exon of GmCLV1A, which is absent in GmTrCLV1A, while qRT-PCR-specific primers for GmTrCLV1A were designed from the first exon. To ensure that the primers were specific and produced only a single band, normal PCR was run using Forrest cDNA. All primer pairs were found to amplify a single product of the correct size. Sequencing of the PCR products confirmed that primers are specific to the genes.
Relative transcript abundance was detected using SYBR Green PCR Master Mix (Applied Biosystems) on an ABI 7900HT cycler (Applied Biosystems) in 384-well plate. The 384-well plates were set up using an Eppendorf epMotion 5075 Robotic system and contained no template (water) control and reverse transcription negative (RT-) controls to verify genomic DNA contamination of the samples. All reactions were carried out in duplicate for three biological replicates. The qRT-PCR conditions used were: initial denaturation of 95 °C for 10 min, then 40 cycles of 95 °C for 15 sec and 60 °C for 1 min followed by a dissociation stage of 95 °C for 2 min to assess the specificity of the PCR. Gene expression levels were normalised to that of GmATP synthase, which was amplified using forward primer 5′-GCGATTCTTAAGCCAGCCTTT-3′ and reverse primer 5′-ACACACCCTGGAAACTGGTGA-3′. PCR efficiency for each sample was calculated using LinRegPCR 7.571.
References
Ferguson, B. J. et al. Molecular analysis of legume nodule development and autoregulation. Journal of Integrative Plant Biology 52, 61–76 (2010).
Gresshoff, P. M. et al. The value of biodiversity in legume symbiotic nitrogen fixation and nodulation for biofuel and food production. Journal of Plant Physiology 172, 128–136, doi:10.1016/j.jplph.2014.05.013 (2015).
Schmutz, J. et al. Genome sequence of the paleopolyploid soybean. Nature 463, 178–183 (2010).
Fletcher, J. C. Shoot and floral meristem maintenance in Arabidopsis. Annual Review of Plant Biology 53, 45–66, doi:10.1146/annurev.arplant.53.092701.143332 (2002).
Clark, S. E. Cell signalling at the shoot meristem. Nature Reviews Molecular Cell Biology 2, 276–284 (2001).
DeYoung, B. J. & Clark, S. E. BAM receptors regulate stem cell specification and organ development through complex interactions with CLAVATA signaling. Genetics 180, 895–904, doi:10.1534/genetics.108.091108 (2008).
Uchida, N., Shimada, M. & Tasaka, M. ERECTA-family receptor kinases regulate stem-cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant and Cell Physiology, doi:10.1093/pcp/pcs109 (2012).
Guo, Y. & Clark, S. E. Membrane distributions of two ligand-binding receptor complexes in the CLAVATA pathway. Plant signaling & behavior 5, 1442–1445 (2010).
Kinoshita, A. et al. RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 137, 3911–3920, doi:10.1242/dev.048199 (2010).
Schoof, H. et al. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644, doi:10.1016/s0092-8674(00)80700-x (2000).
Laux, T., Mayer, K. F. X., Berger, J. & Jurgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122, 87–96 (1996).
Stahl, Y. et al. Moderation of Arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Current Biology 23, 362–371, doi:10.1016/j.cub.2013.01.045 (2013).
Durbak, A. R. & Tax, F. E. CLAVATA signaling pathway receptors of Arabidopsis regulate cell proliferation in fruit organ formation as well as in meristems. Genetics 189, 177–U846, doi:10.1534/genetics.111.130930 (2011).
Clark, S. E., Williams, R. W. & Meyerowitz, E. M. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575–585 (1997).
Jeong, S., Trotochaud, A. E. & Clark, S. E. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11, 1925–1933 (1999).
Muller, R., Bleckmann, A. & Simon, R. The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20, 934–946, doi:10.1105/tpc.107.057547 (2008).
Fletcher, L. C., Brand, U., Running, M. P., Simon, R. & Meyerowitz, E. M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283, 1911–1914 (1999).
