Abstract
Pancreatic islet transplantation is a promising therapy for patients with type 1 diabetes. However, the duration of long-term graft survival is limited due to inflammatory as well as non-inflammatory processes and routine clinical tests are not suitable to monitor islet survival. 111In-exendin-SPECT (single photon emission computed tomography) is a promising method to non-invasively image islets after transplantation and has the potential to help improve the clinical outcome. Whether 111In-exendin-SPECT allows detecting small differences in beta-cell mass (BCM) and measuring the actual volume of islets that were successfully engrafted has yet to be demonstrated. Here, we evaluated the performance of 111In-exendin-SPECT using an intramuscular islet transplantation model in C3H mice. In vivo imaging of animals transplanted with 50, 100, 200, 400 and 800 islets revealed an excellent linear correlation between SPECT quantification of 111In-exendin uptake and insulin-positive area of islet transplants, demonstrating that 111In-exendin-SPECT specifically and accurately measures BCM. The high sensitivity of the method allowed measuring small differences in graft volumes, including grafts that contained less than 50 islets. The presented method is reliable, convenient and holds great potential for non-invasive monitoring of BCM after islet transplantation in humans.
Similar content being viewed by others
Introduction
Transplantation of islets of Langerhans is a promising treatment for patients with type 1 diabetes (T1D). The short-term results of islet transplantation in normalizing blood glucose levels are encouraging1. However, the rate of insulin-independency drops to less than 15% after 5 years2. Although the loss of transplanted beta-cells quickly renders patients insulin-dependent in a life-time perspective, the remaining graft function still exerts a positive effect on glucose homeostasis, reducing late complications and further progression of micro-vascular diseases3. Furthermore, the islet grafts lower the amount of insulin required to maintain normoglycemia preventing potentially lethal, severe hypoglycemia. However, in view of the considerable side effects caused by the immunosuppressive therapy required to prevent graft rejection, improved survival of the transplanted islet is desirable. In order to optimize islet replacement therapy and to prevent loss of graft function, numerous approaches are currently under investigation, including modified immunosuppressive treatments4, 5 and islet encapsulation strategies6, 7 treatment with growth factors and other hormones, as well as alternative sources for beta-cells (i.e. stem cells, tissue bioengineering)8,9,10,11. In order to monitor the graft volume and optimize new strategies for beta-cells replacement a non-invasive technology to visualize viable transplanted islets in vivo is warranted. Such a method should be quantitative and sensitive in order to allow the detection of small changes in the number of surviving islets.
A promising approach to visualize transplanted islets in vivo has been demonstrated by Saudek et al., using Magnetic Resonance Imaging (MRI). The group described MRI of islets that had been labeled with super-paramagnetic iron oxide particles (SPIOs) prior to transplantation12. The feasibility of longitudinal non-invasive monitoring of islet transplants was further demonstrated in animal models by Evgenov and co-workers, successfully monitoring islet transplants for up to 188 days after surgery13. Pre-labeling of the islets with SPIOs is therefore a promising method with clinical potential14.
Alternatively, radionuclide imaging modalities were used because of their high detection sensitivity of transplanted islets. First results of PET imaging of an islet graft were published in 2006, using islets which were transfected with an insulin promoter-dependent reporter gene that leads to trapping of the PET probe 18F-FHBG in islet grafts15. In another experiment, islets were pre-labeled with 18F-fluorodeoxyglucose (18F-FDG) and post-transplantation events could be monitored for up to 6 hours after transplantation16.
More recently, specific targeting of beta-cells after in vivo injection of radiolabeled exendin followed by SPECT imaging was reported as a promising strategy to non-invasively visualize and quantify BCM in the pancreas of rodents, as well as in healthy and diabetic individuals17, 18. Similar exendin-based radiotracers were applied for non-invasive imaging of islet grafts in rodent transplantation models as well as in human skeletal muscle19,20,21. Although the use of such tracers in a clinical setting of islet transplantation is highly warranted, establishing the correlation between true BCM and the uptake of the radiotracer in vivo is the essential validation step before such studies should be conducted in humans. Such decisive studies have not been performed for GLP-1R imaging of islet transplants.
In the present study, we measured the uptake of 111In-exendin-3 in transplants consisting of different amounts of islets in the calf muscles of C3H mice by non-invasive SPECT imaging in vivo and validated 111In-exendin as a quantitative biomarker for assessment of transplanted beta-cell volume.
