Abstract
Recent cancer researches pay more attention to younger patients due to the variable treatment response among different age groups. Here we investigated the effectiveness of neoadjuvant radiation on the survival of younger and older patients in stage II/III rectal cancer. Data was obtained from Surveillance, Epidemiology, and End Results (SEER) database (n = 12801). Propensity score matching was used to balance baseline covariates according to the status of neoadjuvant radiation. Our results showed that neoadjuvant radiation had better survival benefit (Log-rank P = 3.25e-06) and improved cancer-specific 3-year (87.6%; 95% CI: 86.4–88.7% vs. 84.1%; 95% CI: 82.8–85.3%) and 5-year survival rates (78.1%; 95% CI: 76.2–80.1% vs. 77%; 95% CI: 75.3–78.8%). In older groups (>50), neoadjuvant radiation was associated with survival benefits in stage II (HR: 0.741; 95% CI: 0.599–0.916; P = 5.80e-3) and stage III (HR: 0.656; 95% CI 0.564–0.764; P = 5.26e-08). Interestingly, neoadjuvant radiation did not increase survival rate in younger patients (< = 50) both in stage II (HR: 2.014; 95% CI: 0.9032–4.490; P = 0.087) and stage III (HR: 1.168; 95% CI: 0.829–1.646; P = 0.372). Additionally, neoadjuvant radiation significantly decreased the cancer-specific mortality in older patients, but increased mortality in younger patients. Our results provided new insights on the neoadjuvant radiation in rectal cancer, especially for the younger patients.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death in the US. About a third of newly diagnosed CRC patients develop from the rectum, and others develop from the colon1. The genomes of some CRC patients are hypermutated and three quarters of these have highly microsatellite instability (MSI). Among the non-hypermutated tumors, colon and rectum cancers have similar patterns of genomic landscape2. Large-scale genome studies have revealed significantly intra-tumor heterogeneity and recurrent mutated genes in rectal cancer, such as APC, TP53, KRAS 2, 3. Additionally, many biomarkers such as Astrocyte elevated gene-1 (AEG-1)4, CD163 5 and clinical factors such as age6, stage7 have been proven to be associated with rectal cancer prognosis. Rectal cancer has higher risk of local recurrence than colon cancer. Therefore, multimodal therapy is required for treatment of rectal cancer.
Patients with rectal cancer that have not spread to distant sites are usually treated with surgery and additional treatment with radiation and chemotherapy may be used before or after surgery. The goal of using adjuvant radiation therapy for rectal cancer is to prevent local recurrence and mortality in patients with locally advanced tumors. Postoperative radiation therapy alone only improves local control; Additional concurrent chemotherapy to post-radiotherapy not only reduces local recurrent rate but also improves survival8. In 1990, US National Institutes for Health recommended postoperative chemoradiotherapy as standard treatment for the completely resected stage II or III rectal cancer patients in the US9. However, the treatment of rectal cancer has been changed over time with postoperative radiotherapy decreasing from 25% to 4% (1980–1988 vs. 1995–2000), while preoperative radiotherapy increasing from 1 to 35% (1980–1988 vs. 1995–2000)10. In 1994, European Consensus Conference recommended preoperative radiotherapy for patients with T3/ T4 and/or node-positive cancer11. Another recent study also shows that a significantly increase in the use of preoperative radiotherapy for rectal cancer from 1998 to 200712. Many large-scale studies prove that preoperative radiotherapy reduces the rate of local recurrence13, 14 and improves overall survival15. Compared with postoperative chemoradiotherapy, preoperative radiotherapy has better local control16, is associated with reduced toxicity14 and is a more effective treatment17.
However, previous studies did not compare the effectiveness of neoadjuvant radiotherapy between subgroups. Clinical factors such as age6 and stage7 have been proven to be associated with rectal cancer prognosis. Therefore, important prognostic clinical factors are often used to stratify patients. For example, in age aspect, CRC screening standard is recommended for adults above 50 years since 199618. Recently studies find the incidence rate of CRC among adults younger than 50 years is increasing19, 20 which is in sharp contrast to overall incidence trend among adults above 50 years21. Additionally, different prognostic molecular biomarkers such as PRL, RBM3 in younger patients6, clinical risk factors such as varied distribution of stage, histological type, grade19, 22, 23 and different responses to the same treatment24 have been shown between two age subgroups. Therefore, it is important to investigate the effectiveness of neoadjuvant radiation in different subgroups.