Hastwell, A. H., Gresshoff, P. M. & Ferguson, B. J. Genome-wide annotation and characterization of CLAVATA/ESR (CLE) peptide hormones of soybean (Glycine max) and common bean (Phaseolus vulgaris), and their orthologues of Arabidopsis thaliana. Journal of Experimental Botany, doi:10.1093/jxb/erv351 (2015).
Brand, U., Fletcher, J. C., Hobe, M., Meyerowitz, E. M. & Simon, R. Dependence of stem cell fate in Arabidopsis an a feedback loop regulated by CLV3 activity. Science 289, 617–619 (2000).
Chen, S. K. et al. The association of homeobox gene expression with stem cell formation and morphogenesis in cultured Medicago truncatula. Planta 230, 827–840, doi:10.1007/s00425-009-0988-1 (2009).
Sarkar, A. K. et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811–814 (2007).
Leibfried, A. et al. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438, 1172–1175, doi:10.1038/nature04270 (2005).
Beveridge, C. A., Mathesius, U., Rose, R. J. & Gresshoff, P. M. Common regulatory themes in meristem development and whole-plant homeostasis. Current Opinion in Plant Biology 10, 44–51, doi:10.1016/j.pbi.2006.11.011 (2007).
Clark, S. E., Running, M. P. & Meyerowitz, E. M. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119, 397–418 (1993).
Clark, S. E., Running, M. P. & Meyerowitz, E. M. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121, 2057–2067 (1995).
Kayes, J. M. & Clark, S. E. CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125, 3843–3851 (1998).
Searle, I. R. et al. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299, 109–112, doi:10.1126/science.1077937 (2003).
Yamamoto, E., Karakaya, H. C. & Knap, H. T. Molecular characterization of two soybean homologs of Arabidopsis thaliana CLAVATA1 from the wild type and fasciation mutant. Biochimica Et Biophysica Acta-Gene Structure and Expression 1491, 333–340 (2000).
Carroll, B. J., McNeil, D. L. & Gresshoff, P. M. Isolation and properties of soybean [Glycine max (L) Merr] mutants that nodulate in the presense of high nitrate concentrations. Proceedings of the National Academy of Sciences of the United States of America 82, 4162–4166 (1985).
Schnabel, E., Journet, E. P., de Carvalho-Niebel, F., Duc, G. & Frugoli, J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol 58, 809–822, doi:10.1007/s11103-005-8102-y (2005).
Wopereis, J. et al. Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant Journal 23, 97–114, doi:10.1046/j.1365-313x.2000.00799.x (2000).
Ferguson, B. J. et al. The soybean (Glycine max) nodulation-suppressive CLE peptide, GmRIC1, functions interspecifically in common white bean (Phaseolus vulgaris), but not in a supernodulating line mutated in the receptor PvNARK. Plant Biotechnology Journal 12, 1085–1097, doi:10.1111/pbi.12216 (2014).
Krusell, L. et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420, 422–426, doi:10.1038/nature01207 (2002).
Nishimura, R. et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature 420, 426–429 (2002).
Delves, A. C. et al. Regulation of the Soybean-rhizobium nodule symbiosis by shoot and root factors. Plant Physiology 82, 588–590 (1986).
Reid, D. E., Ferguson, B. J. & Gresshoff, P. M. Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Molecular Plant-Microbe Interactions 24, 606–618, doi:10.1094/MPMI-09-10-0207 (2011).
Mortier, V. et al. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiology 153, 222–237, doi:10.1104/pp.110.153718 (2010).
Okamoto, S. et al. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant and Cell Physiology 50, 67–77, doi:10.1093/pcp/pcn194 (2009).
Reid, D. E., Ferguson, B. J., Hayashi, S., Lin, Y.-H. & Gresshoff, P. M. Molecular mechanisms controlling legume autoregulation of nodulation. Annals of Botany 108, 789–795, doi:10.1093/aob/mcr205 (2011).
Magori, S. et al. TOO MUCH LOVE, a Root Regulator Associated with the Long-Distance Control of Nodulation in Lotus japonicus. Molecular Plant-Microbe Interactions 22, 259–268, doi:10.1094/mpmi-22-3-0259 (2009).
