Abstract
Sepsis is a serious clinical problem. Negative regulation of innate immunity is associated with sepsis progression, but the underlying mechanisms remains unclear. Here we show that the receptor CD300f promotes disease progression in sepsis. CD300f −/− mice were protected from death after cecal ligation and puncture (CLP), a murine model of septic peritonitis. CD300f was highly expressed in mast cells and recruited neutrophils in the peritoneal cavity. Analysis of mice (e.g., mast cell-deficient mice) receiving transplants of wild-type or CD300f −/− mast cells or neutrophils indicated that CD300f deficiency did not influence intrinsic migratory abilities of neutrophils, but enhanced neutrophil chemoattractant production (from mast cells and neutrophils) in the peritoneal cavity of CLP-operated mice, leading to robust accumulation of neutrophils which efficiently eliminated Escherichia coli. Ceramide-CD300f interaction suppressed the release of neutrophil chemoattractants from Escherichia coli-stimulated mast cells and neutrophils. Administration of the reagents that disrupted the ceramide-CD300f interaction prevented CLP-induced sepsis by stimulating neutrophil recruitment, whereas that of ceramide-containing vesicles aggravated sepsis. Extracellular concentrations of ceramides increased in the peritoneal cavity after CLP, suggesting a possible role of extracellular ceramides, CD300f ligands, in the negative-feedback suppression of innate immune responses. Thus, CD300f is an attractive target for the treatment of sepsis.
Similar content being viewed by others
Introduction
Septic peritonitis is a life-threatening emergency. Most clinical trials of anti-inflammatory agents in septic patients have been unsuccessful. Sepsis is characterized by systemic dysregulated inflammatory responses to infection. Despite extensive studies aimed at controlling or preventing it, sepsis remains a substantial clinical problem with poor prognosis and limited therapeutic options1, 2. The innate immune system is the first line of defense against bacterial infection. Tissue-resident myeloid cells―including mast cells and macrophages―detect invading bacteria or their products via pattern recognition receptors. To recruit neutrophils to infection sites, these myeloid cells release a variety of chemical mediators: neutrophil chemoattractants (e.g., leukotriene B4 [LTB4], macrophage inflammatory protein 2 [MIP2], and keratinocyte-derived chemokine [KC]) and vascular inflammation- and permeability-inducing factors (e.g., leukotrienes [LTs] and histamine). In turn, the recruited neutrophils themselves release neutrophil chemoattractants to further recruit neutrophils, which engulf and kill bacteria, thereby preventing the bacteria from spreading3,4,5,6. Nonetheless, once bacterial infections overcome such coordinated innate responses, sepsis progresses with systemic hyper-inflammation followed by immunosuppression. Most clinical trials of anti-inflammatory agents in septic patients have been unsuccessful2. Excessive activation of innate immunity is counterbalanced by negative signaling cascades7, 8. Therefore, understanding the inhibitory mechanisms in innate host responses is necessary to develop effective treatments of sepsis. Cecal ligation and puncture (CLP) is the standard model of septic peritonitis triggered by self-infection with intestinal bacteria such as Escherichia coli (E. coli). A variety of immune cells and receptors regulate CLP-induced sepsis during its different phases9,10,11. Here, we delineate the critical role of mast cell- and neutrophil-expressed CD300f (also called leukocyte mono-immunoglobulin-like receptor 3 [LMIR3] or CMRF-35-like molecule-1 [CLM-1])12,13,14,15 in innate host responses.
CD300f belongs to the paired activating and inhibitory receptor family CD300 (also called LMIR, CLM, or myeloid-associated Ig-like receptor [MAIR])12,13,14,15,16,17,18. The inhibitory receptor CD300f harbors two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and a single immunoreceptor tyrosine-based switch motif (ITSM) in the cytoplasmic region, and CD300f is expressed in myeloid cells, including mast cells and neutrophils12,13,14,15. Recent studies point to specific lipids or lipid-binding proteins as ligands of CD30013, 19,20,21,22,23,24,25,26. We recently identified extracellular ceramide as a ligand of CD300f13. Ceramides are composed of a long-chain or sphingoid base linked to a fattty acid via an amide bond. Ceramides play an important role not only as structural elements but also as regulators of a variety of cellular processes including differentiation, inflammation, proliferation, and apoptosis27. Ceramide-CD300f interaction inhibits IgE-dependent allergic responses13, ATP-mediated experimental colitis28, or lipopolysaccharide (LPS)-induced skin inflammation29. However, the roles of ceramide-CD300f interaction in sepsis, including septic peritonitis, have remained elusive.
In the present study, we demonstrate that in a model of septic peritonitis, disrupting the ceramide-CD300f interaction remarkably stimulates neutrophil recruitment to sites of infection and protects mice from septic death.
Results
CD300f −/− mice were highly resistant to CLP-induced sepsis
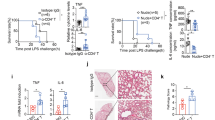
To clarify the role of CD300f in innate host responses, we used CLP, a model of septic peritonitis, in WT and CD300f −/− mice. CD300f −/− mice showed prolonged survival after CLP or mild CLP as compared with WT mice (Fig. 1a,b). Likewise, CD300f −/− mice were less prone to septic death after intraperitoneal inoculation with a minimal lethal dose of E. coli (Fig. 1c). Notably, lower counts of E. coli as well as remarkably lower levels of the proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6 were found in both peritoneal lavage fluid (PLF) and peripheral blood (PB) collected 24 h after CLP in CD300f −/− mice compared with WT mice (Fig. 1d,e). Thus, the vascular spread of E. coli and the concomitant hyper-inflammatory responses were prevented in CD300f −/− mice as early as 24 h after CLP, indicating that CD300f −/− mice were highly resistant to CLP-induced sepsis.