Survival rate is commonly used to determine the treatment advances. To estimate survival rate, clinical characteristics are recoded and analyzed in randomized trials14, 25 or in population based studies26. The public database such as Surveillance, Epidemiology, and End Results (SEER) provides clinical data for different cancer cases, which is a valuable resource for survival analysis. Since SEER is a population-based cancer registry, controlling treatment selection bias can get a more reliable results27. Recently propensity score methods have been widely used to adjust baseline covariates, which can control the treatment selection bias. Propensity score is the probability of a subject receiving the treatment of interest conditional on their observed baseline covariates28. Through propensity score matching, 98% of the treatment bias are removed and an unbiased estimate of treatment effect is achieved in retrospective cohorts29.
Here we aimed to investigate the effectiveness of neoadjuvant radiation in younger and older rectal cancer patients. Clinical data was obtained from SEER, and propensity score matching was used to balance patients with or without neoadjuvant radiation. The effect of neoadjuvant radiation on survival was analyzed among patients mainly stratified by age at diagnosis. Our results might reinforce the consideration of younger patients when receiving neoadjuvant radiation.
Method
Data preprocessing
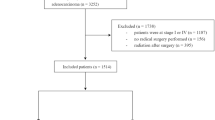
We signed an agreement for the SEER 1973–2012 research data and all clinical data were approved to use by SEER database. Then we downloaded the classified data of colorectal cancers from SEER database (1973~2012), and used R package SAScii to decode the original data into clinical information. In all CRC patients, we restricted our analysis to rectal cancer patients whose death was caused by cancer, date of diagnosis was after 2007, tumor grade was stage II or III, and treatment strategy was surgery alone or radiotherapy before surgery.
Statistical analysis
Propensity score matching
We defined propensity score as the probability of patients being in the neoadjuvant radiation group. Propensity score model is preferable to include prognostically important covariates than these that affecting the treatment-selection process30. Firstly, we select these important prognostic factors: tumor size31, histological type32, differentiation31, age6, number of harvest lymph node33, 34, tumor stage7. Secondly, some confounding variables which included in previous CRC6, 32, 35 or propensity score based research27 and might be related with treatment and outcome were selected. Finally, in combination with records in SEER, nine covariates: race, gender, age, tumor size, tumor number, stage, histological type, grade, lymph node resected were used to build propensity score models. The propensity scores were calculated by a nonparsimonious multivariable logistic regression model, which used neoadjuvant radiotherapy as the outcome of interest and nine clinical features as covariates. A nearest neighbor and 1 to 1 matching algorithm was performed within default caliper (0.2) in SPSS36. Standardized difference (S.D) between neoadjuvant radiation and no-neoadjuvant radiation patients was calculated for every baseline covariate to test corresponding balance29. S.D before and after matching was compared to see whether the S.D was changed to less than 0.1, which indicated a good balance for matched data30. A sensitivity analysis was performed to test the robustness of matched data using the R package rbounds37. R packages powerSurvEpi38, 39 was used to estimate the sample size necessary for 80% statistically power.
Cancer specific survival curve and Cox model construction
In propensity score matched SEER dataset, survival time was calculated from the date of diagnosis to death or to the end of study. Patients who were lost followed-up or still alive at the end of study were censored. Cancer-specific survival curve was generated using Kaplan–Meier estimate, and difference between different treatment groups were analyzed by log-rank test. Additionally, 3-year and 5-year cancer specific survival rates were calculated for neoadjuvant radiation and no-neoadjuvant radiation groups. Cox proportional hazards regression model which contained all measured covariates was performed and hazard ratio (HR) was calculated to assess the importance of neoadjuvant radiotherapy and other covariates. To assess the effects of unmeasured clinical variables on HR, sensitivity analysis40,41,42 was performed by R package obsSens.
All Survival rate, HR and risk ratio (RR) were reported with their corresponding 95% CI. All statistical test were two-sided and a P value of less 0.05 was considered to be statistically significant.
Patient stratification by clinical covariates
Based on the results of Cox model, we selected clinical covariates associated with neoadjuvant radiotherapy. We grouped patients by each covariate, performed cancer specific survival analysis and constructed Cox model in each subgroup.
Effect of age on neoadjuvant treatment analysis
We divided patients into younger and older groups. For each group, annual age-adjusted incidence rates (using the 2000 U.S. standard population) were calculated of rectal cancer per 100,000 from 1992 to 2012 using SEER*Stat software version 8.3.443, 44. Fisher’s exact test was used to compare the distribution of every clinical variable item between younger and older patients. Cancer specific survival curve and Cox proportional hazards regression were performed to evaluate the effectiveness of neoadjuvant radiotherapy. HR was calculated to assess the importance of neoadjuvant radiotherapy and also 3, 5 year survival rates were calculated in different treatment groups. The RR was calculated in different time interval for younger and older groups. We further combined age and stage to stratify patients, and investigate the effectiveness of neoadjuvant radiotherapy in four subgroups.