Crook, A. D., Schnabel, E. L. & Frugoli, J. A. The systemic nodule number regulation kinase SUNN in Medicago truncatula interacts with MtCLV2 and MtCRN. The Plant Journal 88, 108–119, doi:10.1111/tpj.13234 (2016).
Krusell, L. et al. The Clavata2 genes of pea and Lotus japonicus affect autoregulation of nodulation. The Plant Journal 65, 861–871, doi:10.1111/j.1365-313X.2010.04474.x (2011).
Miyazawa, H. et al. The receptor-like kinase KLAVIER mediates systemic regulation of nodulation and non-symbiotic shoot development in Lotus japonicus. Development 137, 4317–4325, doi:10.1242/dev.058891 (2010).
Lin, Y.-H., Ferguson, B. J., Kereszt, A. & Gresshoff, P. M. Suppression of hypernodulation in soybean by a leaf-extracted, NARK- and Nod factor-dependent, low molecular mass fraction. New Phytologist 185, 1074–1086, doi:10.1111/j.1469-8137.2009.03163.x (2010).
Lin, Y.-H., Lin, M.-H., Gresshoff, P. M. & Ferguson, B. J. An efficient petiole-feeding bioassay for introducing aqueous solutions into dicotyledonous plants. Nature Protocols 6, 36–45 (2011).
Wang, Y. et al. Soybean miR172c Targets the Repressive AP2 Transcription Factor NNC1 to Activate ENOD40 Expression and Regulate Nodule Initiation. The Plant Cell Online. doi:10.1105/tpc.114.131607 (2014).
Meksem, K. et al. In The handbook of plant functional genomics 251–265 (Wiley-VCH Verlag GmbH & Co. KGaA, 2008).
Day, D. A., Lambers, H., Bateman, J., Carroll, B. J. & Gresshoff, P. M. Growth comparisons of a supernodulating soybean (Glycine max) mutant and its wild-type parent. Physiologia Plantarum 68, 375–382, doi:10.1111/j.1399-3054.1986.tb03368.x (1986).
Ubersax, J. A. & Ferrell, J. E. Jr. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 8, 530–541 (2007).
Nontachalyapoom, S. et al. Promoters of orthologous Glycine max and Lotus japonicus nodulation autoregulation genes interchangeably drive phloem-specific expression in transgenic plants. Molecular Plant-Microbe Interactions 20, 769–780, doi:10.1094/mpmi-20-7-0769 (2007).
Couzigou, J.-M. et al. NODULE ROOT and COCHLEATA maintain nodule development and are legume orthologs of Arabidopsis BLADE-ON-PETIOLE genes. The Plant Cell Online, doi:10.1105/tpc.112.103747 (2012).
Ferguson, B. J. & Reid, J. B. Cochleata: Getting to the root of legume nodules. Plant and Cell Physiology 46, 1583–1589, doi:10.1093/pcp/pci171 (2005).
Mirzaei, S., Jacqueline, B., Brett, J. F. & Peter, M. G. Transcriptome Proling of the Shoot and Root Tips of S562L, a Soybean GmCLAVATA1A Mutant. Atlas Journal of Biology 3, 183–205 (2014).
Mathews, A., Carroll, B. J. & Gresshoff, P. M. Characterization of non-nodulation mutants of soybean [Glycine max (L.) Merr]: Bradyrhizobium effects and absence of root hair curling. Journal of Plant Physiology 131, 349–361, doi:10.1016/S0176-1617(87)80174-8 (1987).
Carroll, B. J., McNeil, D. L. & Gresshoff, P. M. A supernodulation and nitrate-tolerant symbiotic (NTS) soybean mutant. Plant Physiology 78, 34–40 (1985).
Buzas, D. M. & Gresshoff, P. M. Short- and long-distance control of root development by LjHAR1 during the juvenile stage of Lotus japonicus. Journal of Plant Physiology 164, 452–459, doi:10.1016/j.jplph.2006.03.006 (2007).
De Smet, I., Vosz, U., Jurgens, G. & Beeckman, T. Receptor-like kinases shape the plant. Nat Cell Biol 11, 1166–1173 (2009).