CD300f −/− mice were highly resistant to CLP-induced sepsis. (a,b) WT or CD300f −/− mice were subjected to (a) CLP (n = 13 per genotype) or (b) mild CLP (n = 7 per genotype), and monitored regarding survival. (c) WT or CD300f −/− mice (n = 6 per genotype) were intraperitoneally inoculated with a suspension of E. coli (4.0 × 108 colony-forming units [CFU] per mouse) and monitored regarding survival. (a–d) *p < 0.01 (log-rank test). (d) Bacterial counts (CFUs) in PLF or PB of WT or CD300f −/− mice 24 h after CLP (n = 13 per genotype). (e) The concentrations of IL-6 or TNF-α in PLF or serum of WT or CD300f −/− mice 24 h after CLP (n = 6 per genotype). (d,e) The data are expressed as mean ± SD; *p < 0.01 (Student’s t-test). (a,b,c and e) The data are representative of two independent experiments. WT and KO indicate WT mice and CD300f −/− mice, respectively.
Markedly enhanced accumulation of neutrophils was observed in the peritoneal cavity of CD300f −/− mice after CLP
We then performed histological analysis of cecum sections, which showed no significant differences between WT and CD300f −/− mice after a sham operation (Fig. 2a). WT mice, but not CD300f −/− mice, showed severe destruction or a complete loss of mucosal architecture in cecum sections collected 4 or 24 h after CLP, respectively (Fig. 2a). Cecum sections of CD300f −/− mice revealed prominent submucosal edema and serosal neutrophil infiltration 4 h after CLP, and showed attenuated edema and enhanced neutrophil accumulation after 24 h (Fig. 2a). In line with the histological findings, we observed a marked increase in the number of CD11b+Gr-1high neutrophils in PLF of CD300f −/− mice 4 h after CLP (Fig. 2b). We then measured the levels of MIP2, KC, LTB4, or cysteinyl LTs in PLF of the CLP-operated mice. The results showed higher concentrations of these chemical mediators in PLF of CD300f −/− mice as compared with PLF of WT mice as early as 2 h after CLP (Fig. 2c). These results led us to hypothesize that the high levels of neutrophil chemoattractants and vascular inflammation- and permeability-inducing factors recruit substantial numbers of neutrophils to the peritoneal cavity of CD300f −/− mice.
Neutrophil recruitment to infection sites was enhanced in CLP-operated CD300f −/− mice. (a) Cecum sections from WT or CD300f −/− mice at 4 h (top) after a sham operation and 4 h (middle) or 24 h (bottom) after CLP were stained with hematoxylin and eosin. Scale bars are 100 μm. The data are representative of five independent experiments. (b) Numbers of neutrophils recruited into the peritoneal cavity of the indicated mice 4 h after a sham operation (total n = 6) or CLP (total n = 20). Data were pooled from two independent experiments. (c) The concentrations of MIP2, KC, LTB4, or cysteinyl LTs in PLF in the indicated mice 2 h after CLP (n = 5–6 per genotype). The data are representative of three independent experiments and are expressed as mean ± SD; *p < 0.01 (Student’s t-test).
Ceramide-CD300f interaction suppressed the release of neutrophil chemoattractants from mast cells and neutrophils in response to the presence of E. coli
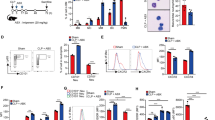
To clarify the role of the interaction between CD300f and its ligand ceramide in CLP, we examined the levels of extracellular ceramide species in PLF or plasma from WT mice30. The results revealed that several kinds of ceramide (N-palmitoyl-D-erythro-sphingosine [d18:1/16:0], N-stearoyl-D-erythro-sphingosine [d18:1/18:0], N-arachidoyl-D-erythro-sphingosine [d18:1/20:0], N-behenoyl-D-erythro-sphingosine [d18:1/22:0], N-lignoceroyl-D-erythro-sphingosine [d18:1/24:0], and N-nervonoyl-D-erythro-sphingosine [d18:1/24:1]) were present in PLF; the levels of ceramide species in PLF, but not in plasma, increased after CLP, implying a possible role of the ceramide-CD300f binding in this phenomena (Fig. 3a and Supplementary Fig. S1). Next, to identify the cell populations responsible for the enhanced neutrophil accumulation in CLP-operated CD300f −/− mice, we examined CD300f expression in peritoneal myeloid cells of WT mice. Notably, CD300f was highly expressed in resident mast cells and in the recruited neutrophils, whereas it was barely expressed in peritoneal macrophages (Fig. 3b). We then tested whether the binding of ceramide to CD300f inhibits the release of neutrophil chemoattractants from these myeloid cells in response to the presence of E. coli. To this end, bone marrow-derived mast cells (BMMCs) were stimulated with E. coli on plates coated with ceramide or phosphatidylcholine (PC) as a control. The CD300f deficiency failed to alter the release of the indicated chemical mediators from the stimulated BMMCs in the absence of ceramide (Fig. 3c–e). On the other hand, the ceramide-CD300f interaction specifically inhibited the release of MIP2, KC, LTB4, LTC4, or β-hexosaminidase (a marker of degranulation) from WT BMMCs, but not from CD300f −/− BMMCs, in response to the presence of E. coli (Fig. 3c–e). Similar experiments with BMMC transfectants confirmed that the disruption of ITIMs and ITSM of CD300f attenuated the inhibition of E. coli-stimulated mast cell activation by the ceramide-CD300f binding (Supplementary Fig. S2a–c)13. Thus, the ceramide-CD300f interaction suppressed E. coli-stimulated mast cell activation via ITIMs and ITSM of CD300f. Similarly, the ceramide-CD300f interaction also suppressed the release of neutrophil chemoattractants from neutrophils stimulated by E. coli (Fig. 3f and Supplementary Fig. S3). In contrast, the release of these neutrophil chemoattractants was not inhibited by the ceramide-CD300f interaction in peritoneal macrophages, presumably due to the low levels of CD300f expression (Fig. 3g). On the other hand, neither LMIR3 expression nor the ceramide-CD300f interaction significantly influenced the bactericidal activity of neutrophils or BMMCs against E. coli (Supplementary Fig. S4a,b); this finding is suggestive of a limited role of the ceramide-CD300f binding in innate immune responses.