Results
Data source and propensity score matching
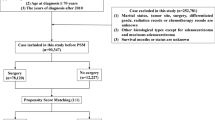
We performed a comprehensive study to access the impact of neoadjuvant radiation on survival in rectal cancer patients (Fig. 1). Firstly, clinical data of 545474 CRC patients were obtained from the SEER database. Stage-specific survivals were significantly different in five stages, which was consistent with a previous study7. Adults over age 50 are high-risk population of CRC who are recommended to receive CRC screening. Our data also showed that 80% rectal patients were above age 50 (see Supplementary Fig. 1AB). We also calculated annual age-adjusted incidence rate per 100,000 between younger and older groups for rectal cancer during 1992~2012. Trend of incidence rate decreased in adults over age 50, while rose for adults less 50 (see Supplementary Fig. 1C), which was consistent with the trend in previous CRC studies19, 20. Therefore, age 50 was used to divide patients into younger (age < = 50, n = 2511) and older groups (age >50, n = 10290). Totally, There were 12801 rectal patients in stage II or III, or whom 6117 (48.25%) had neoadjuvant radiation before surgery since 2007. Their clinical characteristics were summarized in Table 1.
There were systematic differences in baseline characteristics between neoadjuvant radiation and no-neoadjuvant radiation patients in the overall samples. The S.Ds of gender, age, lymph node resected etc were larger than 0.1 between neoadjuvant radiation and no-neoadjuvant radiation patients which means the distribution of those items were unbalanced (Table 1 left). Therefore, a propensity score model was built to get a similar distribution of these measured baseline covariates. After propensity score matching, the standardized differences of all covariates were less than or close to 0.1 (Table 1 right), which indicated the covariates were balanced between two treatment groups. This balanced cohort totally had 9968 (77.8%) patients, and the number of two group patients were same (n = 4984). Furthermore, we did the sensitivity analysis to test whether the matched dataset was robust to clinical variables that were not considered in the propensity score model. The results showed our matched data was robust to the unconfouned variants (Γ = 7, P = 2.1e-05), as described in previous studies37. To determine whether the sample size was enough to reach a power of 0.8, we estimated the number of patients for survival analysis and the number of deaths for Cox model. Result showed that at least 1096 patients and 302 deaths were needed in each treatment subgroup. Therefore, our propensity score matched dataset (4984 in each group) could achieve higher power.
Cancer-specific survival analysis and Sensitivity analysis
Survival benefit of neoadjuvant radiation
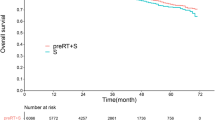
In the propensity score matched cohort, the median follow-up time was 27 months (ranged 0–71 months). The 3-year and 5-year cancer-specific survival (CSS) rates were 87.6%(95% CI: 86.4–88.7%), 78.1%(95% CI: 76.2–80.1%) in neoadjuvant radiation group and 84.1%(95% CI: 82.8–85.3%), 77%(95% CI: 75.3–78.8%) in no-neoadjuvant radiation group. The CSS rates of neoadjuvant radiation group was significantly better than no-neoadjuvant group (Fig. 2A, Log-rank P = 3.25e-06). The neoadjuvant radiotherapy was associated with reduced disease-specific mortality at 6 months (RR, 0.25; 95% CI, 0.18–0.35), 1 year (RR, 0.44; 95% CI, 0.35–0.55) and 2 year (RR, 0.59; 95% CI, 0.50–0.69). In order to investigate how the clinical factors jointly affected survival, multivariable proportional Cox model was constructed. When considering all clinical factors together, neoadjuvant radiation also achieved a significantly disease-specific survival benefit (HR, 0.741; 95% CI: 0.646–0.811; P = 2.27e-07). Additionally, grade, number of harvested lymph nodes, histological types, tumor size, stage and age also had significant effect on survival (Fig. 2B).
Comparison between neoadjuvant (n = 4984) and no-neoadjuvant groups (n = 4984) in the propensity score matched cohort. (A) Kaplan-Meier curves for cancer-specific survival. (B) HR of every clinical covariate from Cox model. (C) Sensitivity analysis estimating the effect of unmeasured confounders on hazard ratio. Red star means significantly render treatment effect (the lower confidence bound of CI is larger than 1).
Although Cox model included 23 clinical items that presumably captured most of potential cofounding, some unknown or unmeasured covariates associated with both treatment and outcome still existed. In order to eliminating the effect of unmeasured covariates, we did a sensitivity analysis for the Cox model. Supposing there existed an unmeasured covariant, we changed its parameters (unmeasured confounder HR, prevalence in neoadjuvant group, and prevalence in no radiotherapy group) to see whether we could get similar conclusion for neoadjuvant radiation (Fig. 2C). In most conditions, the unmeasured covariant did not change our conclusion (yellow). The opposite effect of neo-radiotherapy only occurred in some extreme conditions (red). For example, unmeasured confounders whose HR was 2.0 or greater and the prevalence in no radiotherapy group was larger than 0.5. Thus, the beneficial effect of radiotherapy was robust to departures from ignobility.