Results
111In-exendin accumulation in the muscle co-localizes with islet transplants
To check whether 111In-exendin-3 signal originates from transplanted islets, C3H mice were transplanted with 800 islets in the calf muscle and were injected with 111In-exendin-3 four weeks after transplantation, where accumulation of the radiotracer in the islets becomes reproducible22. Autoradiographical analysis of muscle sections showed tracer accumulation in well localized regions of the tissue (Fig. 1A) and immunostaining for insulin confirmed that the radioactive signal originated from the islets (Fig. 1B).
111In-exendin-3 uptake in the skeletal muscle is co-localized with islet transplants. (A) Autoradiography of muscle sections shows local accumulation of 111In-exendin-3 (black arrows), the limits of the muscle section are shown by the dashed line. (B) Immunostaining of the corresponding autoradiography section shows co-localization between beta-cells (brown) and 111In-exendin-3.
111In-exendin uptake by transplanted islets correlates linearly with BCM
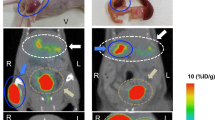
111In-exendin-3 uptake in grafts containing various amounts of islets was detected and clearly delineated by SPECT signal (Fig. 2A). Quantitative analysis of SPECT signal originating from the transplant revealed differences in 111In-exendin-3 accumulation depending on the number of initially transplanted islets, where the uptake was 5.9 kBq ± 2.4, 22.9 kBq ± 4.8, 30.1 kBq ± 10.1, 60.9 kBq ± 9.8 and 88.7 kBq ± 11.5, in muscles transplanted with 50, 100, 200, 400 and 800 islets, respectively (Fig. 2B). Immunohistochemical determination of graft volume was performed in all groups of mice (Fig. 2C). Plotting of SPECT data against the insulin staining volume revealed an excellent linear correlation between 111In-exendin uptake and transplant size (pearson r = 0.89).
Uptake of 111In-exendin by islet transplants is in linear correlation with transplant size. (A) SPECT imaging detected the grafts initially transplanted with 50, 100, 200, 400 or 800 islets with high sensitivity, 4 weeks after transplantation (white arrows). (B) 111In-exendin uptake (expressed in kilobecquerels, kBq) was dependent on transplant size (n = 4–5). Data are shown as means ± SEM (n = 5). (C) SPECT signal (expressed in kBq) correlated linearly with graft insulin-positive volume (µm3). (Pearson r = 0.89, 95% confidence interval shown as a dotted line).
Discussion
The aim of this study was to evaluate the relation between 111In-exendin uptake in islet transplants compared to actual BCM in a muscle model of islet transplantation. Tracer uptake and beta-cell volume showed an excellent linear correlation. In addition, reliable visualization of transplants consisting of low numbers of islets indicates towards high sensitivity of this method. This high sensitivity in combination with the excellent correlation of BCM and tracer uptake demonstrate the potential of this method to quantify small differences in viable beta-cell volume.
Previously, imaging of liver islet transplants using 64Cu-labelled exendin has been demonstrated in NOD/SCID mice19. Twelve days follow-up of the grafts revealed significantly higher uptake of 64Cu-labeled exendin-4 in the liver of transplanted animals when compared to the control group. Here, we used the skeletal muscle transplantation as a highly controlled model for histological verification of the insulin positive area (which is challenging in the liver transplantation model given that islets are distributed throughout a large part of the organ), a prerequisite enabling us to, for the first time, reveal an excellent linear correlation between the actual numbers of beta-cells that were successfully engrafted, and the uptake of the tracer in the islet grafts.
Recently, it was reported that injection of 123I-IBZM enables the quantification of BCM in islet grafts, where tracer uptake could be linearly correlated with graft volume23, 24. However, a far lower correlation was observed between 123I-IBZM uptake and insulin positive graft volumes when compared to 111In-exendin, indicating that GLP-1R could allow more accurate assessments of islet graft survival. Moreover, our data indicate that 111In-exendin has a much higher sensitivity for detection of islet grafts when compared to 123I-IBZM as we could easily visualize islet grafts after transplantation of 50 islets while 1000 islets were needed for in vivo visualization by IBZM. In fact, more than 50% of the islets could be lost in the first days after transplantation25, indicating that 111In-exendin-SPECT was able to detect grafts containing far less than the 50 islets being initially transplanted. The superior detection sensitivity of 111In-exendin-SPECT could be explained by the higher abundance of the GLP-1R on the surface of the beta-cells when compared to the dopamine 2 receptor21, 23. Hence, 111In-exendin-SPECT has the potential to detect small grafts even after post transplantation beta-cell loss. This enables the possibility to evaluate and optimize treatment to preserve the remaining islets in patients that became insulin dependent after islet transplantation, helping to preserve their positive effect on glucose homeostasis.