Ceramide-CD300f interaction suppressed the release of factors (inducing neutrophil migration) from mast cells and neutrophils in response to the presence of E. coli. (a) The concentrations of all and indicated ceramide species in PLF before or 4 h after a sham operation or CLP in WT mice (n = 5 per group). (b) Surface expression levels of CD300f in peritoneal mast cells (MC) or macrophages (Mϕ) before CLP or in neutrophils 4 h after CLP in WT mice. C-G, WT or CD300f −/− BMMCs (c–e) neutrophils (f), or peritoneal macrophages (g) were stimulated with 5 × 107 CFU/ml heat-killed E. coli on plates coated with ceramide, PC, or vehicle. Production of (c,f) MIP2, KC, and LTB4, (d) LTC4, (e) a release of β-hexosaminidase, or (f) MIP2 and KC. (b–g) The data are representative of four independent experiments. The data are expressed as mean ± SD; *p < 0.01 (Student’s t-test).
CD300f −/− neutrophils recruited to the peritoneal cavity contributed to enhanced neutrophil accumulation in the CLP model
To clarify the in vivo role of CD300f-deficient neutrophils in promoting neutrophil accumulation in our CLP model, we intraperitoneally injected equal numbers of WT or CD300f −/− neutrophils (Ly5.2+) into CLP-operated WT mice (Ly5.1+). Transfusion of 107 neutrophils, irrespective of the expression of CD300f, equally improved survival of the CLP-operated mice (Supplementary Fig. S5), suggesting that an adequate number of recruited neutrophils can prevent CLP-induced sepsis. On the other hand, transfusion of 106 CD300f −/− neutrophils, but not of WT neutrophils, increased survival and the number of Ly5.1+ neutrophils recruited to (and the levels of MIP2, KC, and LTB4 in) the peritoneal cavity of the CLP-operated WT mice (Fig. 4a–c). We then determined whether the CD300f deficiency enhances the intrinsic migratory ability of neutrophils themselves. Transwell migration assays revealed that PLF of CLP-operated CD300f −/− mice attracted more neutrophils than did PLF of CLP-operated WT mice, and that equivalent numbers of WT or CD300f −/− neutrophils migrated into the same PLF (Fig. 4d). After that, to next compare the migratory ability between WT and CD300f −/− neutrophils in vivo, CFSE-labeled CD300f −/− neutrophils mixed in the ratio of 1:4, 1:1, or 4:1 with WT neutrophils were intravenously injected into WT mice 1 h before CLP. The proportions of CD300f −/− cells among CFSE-positive peritoneal neutrophils were also similar to their proportions among the CFSE-positive PB neutrophils (Fig. 4e). These data indicated that neutrophil chemoattractants released by CD300f −/− neutrophils rather than the intrinsic migratory ability of CD300f −/− neutrophils contributed to the enhanced accumulation of neutrophils in CLP-operated CD300f −/− mice.
CD300f −/− neutrophils recruited to the peritoneal cavity contributed to enhanced neutrophil migration in a CLP model. (a–c) CLP-operated WT (Ly5.1+) mice were intraperitoneally injected with 106 cells of WT (Ly5.2+) or CD300f −/− (Ly5.2+) neutrophils or PBS as a control (n = 6 per genotype). (a) The mice were monitored regarding survival; *p < 0.01 compared to the mice inoculated with WT neutrophils (log-rank test). (b,c) Numbers of Ly5.1+ neutrophils (b) or levels of MIP2, KC, or LTB4 (c) in PLF (each, n = 4). (d) Numbers of WT or CD300f −/− neutrophils that migrated into the lower wells containing PLF derived from either WT or CD300f −/− mice 4 h after CLP. (e) The proportions (%) of CD300f −/− neutrophils among CSFE-positive neutrophils present in PB or PLF from the chimeric mice (each, n = 4) 4 h after CLP. Values on the X and Y axes represent the percentage in PB and PLF, respectively. (a–e) The data are representative of two independent experiments. The data are expressed as mean ± SD; *p < 0.01 (Student’s t-test).
CD300f −/− mast cells played an important role in the enhanced neutrophil accumulation in the CLP model
To clarify the functions of CD300f −/− mast cells in the defense against sepsis, we used CLP in mast cell-deficient (Kit W-sh/W-sh) mice reconstituted intraperitoneally with WT or CD300f −/− BMMCs, with the two types of mice having equivalent numbers of peritoneal mast cells (Supplementary Fig. S6a,b). The reconstitution with CD300f −/− BMMCs, but not with WT BMMCs, improved the survival of CLP-operated Kit W-sh/W-sh mice (Fig. 5a), implicating CD300f −/− mast cells in the prolonged survival of the CLP-operated CD300f −/− mice. In addition, CD300f −/− BMMC-reconstituted Kit W-sh/W-sh mice were found to have greater numbers of neutrophils as well as higher levels of MIP2, KC, LTB4, and cysteinyl LTs in PLF than did WT BMMC-reconstituted Kit W-sh/W-sh mice (Fig. 5b,c). Similarly, a transplant of CD300f −/− BMMCs, but not WT BMMCs, enhanced neutrophil accumulation and improved survival after CLP in a cross of Mcpt5-Cre mice with the R-DTA mice (Mcpt5-Cre/R-DTA mice); these mice were genetically engineered to be mast cell-deficient (Fig. 5d,e and Supplementary Fig. S7)31. Moreover, we compared disease progression between Kit W-sh/W-sh mice and Kit W-sh/W-sh CD300f −/− mice. The latter mice showed slightly increased survival after CLP as compared with the former (Fig. 5f), indicating that CD300f −/− myeloid cells other than mast cells, possibly neutrophils, contributed to the resistance of CD300f −/− mice to CLP lethality. Moreover, reconstitution with CD300f −/− BMMCs, but not with their WT counterparts, increased the number of neutrophils in the peritoneal cavity and improved survival in CLP-operated CD300f −/− Kit W-sh/W-sh mice (Supplementary Fig. S8a–c). On the other hand, a transplant of BM-derived macrophages, irrespective of CD300f expression, equally improved survival after CLP in WT mice with macrophage/monocyte depletion by clodronate liposomes (Supplementary Fig. S9), confirming that monocytes/macrophages play a role in the resistance to CLP-induced sepsis; however, CD300f deficiency in monocytes/macrophages does not significantly influence that.