Impact of neoadjuvant radiation on survival in different clinical subgroups
Then we grouped patients according to the clinical covariates, and compared the impact of neoadjuvant radiation on survival in different subgroups. Cancer specific survival and Cox model showed that neoadjuvant radiation played significant roles in all stage-related and age-related subgroups, but it did not significantly affect all subgroups of other clinical covariates (see Supplementary Fig. 2A1,D2, Supplementary Table S1).
In both stage II (Fig. 3A) and III (Fig. 3B) patients, the cancer specific survival of neoadjuvant radiation was significantly better than in the no-neoadjuvant group (Log rank P = 0.048, 1.29e-05). In stage II patients (n = 4264), the 3-year and 5-year survival rates were 90.6% (95% CI: 89–92.2%) and 84.45% (95% CI: 81.96–87.02%) in neoadjuvant group; 89% (95% CI: 87.4–90.6%), 84.48% (95% CI: 82.29–86.72%) in no-neoadjuvant group. In stage III patients (n = 5704), the 3-year and 5-year survival rates were 85.2% (95% CI: 83.6–86.9%) and 73.3% (95% CI: 70.48–76.2%) in neoadjuvant group; 80.2% (95% CI: 78.4–82.1%), 71.2% (95% CI: 68.67–73.83%) in no-neoadjuvant group. The multivariable Cox model also showed a significant CSS benefits for neoadjuvant radiation in both stage groups (Fig. 3C, 0.741, 0.656; 95% CI: 0.609–0.917, 0.615–0.810; P = 0.0213, 3.29e-06).
Impact of neoadjuvant radiation on survival in younger and older patients
Different from stage-subgroups, neoadjuvant radiation had opposite roles in two subgroups of age. Neoadjuvant radiation improved survival in older patients (P < 1.06e-08, see Supplementary Fig. 2E2), but no-neoadjuvant group had better survival than neoadjuvant group in younger patients (P = 0.04, see Supplementary Fig. 2E1). In order to confirm the opposite role of neoadjuvant radiation on age, we further excluded the impact of other covariates by using propensity score matching in age-related groups. Since the standardized differences of all covariates were already balanced well in older group (see Supplementary Table S2 right), there was no need to do propensity score matching. One covariate deviated from the balance status in younger group (see Supplementary Table S2 left), therefore we did propensity score matching in younger group and got a new dataset (see Supplementary Table S2 middle part). Survival analysis in this new dataset showed the same trend as the original data (Log rank younger: P = 0.022, older: P < 1.06e-08, Fig. 4A/B). In younger group (n = 1846), 3-year and 5-year survival rates were 91.5% (95% CI: 89.29–93.81%), 87.4% (95% CI: 84.34–90.58%) in no-neoadjuvant group, versus 88.05% (95% CI: 85.44–90.74%) and 79.42% (95% CI: 75.18–83.39%) in neoadjuvant group. In older group (n = 7985), 3-year and 5-year survival rates were 82.3% (95% CI: 80.8–83.7%), 74.41% (95% CI: 72.37–76.5%) in no-neoadjuvant group, versus 87% (95% CI: 85.6–88.3%) and 77.72% (95% CI: 75.53–79.98%) in neoadjuvant group. Then we adjusted other covariates by Cox proportional model and got the same results: neoadjuvant radiation had a survival benefit in older group (HR: 0.662; 95% CI: 0.586–0.749; P = 1.39e-09) while not in younger group (HR: 1.294; 95% CI: 0.946–1.770; P = 0.105). Furthermore, we calculated the disease-specific mortality rate in different year interval. For younger group, the RR between neoadjuvant radiation and no-neoadjuvant group was less than 1 in the first two years, but it increased to more than 1 since the 3rd-year. Neoadjuvant radiation was significantly associated with increased disease-specific mortality in the 6th-years. However, in older group neoadjuvant radiation was associated with reduced disease-specific mortality significantly (RR < 1) (Fig. 4C). We noted that cancer specific survival and mortality rate after 3 years were slightly different from that within 3 years (Figs 2A, 3A,B and 4C). To investigate this difference thoroughly, we split patients into short-term (<3 years) group and long-term (> = 3years) group. Result showed that neoadjuvant radiation improved short-term survival in both younger and older patients, especially for older patients. However, there was no long-term survival benefit in older group and younger group, especially for younger group (see Supplementary Fig. 3). Additionally, based on 3th year survival we calculated the 5 year conditional survival which was a kind of more accurate quantification of prognosis for long-term survivors7. Result showed that neoadjuvant group has poorer 5 year conditional survival than no-neoadjuvant group (28.43% vs. 30.05%) in younger patients and neoadjuvant group had better 5 year conditional survival than no-neoadjuvant group (26.62% vs. 27.08%) in older patients. The underlying reasons for this long term and short term difference might be variable. Previous study shows that in younger patients who develop the local recurrence die faster than older patients45. One possible reason for this might be that in younger patients neoadjuvant radiation group had higher local recurrence rate than no-neoadjuvant radiation group. So in the long term when both groups developed distant metastases and died of disease, neoadjuvant group had poorer survival. However, in older group, although neoadjuvant radiation had better ability to control the local recurrence in the short term, in the long term they had similar local recurrence rate and survival rate.