The skeletal muscle was selected as a transplantation site to evaluate whether 111In-exendin-3 can be used to monitor beta-cell volume after transplantation. So far, this site has been used for islet transplantation in an experimental setting in small animal models and in a few patients20. In the clinical setting, intra-portal islet transplantation still predominates26. The ability to detect islet transplants in the liver with exendin-3 has previously been demonstrated in a proof of concept study in mice19. Further studies are warranted to assess whether our finding that the 111exendin-3 uptake corresponds to the beta-cell volume after transplantation is also valid after intrahepatic islet transplantation.
In conclusion, we have demonstrated for the first time that 111In-exendin-3 can be used to determine beta-cell volume after intramuscular islet transplantation. 111In-exendin-3 uptake linearly correlates with the amount of living beta-cells, and allows the detection of islet grafts consisting of less than 50 islets by SPECT imaging. Our data indicates that this approach holds great potential for accurate and sensitive quantification of viable beta-cells in a transplantation setting. Clinical studies evaluating the potential of this promising radiotracer for imaging of islets grafts in humans are under preparation.
Research Design and Methods
Animals
Female C3H/HeNCrl mice (22–30 g) were purchased from Charles River (Calco, Itlay). All experiments were conducted in accordance with Radboud University guidelines on humane care and use of laboratory animals and were approved by the Animal Ethical Committee of the Radboud University, Nijmegen, The Netherlands.
Pancreatic islet isolation and transplantation
Pancreatic islets were isolated from 6–8 weeks old mice by a collagenase digestion method. Briefly, mice were euthanized and 2 ml of cold RPMI 1640 (Invitrogen, Carmarillo, CA, USA) containing collagenase type V (1 mg/ml; Sigma Aldrich, St Louis, MO, USA) were infused into the pancreatic duct in situ. Perfused pancreata were collected in serum-free RPMI medium and kept on ice until enzymatic digestion at 37 °C for 12 min. Islets were purified on a discontinuous Ficoll gradient of following densities: 1.118, 1.096 and 1.037 g/ml (Cellgro by Mediatech Inc., Manassas, VA, USA) and islets were collected between the second and the third fraction. Islets were cultured overnight in a humidified 5% CO2 atmosphere at 37 °C in RPMI 1640 medium supplemented with L-glutamine (Sigma Aldrich, St. Louis, MO, USA), penicillin-streptomycin (10 mg/ml; Sigma Aldrich) and 10% (v/v) fetal calf serum (HyClone, Celbio, Logan, UT, USA). Islets were counted and hand-picked under bright field microscope and 50, 100, 200, 400 or 800 islets were transplanted in the calf muscle (n = 5 per group), parallel to the fibula, using needles with a 0.8 mm diameter. The exact number of transplanted islets was determined by subtracting the remaining islets in the tube from the initially counted number.
Radiolabeling of exendin-3
[Lys 40(DTPA)]-exendin-3 was purchased from Peptide Specialty Laboratories (Heidelberg, Germany). Tracer labeling with In-111 was performed as previously described17. Radiochemical purity was determined by ITLC and radiolabeled exendin-3 was purified by solid-phase extraction using a HLB-column as previously reported17.
SPECT acquisition
Mice with 50, 100, 200, 400 and 800 islets were scanned after 4 weeks. All mice (n = 4–5) were injected with approximately 15 MBq of 111In-exendin-3 (peptide dose 0.1 µg in 200 µl PBS, 0.5% BSA) in the tail vein. SPECT scans were acquired 1 h post-injection on a U-SPECT-II/CT dedicated small-animal scanner (MILabs, Utrecht, Netherlands) for 50 min with a high sensitivity mouse collimator (1.0 mm pinholes). Computed tomography (CT) was performed subsequently for anatomical reference. Standards of 74 kBq, 55 kBq, 37 kBq and 18 kBq in 50 µl volume each, were scanned under the same parameters as reference for quantification. Images were reconstructed with voxel size of 0.4 mm, 3 iterations and 16 subsets, using U-SPECT-II reconstruction software (MILabs, Utrecht, The Netherlands). The VOI was drawn over the islet transplant region, total voxel intensity registered in the islet graft was corrected by the mean of 3 measurements of contra-lateral control muscle, to subtract the background signal originating from the muscle tissue. The absolute activity (in kBq) was calculated by multiplying the corrected voxel intensity value with the calibration factor determined by quantitative analysis of standards with known radioactivity and data were normalized by the injected dose.