CD300f −/− mast cells played an important role in enhanced accumulation of neutrophils in the CLP model. (a) Kit W-sh/W-sh mice that had received an intraperitoneal transplant of WT or CD300f −/− BMMCs (n = 12 per group) or were injected with PBS (control, n = 7) were subjected to CLP and monitored regarding survival; *p < 0.01 compared to the mice transplanted with WT BMMCs (log-rank test). (b,c) Numbers of neutrophils recruited into the peritoneal cavity (b) and concentrations of MIP2, KC, LTB4, or cysteinyl LTs (c) in the indicated mice (n = 5 to 9 per group) 4 h after CLP. (d,e) Mcpt5-Cre/R-DTA mice that had received an intraperitoneal transplant of WT or CD300f −/− BMMCs (n = 8 per group) or were injected with PBS (control, n = 5) were subjected to CLP and monitored regarding survival. *p < 0.01 compared to the mice with a transplant of WT BMMCs (long-rank test). (e) Numbers of neutrophils recruited into the peritoneal cavity of the indicated mice (n = 4 per group) 4 h after CLP. (a–e) The data are representative of two independent experiments. (b,c,e) The data are expressed as mean ± SD; *p < 0.01 (Student’s t-test). (f) Kit W-sh/W-sh or Kit W-sh/W-sh CD300f −/− mice (total n = 18 per group) were subjected to CLP model and monitored regarding survival; *p < 0.05 (log-rank test). Data were pooled from two independent experiments.
Treatment with an anti-ceramide Ab or CD300f-Fc ameliorated septic mortality after CLP
We then intraperitoneally injected CD300f-Fc or a control Fc into mice immediately after CLP and monitored survival. The CD300f-Fc-treated mice survived remarkably longer after CLP, as compared with control mice (Fig. 6a). Similarly, intraperitoneal injection of an anti-ceramide Ab, but not of a control Ab, into WT mice immediately after CLP effectively prevented septic deaths (Fig. 6b). WT mice given an injection of the anti-ceramide Ab at 1 or 3 h after CLP showed survival rates that were similar to those of the mice treated with the anti-ceramide Ab immediately after CLP (Fig. 6c). In addition, even when WT mice received an intraperitoneal injection of the anti-ceramide Ab at 7 h after CLP, this treatment was still effective against septic deaths (Fig. 6c). Accordingly, treatment with the anti-ceramide Ab, but not with a control Ab, increased the numbers of neutrophils recruited to the peritoneal cavity of WT mice 2 h after CLP; these numbers were comparable to those found in the case of CLP-operated CD300f −/− mice (Fig. 6d). In addition, treatment with the anti-ceramide Ab, but not with a control Ab, elevated the levels of MIP-2, KC, LTB4, and cysteinyl LTs in PLF of WT mice 2 h after CLP (Fig. 6e,f). In contrast, the same treatment failed to influence neutrophil recruitment or chemical-mediator production in CLP-operated CD300f −/− mice (Fig. 6d–f). In contrast, treatment with vesicle containing ceramide, but not PC or phosphatidylserine (PS), decreased the survival of WT mice after mild CLP (Fig. 6g). In line with this finding, only treatment with ceramide-containing vesicle decreased the number of neutrophils recruited to the peritoneal cavity of WT mice, but not of CD300f −/− mice (Fig. 6h). Thus, increasing the concentration of extracellular ceramides aggravated experimental sepsis by reducing neutrophil recruitment to the local sites of infection, whereas disruption of the ceramide-CD300f interaction prevented sepsis by stimulating neutrophil recruitment.
Treatment with an anti-ceramide Ab or CD300f-Fc ameliorated septic mortality after CLP. (a,b) Ab- or Fc protein-treated WT mice were subjected to CLP and monitored regarding survival. (a) The mice were intraperitoneally injected with 300 μg of either CD300f-Fc or control Fc immediately after CLP (n = 10); *p < 0.01 (log-rank test). (b) The mice were intraperitoneally injected with 4 μg of either the anti-ceramide Ab or isotype control Ab immediately after CLP (n = 12); *p < 0.01 (log-rank test). (c) Mice were intraperitoneally injected with 4 μg of either anti-ceramide Ab at 0, 1, 3, or 7 h after CLP (n = 7) or isotype control Ab at 0 h after CLP (n = 14); *p < 0.01 or **p < 0.05 compared to control mice (log-rank test). (d–f) WT or CD300f −/− mice were intraperitoneally injected with 4 μg of either the anti-ceramide Ab or isotype control Ab immediately after CLP. (d) Numbers of neutrophils recruited into the peritoneal cavity 4 h after CLP (n = 5). (e,f) Concentrations of (e) MIP-2, KC, or LTB4 or (f) cysteinyl LTs in PLF from mice 4 h after CLP (n = 5 to 6). (g,h) WT or CD300f −/− mice were intraperitoneally injected with 100 μg of vesicle containing ceramide, PC, PS, or PBS as a control immediately after CLP. (g) Mice (total n = 10 per group) were monitored regarding survival; *p < 0.05 compared to control mice (log-rank test). Data were pooled from two independent experiments. (h) Numbers of neutrophils recruited into the peritoneal cavity 4 h after CLP (n = 5). (d,e,f,h) The data are representative of two independent experiments. The data are expressed as mean ± SD; *p < 0.01 (Student’s t-test).