Survival curves, RR and stage distribution in younger and older groups. (A) Kaplan-Meier curves for CSS between neoadjuvant (n = 923) and no-neoadjuvant groups (n = 923) in younger patients. (B) Kaplan-Meier curves for CSS between neoadjuvant (n = 3957) and no-neoadjuvant groups (n = 4028) in older patients. (C) Risk ratio of different year intervals in younger and older groups (* means P < 0.05 compared with its own control in younger or older groups). (D) Percentage of stage distribution in younger and older groups. (** means P < 0.01 compared with older group).
Additionally, we analyzed the difference of every covariate between two age groups. Fisher’s exact test showed that there were significantly different distributions in gender, tumor number, stage, tumor size, lymph nodes resected, histological type and grade between younger group and older group (see Supplementary Table S3), which in same covariates was consistent with previous research19, 23. Tumors of younger patients tended to be in later stage (Fig. 4D). Since both age and stage were significantly associated with the benefit of neoadjuvant radiation, we further split patients by age and stage to investigate their combination effects. Survival curves were constructed for four subgroups. In younger group (Fig. 5A,B), neoadjuvant radiation did not have survival benefit no matter of the stages, and this trend was worse in stage II (Log-rank P = 0.049, P = 0.163). In older group (Fig. 5C,D), neoadjuvant radiation have survival benefit no matter of the stages, and this trend was better in stage III (Log-rank P < 1.06e-07, P = 0.013). Finally, we calculated the neoadjuvant radiation’s HRs in four groups (Table 2). Result indicated that neoadjuvant radiation played different roles in different sub-groups. Patients in later stage of disease and in older group tended to obtain more survival benefits from the neoadjuvant radiation.
Discussion
In the present study, we used propensity score matching to get a large baseline covariates balanced cohort of rectal cancer, and investigated effectiveness of neoadjuvant radiotherapy on survival. And Cox model confirmed previous reported significant prognostic factors (Fig. 2B, see Supplementary Table S4), such as tumor size31, histological type32, differentiation31, age6, number of harvest lymph node33, 34. In all results, neoadjuvant radiation had cancer specific survival benefit not only in the whole cohort but also in different stage subgroups. Previous studies shows that stage III patients tend to have more survival benefits from neoadjuvant/adjuvant therapy compared with stage II15, 26, which was consistent with our result. However, neoadjuvant radiotherapy had opposite survival benefit in younger and older groups.
We analyzed the effectiveness of neoadjuvant radiotherapy according to age. Neoadjuvant radiotherapy in younger group showed increasing survival risk. From our disease-specific mortality analysis, neoadjuvant radiotherapy was associated with increase disease-specific mortality starting from 3 years (Fig. 4C). Recently the incidence of CRC tends to be increasing among adults younger than 50 years19, and previous studies shows that rectal and distal colon are identified as predilection location in younger CRC patients19, 23. Age is an important factor when considering the treatment for patients24. Therefore they suggests starting rectal cancer screening before age 5046. These studies showed that more attention has been paid to younger patients. Our results on neoadjuvant radiotherapy also suggested that younger patients should be distinguished from older patients when making treatment decision.
Since this work was based on population-based cancer registry, there exist some limitations. Population study has limited internal validity compared with randomized controlled trials (RCTs), exists many confounding variables and study design is limited47. Many pitfalls exist in population-based state cancer registry data, such as: multiple cancer diagnoses, duplicate reports, reporting delays, misclassification of race/ethnicity, and pitfalls in estimations of cancer incidence rates, etc48. So analysis results based on population-based study are prone to multiple biases. In this study, we adopted propensity score matching method to mitigate some potential sources of biases. After matching, sensitivity analysis showed (Fig. 2C) that unmeasured confounders did not influence our conclusions except their hazard ratio higher than 1.3. Such result was similar with previous study27, 41 and indicated the robustness of our results.