Morphometric analysis of the transplant
Immediately after SPECT acquisitions, mice were euthanized, muscles were fixed in 4% paraformaldehyde and embedded in paraffin, then sectioned into 4 µm slices for autoradiography analysis or for determination of insulin volume by immunohistochemistry. For autoradiography analysis, muscle sections were exposed to an imaging plate (Fuji Film BAS-SE 2025, Raytest, Straubenhardt, Germany) for 7 days and images were visualized with a radioluminography laser imager (Fuji Film BAS 1800 II system, Raytest, Straubenhardt, Germany) and were finally stained with hematoxylin-eosin (HE) to confirm the presence of islets. For determination of insulin volume, insulin staining was performed in muscle sections. Antigen retrieval was done using 10 mM sodium citrate buffer, pH 6.0, for 10 min (Thermo Scientific PT module, Lab Vision, USA). Blocking of endogenous peroxidase activity was performed by incubation with 0.6% H2O2 in 40% methanol/60% PBS for 30 min at RT in the dark. An additional blocking step was done with 5% swine serum in PBS for 30 min at RT. Primary anti-insulin antibody (cat. sc 9168, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was applied at a dilution of 1:50 (in PBS containing 1% BSA w/v). Subsequently, sections were washed with PBS and incubated with secondary horseradish peroxidase-conjugated swine-anti-rabbit IgG (1:50) (cat. P0217, Dakopatts, Copenhagen, Denmark) in PBS containing 1% BSA w/v for 30 min at RT. The staining was visualized with diaminobenzidine (PowerVisionTM DAB substrate system, Immunologic, Duiven, The Netherlands) and nuclei were counterstained with haematoxylin. To determine the volume of the transplant, sections were scanned with Pannoramic250 Flash II scanner (3D Histech, Budapest, Hungary), beta-cell surface was manually drawn around insulin positive region using Photoshop CS6. Finally, the volume was determined by multiplying the insulin positive surface per section by the inter-section distance, which is 40 µm.
Statistical analysis
Statistical analyses were performed using with Graphpad Prism 5 (San Diego CA, USA). The results were presented as mean ± SEM. Correlations were assessed using a two-tailed Pearson’s correlation coefficient. The level of significance was set at P < 0.05.
References
Shapiro, A. M. J. et al. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. New England Journal of Medicine 343, 230–238, doi:10.1056/NEJM200007273430401 (2000).
Ryan, E. A. et al. Five-Year Follow-Up After Clinical Islet Transplantation. Diabetes 54, 2060–2069, doi:10.2337/diabetes.54.7.2060 (2005).
Thompson, D. M. et al. Reduced Progression of Diabetic Microvascular Complications With Islet Cell Transplantation Compared With Intensive Medical Therapy. Transplantation 91, 373–378, doi:10.1097/TP.0b013e31820437f3 (2011).
Fandrich, F. & Ungefroren, H. Customized cell-based treatment options to combat autoimmunity and restore beta-cell function in type 1 diabetes mellitus: current protocols and future perspectives. Advances in experimental medicine and biology 654, 641–665 (2010).
Cozzi, E. & Bosio, E. Islet xenotransplantation: current status of preclinical studies in the pig-to-nonhuman primate model. Current opinion in organ transplantation 13, 155–158 (2008).
Lee, B. R. et al. In situ formation and collagen-alginate composite encapsulation of pancreatic islet spheroids. Biomaterials 33, 837–845 (2012).
Basta, G. et al. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: four cases. Diabetes care 34, 2406–2409 (2011).
Guney, M. A. et al. Connective tissue growth factor acts within both endothelial cells and beta cells to promote proliferation of developing beta cells. Proceedings of the National Academy of Sciences of the United States of America 108, 15242–15247 (2011).
Courtney, M. et al. In vivo conversion of adult alpha-cells into beta-like cells: a new research avenue in the context of type 1 diabetes. Diabetes, obesity & metabolism 13(Suppl 1), 47–52 (2011).
Brolen, G. K., Heins, N., Edsbagge, J. & Semb, H. Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes 54, 2867–2874 (2005).
D’Amour, K. A. et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature biotechnology 24, 1392–1401 (2006).