Discussion
A striking feature of CD300f −/− mice subjected to CLP is the dramatically enhanced accumulation of neutrophils after elevated production of neutrophil chemoattractants (e.g., MIP2, KC, and LTB4) and vascular permeability- and inflammation-inducing factors (e.g., cysteinyl LTs) in the peritoneal cavity, where feces-derived bacteria (e.g., E. coli) evoked inflammation. Given that CD300f deficiency enhances LPS-induced skin inflammation characterized by edema formation and neutrophil accumulation29, it is possible that E. coli-derived LPS plays a certain role in the underlying pathogenesis. Notably, peritoneal exudates from CLP-operated CD300f −/− mice attracted more neutrophils, irrespective of CD300f expression, than did those from WT counterparts in an in vitro migration assay. In addition, WT and CD300f-deficient neutrophils had equivalent abilities to migrate from the circulating blood to the peritoneal cavity in our CLP model. Therefore, it was reasonable to assume that the enhanced neutrophil accumulation in CD300f −/− mice was primarily due to elevated local production of factors inducing neutrophil migration at an early time-point after CLP; peritoneal CD300f −/− myeloid cells should contribute to this phenomenon. On the other hand, flow cytometric analysis revealed high levels of CD300f expression in peritoneal mast cells and in recruited neutrophils, but not in peritoneal macrophages. Consistent with this, the binding of plate-coated ceramide to CD300f inhibited the release of factors (inducing neutrophil migration) from mast cells and neutrophils, but not from peritoneal macrophages, in response to the presence of E. coli. Accordingly, we hypothesized that the ceramide-CD300f binding in resident mast cells and recruited neutrophils would suppress the local production of factors inducing neutrophil migration in the CLP model. Indeed, we confirmed that ceramide species were present in PLF. Moreover, CLP substantially increased the amounts of peritoneal ceramide species; this finding is suggestive of a possible role of extracellular ceramides in the negative-feedback suppression of innate immune responses32. Expression patterns of ceramide species were different between plasma and PLF, implying that extracellular ceramides may be derived from damaged, necrotic, or apoptotic cells, but not from extravasated blood, in the peritoneal cavity after CLP. Moreover, treatment with CD300f-Fc or the anti-ceramide Ab, which disrupt the ceramide-CD300f interaction in vivo 13, increased the number of neutrophils recruited to and the levels of neutrophil chemoattractants in the peritoneal cavity of WT mice. In contrast, treatment with ceramide-containing vesicle, which locally upregulated ligands for CD300f13, decreased neutrophil accumulation and the concentrations of the above mentioned mediators in WT mice. All these observations support our hypothesis that the ceramide-CD300f interaction suppresses innate immune responses in the CLP model.
Inoculation of an adequate number (107) of either WT or CD300f −/− neutrophils equally improved survival of WT mice after CLP, suggesting that robust accumulation of neutrophils prevented sepsis after CLP in CD300f −/− mice. On the other hand, inoculation of low numbers (106) of CD300f −/− neutrophils, but not WT neutrophils, increased the number of recipient neutrophils recruited to the peritoneal cavity, concentrations of neutrophil chemoattractants in PLF, and survival after CLP in WT mice. In addition, CD300f −/− Kit W-sh/W-sh mice were less prone to CLP-induced death than Kit W-sh/W-sh mice were. These results implicated CD300f −/− neutrophils in the resistance of CD300f −/− mice to septic death. We can theorize that once recruited to inflamed tissues containing extracellular ceramides, CD300f −/− neutrophils release more neutrophil chemoattractants than WT neutrophils do, thus accelerating neutrophil accumulation in the CLP model.
The crucial role of CD300f-deficient mast cells in CLP-operated CD300f −/− mice was illustrated by several lines of evidence: the concentrations of neutrophil chemoattractants and cysteinyl LTs in PLF, the number of neutrophils recruited to the peritoneal cavity, and survival after CLP were increased by implantation of CD300f −/− mast cells, but not WT mast cells, in two types of mast cell-deficient mice (Kit W-sh/W-sh or Mcpt5-Cre/R-DTA). In addition, similar results were obtained in CD300f −/− Kit W-sh/W-sh mice, confirming the non-redundant and indispensable role of CD300f −/− mast cells in this model. Recent studies suggested that whether mast cells have a beneficial or detrimental effect on survival during bacterial infection depends on the circumstances, including the type and severity of infection33, 34. In fact, under our experimental conditions, WT mast cells appeared to not promote survival after CLP. It was evident, however, that CD300f −/− mast cells drive neutrophil recruitment and enhance survival after CLP; these data are suggestive of an outstanding role of CD300f −/− mast cells in prevention of sepsis. Prominent mucosal edema in the cecum of CLP-operated CD300f −/− mice can be attributed to upregulation of factors increasing vascular permeability, including cysteinyl LTs, which are released mainly by mast cells. On the other hand, the increased concentrations of mast cell-derived chemical mediators known to improve survival after CLP35, 36 may also contribute to the resistance of CD300f −/− mice to septic peritonitis.
Collectively, these results support our hypothesis that resident mast cells collaborate with the recruited neutrophils to accelerate neutrophil accumulation at sites of infection in CD300f −/− mice although a contributory role of other CD300f −/− myeloid cells cannot be ruled out. On the other hand, the ceramide-CD300f binding failed to significantly influence the in vitro bactericidal abilities of mast cells or neutrophils in response to the presence of E. coli. Hence, we can conclude that the resistance of CD300f −/− mice to CLP lethality is likely to the result of efficient elimination of E. coli by vast numbers of neutrophils recruited to the peritoneal cavity where large amounts of the factors inducing neutrophil recruitment were released by resident mast cells and by recruited neutrophils.
CD300f was reported to recognize PS21, 25, but our results did not implicate this interaction in the CD300f-mediated inhibition of innate immune responses in our CLP model. This conclusion seems to be supported by a recent finding that apoptotic cell-mediated inhibition of cytokine and chemokine production in CLP-operated mice depends on the PS-CD300a interaction22. It was therefore possible to assume that the ceramide-CD300f interaction cooperated with the PS-CD300a interaction to inhibit innate immune responses in our CLP model. Thus, the ceramide-CD300f binding inhibits CLP-induced neutrophil accumulation to sites of infection as well as IgE-dependent allergic reactions, ATP-mediated experimental colitis, or LPS-induced skin inflammation; however, the ceramide-CD300f binding is either detrimental or beneficial to human health in the former and latter case, respectively13.