Although many covariates had being considered in our analysis, some important clinical factors and prognostic biomarkers were not included due to the lack of information in SEER database, such as: MSI, expression of biomarkers, local recurrence, details of radiotherapy such as dose, field, radiation treatment technique etc. Previous study shows that different age group (< = 50, >50) has different prognostic biomarkers, such as the PRL, RBM3, Wrap53, p53 and DNA status. PRL expression was negatively related with CSS in young CRC patients6. Receiving neoadjuvant radiotherapy, strong PRL expression and FXYD-3 expression is unfavorable prognostic in patients49, 50. Some other genes has been assessed the predictor’s role in neoadjuvant radiochemotherapy such as p21, VEGF etc51. Besides, Previous research finds MSI is very important good marker of prognosis in stage II/III CRC52. A former study showed that MSI could not predict therapeutic response to neoadjuvant radiotherapy53. However, this relationship has not fully demonstrated. In younger and older rectal patient groups, there is different percentage of MSI and different studies has different MSI percentage in younger and older rectal patients54, 55. Besides, in younger patients, 2–7% MSI are associated with Lynch syndrome56. Apart from these biomarkers, local recurrence is also an important prognostic factor and clinical indicator for quality of neoadjuvant treatment in rectal cancer. Local recurrence is an painful event for rectal cancer patients and limited success is achieved by further salvage surgery treatment57. A study of cohort who underwent R0 or R1 resections showed that patients die faster if diagnosing with local recurrence and local recurrence is the single most important indicator for reduced survival in this study45. Neoadjuvant radiation can improve local control for rectal cancer conclusively15, 58. Different neoadjuvant regimens followed by different surgery has different local control rate, and the standard care for stage II and stage III rectal cancer is neoadjuvant radiotherapy followed by Total Mesorectal Excision (TME), which leads to a decrease in local recurrence rates to 6%25. Other study shows that short-course preoperative radiotherapy can reduce local recurrence and can maintain this ability when combined with TME59. A randomized study showed that short-course and long-course neoadjuvant radiotherapy are not statistically significant in local recurrence rate13.
In future, we would like to do more studies about neoadjuvant radiotherapy. Firstly, results obtained from our population-based study are needed to be validated by more comprehensive and accurate data. Therefore, we expect to analyze data from randomized clinical trials via scientific collaboration. Secondly, we would like to explore the molecular-level differences between younger and older patients before and after neoadjuvant radiation. This may help our understanding of the different effects of neoadjuvant radiotherapy according to age. Thirdly, we will investigate the challenging problem of predicting response to neoadjuvant radiotherapy. We hope find an optimal radiotherapy plan for individual patient by combining clinical factors and molecular markers.
In conclusion, our study provided new insights on the neoadjuvant radiation in rectal cancer, especially for the younger patients; And provided reliable information to guide future knowledge translation of neoadjuvant radiation in younger patients, especially for long term ineffectiveness of neoadjuvant radiation in younger patients. Additionally, our results provided evidence of effectiveness of neoadjuvant radiotherapy for older patients, especially in short term cancer specific survival benefit.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J Clin 66, 7–30, doi:10.3322/caac.21332 (2016).
Muzny, D. M. et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–7, doi:10.1038/nature11252 (2012).
Hardiman, K. M. et al. Intra-tumor genetic heterogeneity in rectal cancer. Lab Invest 96, 4–15, doi:10.1038/labinvest.2015.131 (2016).
Gnosa, S. et al. AEG-1 expression is an independent prognostic factor in rectal cancer patients with preoperative radiotherapy: a study in a Swedish clinical trial. Br J Cancer 111, 166–173, doi:10.1038/bjc.2014.250 (2014).
Shabo, I., Olsson, H., Sun, X.-F. & Svanvik, J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. International Journal of Cancer 125, 1826–1831, doi:10.1002/ijc.24506 (2009).
Wang, M. J. et al. The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci Rep 5, 10645, doi:10.1038/srep10645 (2015).
Wang, S. J., Fuller, C. D., Emery, R. & Thomas, C. R. Conditional Survival in Rectal Cancer: A SEER Database Analysis. Gastrointest Cancer Res 1, 84–89 (2007).
Krook, J. E. et al. Effective Surgical Adjuvant Therapy for High-Risk Rectal Carcinoma. New England Journal of Medicine 324, 709–715, doi:10.1056/NEJM199103143241101 (1991).
Hall, W. H. Adjuvant therapy for patients with colon and rectal cancesr. JAMA 264, 1444–1450, doi:10.1001/jama.1990.03450110090034 (1990).
Martijn, H. et al. Improved survival of patients with rectal cancer since 1980: a population-based study. European Journal of Cancer 39, 2073–2079, doi:10.1016/S0959-8049(03)00493-3 (2003).
Hay, J. & Msika, S. Radiotherapy and chemotherapy in the treatment of cancer of the rectum. Journal de chirurgie 137, 13–15 (2000).