Jirak, D. et al. MRI of transplanted pancreatic islets. Magn Reson Med 52, 1228–1233 (2004).
Evgenov, N. V., Medarova, Z., Dai, G., Bonner-Weir, S. & Moore, A. In vivo imaging of islet transplantation. Nature medicine 12, 144–148 (2006).
Saudek, F. et al. Magnetic Resonance Imaging of Pancreatic Islets Transplanted Into the Liver in Humans. Transplantation 90, 1602–1606, doi:10.1097/TP.0b013e3181ffba5e (2010).
Lu, Y. et al. Noninvasive imaging of islet grafts using positron-emission tomography. Proceedings of the National Academy of Sciences of the United States of America 103, 11294–11299 (2006).
Toso, C. et al. Positron-emission tomography imaging of early events after transplantation of islets of Langerhans. Transplantation 79, 353–355 (2005).
Brom, M. et al. Non-invasive quantification of the beta cell mass by SPECT with 111In-labelled exendin. Diabetologia 57, 950–959, doi:10.1007/s00125-014-3166-3 (2014).
Brom, M., Joosten, L., Frielink, C., Boerman, O. & Gotthardt, M. 111In-exendin uptake in the pancreas correlates with the beta cell mass and not with the alpha cell mass. Diabetes, doi:10.2337/db14-1212 (2014).
Wu, Z. et al. Development and evaluation of 18F-TTCO-Cys40-Exendin-4: a PET probe for imaging transplanted islets. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 54, 244–251, doi:10.2967/jnumed.112.109694 (2013).
Pattou, F., Kerr-Conte, J. & Wild, D. GLP-1–Receptor Scanning for Imaging of Human Beta Cells Transplanted in Muscle. New England Journal of Medicine 363, 1289–1290, doi:10.1056/NEJMc1004547 (2010).
van der Kroon, I. et al. Noninvasive imaging of islet transplants with 111In-exendin-3. Journal of Nuclear Medicine, doi:10.2967/jnumed.115.166330 (2016).
Eter, W. A. et al. Graft revascularization is essential for non-invasive monitoring of transplanted islets with radiolabeled exendin. Scientific Reports 5, 15521, doi:10.1038/srep15521 (2015).
Willekens, S. et al. Quantitative imaging of transplanted pancreatic islets with SPECT using I-123 labeled benzamide. Journal of Nuclear Medicine 56, 592 (2015).
Willekens, S. M. A. et al. SPECT of Transplanted Islets of Langerhans by Dopamine 2 Receptor Targeting in a Rat Model. Molecular Pharmaceutics 13, 85–91, doi:10.1021/acs.molpharmaceut.5b00518 (2016).
Biarnés, M. et al. β-Cell Death and Mass in Syngeneically Transplanted Islets Exposed to Short- and Long-Term Hyperglycemia. Diabetes 51, 66–72, doi:10.2337/diabetes.51.1.66 (2002).
Scharp, D. W. et al. Insulin Independence After Islet Transplantation Into Type I Diabetic Patient. Diabetes 39, 515 (1990).
Acknowledgements
We kindly thank Bianca Lemmers, Kitty Lemmens, Iris Lamers-Elemans (Central Animal Facility, Radboud University Medical Center) for their excellent technical assistance in the animal experiments. The research leading to these results has received funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007–2013), project BetaTrain, under REA grant agreement no. 289932, and project BetaImage, under grant agreement no. 222980.
Author information
Authors and Affiliations
Contributions
W.A.E. contributed to the design of the study, conducted experiments, researched data and wrote the manuscript. I.V.D.K., S.W., D.B., L.J. and C.F. conducted experiments, M.B. and K.A. contributed in the design of the study, data analysis and the writing of the manuscript. O.B. contributed to the design of the study. M.B. and M.G. contributed to the discussion and the design of the study. All authors reviewed/edited the manuscript. M.G. is responsible for the overall integrity of the study. All authors agreed upon the submission version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Martin Gotthardt is patent holder in the field.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eter, W.A., Van der Kroon, I., Andralojc, K. et al. Non-invasive in vivo determination of viable islet graft volume by 111In-exendin-3. Sci Rep 7, 7232 (2017). https://doi.org/10.1038/s41598-017-07815-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07815-3
This article is cited by
-
Imaging in Type 1 Diabetes, Current Perspectives and Directions
Molecular Imaging and Biology (2023)
-
Integrated whole liver histologic analysis of the allogeneic islet distribution and characteristics in a nonhuman primate model
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.