It is worth mentioning that even post-CLP treatment with the anti-ceramide Ab improved the survival of the septic mice. Although most of the anti-inflammatory therapies for sepsis have failed in clinical trials, novel strategies to relieve immunosuppression in sepsis are emerging2. In this sense, CD300f is a promising target because blocking of CD300f function stimulates innate host responses that can overcome both primary bacterial infections and secondary cosmic infections. Because human CD300f binds both ceramide and sphingomyelin20, a novel drug specifically disrupting this binding may be effective against bacterial infections. In addition, it is possible that combined targeted therapies will synergistically improve the survival rates of septic patients.
In conclusion, ceramide-CD300f interaction inhibits innate host responses in a model of septic peritonitis. Disruption of this interaction stimulates neutrophil accumulation at infection sites. Accordingly, the development of an intervention involving CD300f seems to be an appealing approach to the prophylaxis of bacterial sepsis.
Methods
Mice
C57BL/6 J (Ly5.1+), C57BL/6 J (Ly5.2+), CD300f −/−, Kit w-sh/w-sh, Kit w-sh/w-sh CD300f −/−, and Mcpt5-Cre/R-DTA mice were used in this study13, 31.
Ethics statement
All animal experiments were approved by the ethical committee of the University of Tokyo (approval no 20–8) and Juntendo University (approval no 270015). All the methods were carried out in accordance with the approved guidelines and regulations.
Antibodies (Abs) and other reagents
The following Abs were used: rat anti-LMIR3/CD300f (3–14–11) was obtained from ACTGen; anti-Flag (M2) and mouse IgG1 (MOPC21) were purchased from Sigma-Aldrich; fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (M1/70), Gr-1 (RB6-8C5), F4/80 (BM8), or the high-affinity IgE receptor (FcεRI)-α (MAR-1), phycoerythrin (PE)-conjugated anti-Gr-1 (RB6-8C5), CD45.1 (A20), CD45.2 (104), Ly-6G (1A8), CD11b (M1/70), or c-kit (2B8) or streptavidin- or allophycocyanin (APC)-conjugated anti-c-kit (2B8) and rat IgG2a (eBR2a) were from eBioscience; anti-ceramide Ab (MID 15B4) was from Enzo Life Sciences, mouse IgM (MOPC-104E) was from BioLegend, rat IgG1/2a (G28-5) was from BD Biosciences. Clodronate liposomes were from Sigma-Aldrich. All the cytokines were obtained from R&D Systems. 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (phosphatidylcholine [PC]) and 1,2-Dipalmitoyl-sn-glycero-3-phosphoserine (phosphatidylserine [PS]) were from Echelon Biosciences Inc. All other reagents were from Sigma unless stated otherwise13.
Cells
BMMCs and BMMC transfectants were generated as described elsewhere13, 37. Neutrophils were isolated from mouse BM by means of a three-layer gradient as described previously12, 38, 39. Peritoneal macrophages and BM-derived macrophages were isolated as described elsewhere17, 26.
Cell stimulation
Lipids were diluted to a concentration of 20 μg/ml in methanol. MaxiSorp 96-well plates (Nunc, catalog No. 430341) were coated with 50 μl of each solution, air-dried, and washed twice with the medium, as previously described13. BMMCs, neutrophils, or peritoneal macrophages were pre-incubated on a lipid-coated plate for 1 h before stimulation with 5 × 107 CFU/ml heat-killed E. coli 13.
Peritonitis induced by CLP or by inoculation of E. coli
CLP was performed as previously described10, 11, 40,41,42. Briefly, male mice were anesthetized, and the cecum was exposed through a 1.0- to 1.5-cm abdominal midline incision. The cecum was ligated at half the distance between the distal pole and the base of the cecum, followed by a single puncture with either a G-18 needle (CLP with 80–100% lethality among wild-type [WT] mice) or a G-22 needle (mild CLP with 40–60% lethality among WT mice). Sham-operated mice underwent an identical laparotomy without CLP. The operated mice received a subcutaneous injection of sterile saline (1 ml) for fluid resuscitation after the abdominal incision was closed. Survival after CLP was assessed every 12 h for 7 d. Peritoneal lavage fluid (PLF) (1 ml PBS), serum, or cecum samples were collected from the mice euthanized 2, 4, or 24 h after CLP. In some experiments, 4 μg of either the anti-ceramide Ab or isotype control Ab or 300 μg of either CD300f-Fc or control Fc was intraperitoneally injected immediately after or at the indicated time points after CLP. To induce E. coli peritonitis, E. coli was isolated from mouse feces with Drigalski agar (Nissui), was grown in Luria-Bertani broth overnight, washed with PBS four times, and re-suspended in PBS (109 colony forming unit [CFU]/ml)1, 40, 43. Female mice were intraperitoneally injected with a suspension of E. coli (4.0 × 108 CFU per mouse as the minimum lethal dose). For some experiments, E. coli was killed by heat (95 °C for 2 min)33.
Analysis of in vivo neutrophil migration
Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD300f −/− (Ly5.2+) neutrophils mixed with WT (Ly5.1+) neutrophils were intravenously injected into WT (Ly5.1+) mice 1 h before CLP. The peritoneal lavage cells or PB cells 4 h after CLP were stained with PE-conjugated anti-Ly5.1 (CD45.1) Ab or ant-Ly5.2 (CD45.2) Ab, and the proportions of CD300f −/− (Ly5.2+) cells among CFSE-positive neutrophils recruited to the peritoneal cavity or among CFSE-positive PB neutrophils were calculated by means of fluorescence-activated cell sorting (FACS).