Fitzgerald, T. L., Biswas, T., O’Brien, K., Zervos, E. E. & Wong, J. H. Neoadjuvant Radiotherapy for Rectal Cancer: Adherence to Evidence-Based Guidelines in Clinical Practice. World Journal of Surgery 37, 639–645, doi:10.1007/s00268-012-1862-z (2013).
Ngan, S. Y. et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 30, 3827–3833, doi:10.1200/JCO.2012.42.9597 (2012).
Sauer, R. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine 351, 1731–1740, doi:10.1056/NEJMoa040694 (2004).
Camma, C. et al. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA 284, 1008–1015, doi:10.1001/jama.284.8.1008 (2000).
Sauer, R. et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 30, 1926–1933, doi:10.1200/JCO.2011.40.1836 (2012).
Sebag-Montefiore, D. et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. The Lancet 373, 811–820, doi:10.1016/S0140-6736(09)60484-0 (2009).
Force, U. P. S. T. Guide to clinical preventive services: report of the US Preventive Services Task Force (Lippincott Williams & Wilkins, 1996).
You, Y. N., Xing, Y., Feig, B. W., Chang, G. J. & Cormier, J. N. Young-onset colorectal cancer: is it time to pay attention? Archives of internal medicine 172, 287–289, doi:10.1001/archinternmed.2011.602 (2012).
Siegel, R. L., Jemal, A. & Ward, E. M. Increase in Incidence of Colorectal Cancer Among Young Men and Women in the United States. Cancer Epidemiology Biomarkers & Prevention 18, 1695–1698, doi:10.1158/1055-9965.EPI-09-0186 (2009).
Edwards, B. K. et al. Annual report to the nation on the status of cancer, 1975‐2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116, 544–573, doi:10.1002/cncr.24760 (2010).
Marble, K., Banerjee, S. & Greenwald, L. Colorectal carcinoma in young patients. Journal of Surgical Oncology 51, 179–182, doi:10.1002/(ISSN)1096-9098 (1992).
O’Connell, J. B., Maggard, M. A., Livingston, E. H. & Yo, C. K. Colorectal cancer in the young. American journal of surgery 187, 343–348, doi:10.1016/j.amjsurg.2003.12.020 (2004).
Rutten, H. J., den Dulk, M., Lemmens, V. E., van de Velde, C. J. & Marijnen, C. A. Controversies of total mesorectal excision for rectal cancer in elderly patients. The Lancet. Oncology 9, 494–501, doi:10.1016/S1470-2045(08)70129-3 (2008).
van Gijn, W. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. The Lancet. Oncology 12, 575–582, doi:10.1016/S1470-2045(11)70097-3 (2011).
Neugut, A. I. et al. Use of adjuvant chemotherapy and radiation therapy for rectal cancer among the elderly: a population-based study. Journal of clinical oncology 20, 2643–2650, doi:10.1200/JCO.2002.08.062 (2002).
Sugawara, A. & Kunieda, E. Effect of adjuvant radiotherapy on survival in resected pancreatic cancer: a propensity score surveillance, epidemiology, and end results database analysis. J Surg Oncol 110, 960–966, doi:10.1002/jso.23752 (2014).
d’Agostino, R. B. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17, 2265–2281, doi:10.1002/(ISSN)1097-0258 (1998).
Austin, P. C. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Statistics in medicine 33, 1242–1258, doi:10.1002/sim.5984 (2014).
Austin, P. C., Grootendorst, P. & Anderson, G. M. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Statistics in medicine 26, 734–753, doi:10.1002/sim.2580 (2007).
Safaee, A., Moghimi_dehkordi, B., Fatemi, S., Ghiasi, S. & Zali, M. Pathology and prognosis of colorectal cancer. Iranian Journal of cancer prevention 2, 137–141 (2012).
Hugen, N. et al. Prognosis and value of adjuvant chemotherapy in stage III mucinous colorectal carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology 24, 2819–2824, doi:10.1093/annonc/mdt378 (2013).
Cianchi, F. et al. Lymph node recovery from colorectal tumor specimens: recommendation for a minimum number of lymph nodes to be examined. World journal of surgery 26, 384–389, doi:10.1007/s00268-001-0236-8 (2002).
Tsai, H.-L. et al. The prognostic significance of total lymph node harvest in patients with T2–4N0M0 colorectal cancer. Journal of Gastrointestinal Surgery 11, 660–665, doi:10.1007/s11605-007-0119-x (2007).
Fitzgerald, T. L., Biswas, T., O’Brien, K., Zervos, E. E. & Wong, J. H. Neoadjuvant radiotherapy for rectal cancer: adherence to evidence-based guidelines in clinical practice. World J Surg 37, 639–645, doi:10.1007/s00268-012-1862-z (2013).