Replogle, A. et al. Synergistic interaction of CLAVATA1, CLAVATA2, and RECEPTOR-LIKE PROTEIN KINASE 2 in cyst nematode parasitism of Arabidopsis. Molecular Plant-Microbe Interactions 26, 87–96, doi:10.1094/MPMI-05-12-0118-FI (2012).
Szczyglowski, K. et al. Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Molecular Plant-Microbe Interactions 11, 684–697, doi:10.1094/mpmi.1998.11.7.684 (1998).
Cooper, J. L. et al. TILLING to detect induced mutations in soybean. BMC Plant Biology 8, (24 January 2008), doi:10.1186/1471-2229-8-9 (2008).
Letunic, I., Doerks, T. & Bork, P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Research 40, D302–D305, doi:10.1093/nar/gkr931 (2012).
Schultz, J., Milpetz, F., Bork, P. & Ponting, C. P. SMART, a simple modular architecture research tool: Identification of signaling domains. Proceedings of the National Academy of Sciences of the United States of America 95, 5857–5864 (1998).
Kelley, L. A. & Sternberg, M. J. E. Protein structure prediction on the Web: a case study using the Phyre server. Nature Protocols 4, 363–371, doi:10.1038/nprot.2009.2 (2009).
Delano, W. The PyMOL molecular graphics system. DeLano, Scientific, San Carlos, CA, USA (2002).
Drummond, A. J. et al. Geneious v5.6, Available from http://www.geneious.com. (2012).
Herridge, D. F. Relative abundance of ureides and nitrate in plant-tissue of soybean as a quantitative assay of nitrogen-fixation. Plant Physiology 70, 1–6, doi:10.1104/pp.70.1.1 (1982).
Delves, A. C., Higgins, A. V. & Gresshoff, P. M. Shoot control of supernodulation in a number of mutant soybean, GLYCINE-MAX (L) MERR. Australian Journal of Plant Physiology 14, 689–694 (1987).
Lin, M.-H., Gresshoff, P. M. & Ferguson, B. J. Systemic regulation of soybean nodulation by acidic growth conditions. Plant Physiology 160, 2028–2039, doi:10.1104/pp.112.204149 (2012).
LaMotte, C. E., Curry, TrsM., Palmer, R. G. & Albertsen, M. C. Developmental anatomy and morphology of fasciation in the soybean (Glycine max). Botanical Gazette 149, 398–407 (1988).
Alcorn, S. M. & Ark, P. A. Softening paraffin-embedded plant tissues. Stain Technology 28, 55–56 (1953).
Ramakers, C., Ruijter, J. M., Deprez, R. H. L. & Moorman, A. F. M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters 339, 62–66, doi:10.1016/s0304-3940(02)01423-4 (2003).
Acknowledgements
We thank the Australian Research Council and UQ Central Administration for support through the Centre of Excellence scheme (CEO348212). We would like to thank Dr Andrew James (CSIRO, Brisbane) for his advice on crossing, N. Chen (CILR) for her help in confirming the mutant sequences, and D. Li and A. Tolleneare (both CILR) for technical assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and editing of the manuscript. S.M. contributed to the conception and interpretation and conducted experiments and evaluated results. J.B., B.J.F. and P.M.G. contributed to the conception, interpretation and supervision of the research. K.M., T.E.M. and S.L. were responsible for the original isolation of the S562L mutant by TILLING. J.B. confirmed the mutation in S562L.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mirzaei, S., Batley, J., El-Mellouki, T. et al. Neodiversification of homeologous CLAVATA1-like receptor kinase genes in soybean leads to distinct developmental outcomes. Sci Rep 7, 8878 (2017). https://doi.org/10.1038/s41598-017-08252-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08252-y
This article is cited by
-
Genome-wide identification and characterization of legume T2 Ribonuclease gene family and analysis of GmaRNS9, a soybean T2 Ribonuclease gene, function in nodulation
3 Biotech (2021)
-
Genome reorganization of the GmSHMT gene family in soybean showed a lack of functional redundancy in resistance to soybean cyst nematode
Scientific Reports (2019)
-
CLAVATA1-type receptor-like kinase CsCLAVATA1 is a putative candidate gene for dwarf mutation in cucumber
Molecular Genetics and Genomics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.