Preparation of lipid-containing vesicle
After 1 mg of a dry lipid (C-24 ceramide, PC, or PS) was hydrated with 1 ml of PBS, vesicle was generated by means of Avanti Mini-Etruder (Avanti Polar Lipids, Inc.) according to the manufacturer’s instructions, as previously described13, 20.
Preparation of Fc fusion proteins
These proteins were purified as described1, 7. Endotoxin levels of Fc fusion proteins were less than 0.01 ng/μg protein as determined by the limulus amebocyte lysate test (Lonza)13, 18.
Quantification of cytokines, chemokines, and LTs and the degranulation assay
Enzyme-linked immunosorbent assay (ELISA) kits for IL-6, TNF-α, KC, MIP-2, and LTB4 (R&D Systems) or cysteinyl LTs and LTC4 (Cayman Chemical Company) were used. The release of β-hexosaminidase was estimated as described elsewhere13.
Flow cytometry
Flow cytometric analysis was performed on a FACSCalibur (BD Biosciences) equipped with the CellQuest software and FlowJo software (Tree Star), as previously described13.
DNA constructs
pMXs-internal ribosome entry sites (IRES)-puror (pMXs-IP), pMXs-Flag-CD300f or CD300f-Y241F/Y289F/Y325F-IP, and the pME18S-hIgG1 Fc vector (a kind gift from H. Arase, Osaka University) were described previously13, 44, 45.
Transfection and infection
Retroviral transfection was performed as described elsewhere13, 44, 45. Cells were infected with retroviruses generated by transient transfection of PLAT-E packaging cells.
Reconstitution with BMMC
Mast cell reconstitution was performed as previously described13. In brief, Kit W-sh/W-sh mice or Mcpt5-Cre/R-DTA mice were injected intraperitoneally with either 5 × 106 WT or CD300f −/− BMMCs 6 weeks before CLP. Reconstitution of mast cells was confirmed by toluidine blue staining or fluorescence-activated cell sorting (FACS).
Macrophage reconstitution
Macrophage reconstitution was performed as previously described46. Briefly, clodronate liposomes (50 mg in 200 μl per mouse) were intraperitoneally injected into WT mice to deplete macrophages. Two days later, 107 WT or CD300f −/− BM-derived macrophages were intraperitoneally injected into macrophage-depleted mice 6 h before CLP induction.
Transwell migration assays
These assays were performed using Transwell filters with 3-μm pores (BD Falcon), as previously described26. Briefly, the upper wells contained 1.5 × 106 cells in 0.2 ml of the medium and the lower wells were filled with 0.6 ml of PLF from CLP-operated mice. After incubation for 60 min, neutrophils that migrated into the lower wells were counted.
Killing assays
The indicated cells were incubated with E. coli (4 × 105 cells per 106 CFU of E. coli) for 60 min. Enumeration of viable E. coli cells involved colony-counting methods40, 42.
Quantitation of ceramides
Concentrations of ceramides were measured as previously described30. Briefly, PLF (1 ml PBS) or plasma samples were collected from WT mice before or 4 h after a sham or CLP operation. Total lipids were extracted by the method of Bligh and Dyer. The lipid extracts were dissolved in chloroform:methanol:2-propanol (10:45:45, v/v/v) so that the final concentration of the internal standard was 50 nM. This lipid solution was subjected to reversed-phase liquid chromatography with mass spectrometry (LC/MS). An Agilent 1100 Series LC/MSD SL system equipped with a multi-ion source, ChemStation software, a 1,100-well plate autosampler (Agilent Technologies), and an L-column ODS (2.1 mm i.d. ×150 mm; Chemicals Evaluation and Research Institute) was used. Chromatographic separation of the lipids was attained at a flow rate of 0.2 mL/min using a binary gradient solvent system of mobile phases. Each ceramide species was detected by selected ion monitoring as m/z [M + CH3COO]−. C24-ceramide (d18:1/24:0), C18-ceramide (d18:1/18:0), C16-ceramide (d18:1/16:0) (Toronto Research Chemicals Inc.), N-Nervonoyl-D-erythro-sphingosine (d18:1/24:1) (Cayman Chemical Co.), N-Behenoyl-D-erythro-sphingosine (d18:1/22:0) (Avanti Polar Lipids), and N-Arachidoyl-D-erythro-Sphingosine (d18:1/20:0) (Avanti Polar Lipids) served as standards. N-Heptadecanoyl-D-erythro-sphingosine (d18:1/17:0) (Avanti Polar Lipids) was used as an internal control.
Statistical analyses
The results are expressed as means ± standard deviation (SD). Unpaired Student’s t test was used to analyze the differences between groups. The Kaplan-Meier method and log-rank tests were used to analyze the survival data. *p < 0.01 or **p < 0.05 was assumed to mean statistical significance.
References
Bouchon, A., Facchetti, F., Weigand, M. A. & Colonna, M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107 (2001).
Rittirsch, D., Flierl, M. A. & Ward, P. A. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8, 776–787 (2008).
Peters-Golden, M., Canetti, C., Mancuso, P. & Coffey, M. J. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol 174, 589–594 (2005).
Phillipson, M. & Kubes, P. The neutrophil in vascular inflammation. Nat Med 17, 1381–1390 (2011).
De Filippo, K. et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121, 4930–4937 (2013).
St John, A. L. & Abraham, S. N. Innate immunity and its regulation by mast cells. J Immunol 190, 4458–4463 (2013).
Colonna, M. TREMs in the immune system and beyond. Nat Rev Immunol 3, 445–453 (2013).
Kondo, T., Kawai, T. & Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol 33, 449–458 (2012).
Buras, J. A., Holzmann, B. & Sitkovsky, M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4, 854–865 (2005).
Rittirsch, D., Huber-Lang, M. S., Flierl, M. A. & Ward, P. A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4, 31–36 (2009).
Dejager, L., Pinheiro, I., Dejonckheere, E. & Libert, C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19, 198–208 (2011).
Izawa, K. et al. Functional analysis of activating receptor LMIR4 as a counterpart of inhibitory receptor LMIR3. J Biol Chem 282, 17997–18008 (2007).
Izawa, K. et al. The receptor LMIR3 negatively regulates mast cell activation and allergic responses by binding to extracellular ceramide. Immunity 37, 827–839 (2012).
Chung, D. H. et al. CMRF-35-like molecule-1, a novel mouse myeloid receptor, can inhibit osteoclast formation. J Immunol 171, 6541–6548 (2003).
Xi, H. et al. Negative regulation of autoimmune demyelination by the inhibitory receptor CLM-1. J Exp Med 207, 7–16 (2010).
Kumagai, H. et al. Identification and characterization of a new pair of immunoglobulin-like receptors LMIR1 and 2 derived from murine bone marrow derived mast cells. Bioche. Biophys Res Commun 307, 719–729 (2003).
Yamanishi, Y. et al. Analysis of mouse LMIR5/CLM-7 as an activating receptor: differential regulation of LMIR5/CLM-7 in mouse versus human cells. Blood 111, 688–698 (2008).
Yotsumoto, K. et al. Paired activating and inhibitory immunoglobulin-like receptors, MAIR-I and MAIR-II, regulate mast cell and macrophage activation. J Exp Med 198, 223–233 (2003).
Cannon, J. P., O’Driscoll, M. & Litman, G. W. Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics 64, 39–47 (2011).
Izawa, K. et al. Sphingomyelin and ceramide are physiological ligands for human LMIR3/CD300f, inhibiting FcεRI-mediated mast cell activation. J Allergy Clin Immunol 133, 270–273 (2014).
Choi, S. C. et al. Cutting edge: mouse CD300f (CMRF-35-like molecule-1) recognizes outer membrane-exposed phosphatidylserine and can promote phagocytosis. J Immunol 187, 3483–3487 (2011).
Nakahashi-Oda, C. et al. Apoptotic cells suppress mast cell inflammatory responses via the CD300a immunoreceptor. J Exp Med 209, 1493–1503 (2012).
Simhadri, V. R. et al. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood 119, 2799–2809 (2012).
Takahashi, M. et al. Human CD300C delivers an Fc receptor-γ-dependent activating signal in mast cells and monocytes and differs from CD300A in ligand recognition. J Biol Chem 288, 7662–7675 (2013).
Tian, L. et al. p85α recruitment by the CD300f phosphatidylserine receptor mediates apoptotic cell clearance required for autoimmunity suppression. Nat Commun 5, 3146 (2014).
Yamanishi, Y. et al. TIM1 is an endogenous ligand for LMIR5/CD300b: LMIR5 deficiency ameliorates mouse kidney ischemia/reperfusion injury. J Exp Med 207, 1501–1511 (2010).
Zheng, W. et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758, 1864–1884 (2006).
Matsukawa, T. et al. Ceramide-CD300f binding suppresses experimental colitis by inhibiting ATP-mediated mast cell activation. Gut 65, 777–787 (2016).
Shiba, E. et al. Ceramide-CD300f binding inhibits lipopolysaccharide-induced skin inflammation. J Biol Chem 292, 2924–2932 (2017).
Ohno, Y. et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc Natl Acad Sci U S A 112, 7707–7712 (2015).
Dudeck, A. et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34, 973–984 (2011).
Drobnik, W. et al. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in septic patients. J Lipid Res 44, 754–761 (2003).
Grimbaldeston, M. A. et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol 167, 835–848 (2005).
Piliponsky, A. M. et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol 176, 926–938 (2010).
Maurer, M. et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 432, 512–516 (2004).
Piliponsky, A. M. et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis in a mouse model of sepsis. Nat Med 14, 392–398 (2008).
Kitaura, J. et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Pro Natl Acad Sci USA 100, 12911–12916 (2003).
Mócsai, A., Zhou, M., Meng, F., Tybulewicz, V. L. & Lowell, C. A. Syk is required for integrin signaling in neutrophils. Immunity 16, 547–558 (2002).
Mócsai, A., Ligeti, E., Lowell, C. A. & Berton, G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J Immunol 162, 1120–1126 (1999).
Pinheiro, daS. et al. CD16 promotes Escherichia coli sepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med 13, 1368–1374 (2007).
Walley, K. R., Lukacs, N. W., Standiford, T. J., Strieter, R. M. & Kunkel, S. L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun 64, 4733–4778 (1996).
Moreno, S. E. et al. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J Immunol 177, 3218–3224 (2006).
Tomita, T., Blumenstock, E. & Kanegasaki, S. Phagocytic and chemiluminescent responses of mouse peritoneal macrophages to living and killed Salmonella typhimurium and other bacteria. Infect Immun 323, 1242–1248 (1981).
Kitamura, T. et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol 31, 1007–1014 (2003).
Morita, S., Kojima, T. & Kitamura, T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 7, 1063–1066 (2000).
Han, C., Jin, J., Xu, S., Li, N. & Cao, X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol 8, 734–742 (2010).
Acknowledgements
We thank Hisashi Arase (Osaka University) for providing the plasmids. This study was supported by grants from the Ministry of Education, Science, Technology, Sports and Culture, Japan (JSPS KAKENHI Grant Number 23390257 and 26293231).
Author information
Authors and Affiliations
Contributions
K.I. performed all the experiments and participated in writing the manuscript. A.M., M. I., Y.Y., M.U., Y.H., K.U., T.M., M.T., A.K., E.S., A.T., S.U., K.U., K.M., N.N., Y.Y., T.O., and J.I. assisted with the experiments. D.V., A.R., and S.N. provided mice. Y.K., T.S., H.O., and K.O. analyzed the data. T.K. and J.K. conceived the project, analyzed the data, and actively participated in manuscript writing.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Izawa, K., Maehara, A., Isobe, M. et al. Disrupting ceramide-CD300f interaction prevents septic peritonitis by stimulating neutrophil recruitment. Sci Rep 7, 4298 (2017). https://doi.org/10.1038/s41598-017-04647-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04647-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.