Thoemmes, F. Propensity score matching in SPSS. arXiv preprint arXiv:1201.6385 (2012).
Keele, L. An overview of rbounds: An R package for Rosenbaum bounds sensitivity analysis with matched data. White Paper. Columbus, OH 1–15 (2010).
Freedman, L. Tables of the number of patients required in clinical trials using the logrank test. Statistics in medicine 1, 121–129, doi:10.1002/(ISSN)1097-0258 (1982).
Rosner, B. Fundamentals of biostatistics (Nelson Education, 2015).
Lin, D. Y., Psaty, B. M. & Kronmal, R. A. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 54, 948–963, doi:10.2307/2533848 (1998).
Mitra, N. & Heitjan, D. F. Sensitivity of the hazard ratio to nonignorable treatment assignment in an observational study. Stat Med 26, 1398–1414, doi:10.1002/sim.2606 (2007).
Baer, V. et al. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. Journal of Perinatology 27, 790–796, doi:10.1038/sj.jp.7211833 (2007).
Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version <8.3.4>.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission.
Platell, C. & Spilsbury, K. Influence of local recurrence on survival in patients with rectal cancer. ANZ Journal of Surgery 84, 85–90, doi:10.1111/ans.12214 (2014).
Davis, D. M. et al. Is it time to lower the recommended screening age for colorectal cancer? Journal of the American College of Surgeons 213, 352–361, doi:10.1016/j.jamcollsurg.2011.04.033 (2011).
Booth, C. M. & Tannock, I. F. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer 110, 551–555, doi:10.1038/bjc.2013.725 (2014).
Izquierdo, J. N. & Schoenbach, V. J. The potential and limitations of data from population-based state cancer registries. Am J Public Health 90, 695–698, doi:10.2105/AJPH.90.5.695 (2000).
Wallin, Å. R., Svanvik, J., Adell, G. & Sun, X.-F. Expression of PRL proteins at invasive margin of rectal cancers in relation to preoperative radiotherapy. International Journal of Radiation Oncology* Biology* Physics 65, 452–458, doi:10.1016/j.ijrobp.2005.12.043 (2006).
Loftås, P. et al. Expression of FXYD-3 is an independent prognostic factor in rectal cancer patients with preoperative radiotherapy. International Journal of Radiation Oncology* Biology* Physics 75, 137–142, doi:10.1016/j.ijrobp.2008.10.076 (2009).
Smith, F. M., Reynolds, J. V., Miller, N., Stephens, R. B. & Kennedy, M. J. Pathological and molecular predictors of the response of rectal cancer to neoadjuvant radiochemotherapy. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 32, 55–64, doi:10.1016/j.ejso.2005.09.010 (2006).
Mouradov, D. et al. Survival in stage II/III colorectal cancer is independently predicted by chromosomal and microsatellite instability, but not by specific driver mutations. The American journal of gastroenterology 108, 1785–1793, doi:10.1038/ajg.2013.292 (2013).
Du, C., Zhao, J., Xue, W., Dou, F. & Gu, J. Prognostic value of microsatellite instability in sporadic locally advanced rectal cancer following neoadjuvant radiotherapy. Histopathology 62, 723–730, doi:10.1111/his.12069 (2013).
Liang, J. et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. British journal of surgery 90, 205–214, doi:10.1002/bjs.4015 (2003).
Yantiss, R. K. et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol 33, 572–582, doi:10.1097/PAS.0b013e31818afd6b (2009).
Boland, C. R. & Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 138, 2073–2087, 10.1053/j.gastro.2009.12.064, e2073 (2010).
Alberda, W. J. et al. Outcome in patients with resectable locally recurrent rectal cancer after total mesorectal excision with and without previous neoadjuvant radiotherapy for the primary rectal tumor. Ann Surg Oncol 21, 520–526, doi:10.1245/s10434-013-3306-x (2014).
Colorectal Cancer Collaborative, G. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 358, 1291–1304, doi:10.1016/S0140-6736(01)06409-1 (2001).
Kapiteijn, E. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. The New England journal of medicine 345, 638–646, doi:10.1056/NEJMoa010580 (2001).
Acknowledgements
We appreciate critical reading and valuable comments from Jie Ping. And we appreciate SEER database for providing clinical data. This work was supported by the National Key Research and Development Program on Precision Medicine (2016YFC0901700), National Natural Science Foundation of China (31501077) and National Grand Program on Key Infectious Diseases (2015ZX10004801-005).
Author information
Authors and Affiliations
Contributions
L.L.W. downloaded data and performed propensity score matching analysis. L.L.W., S.C.P. and Q.L.Y. performed survival analysis. C.J. provided clinical thoughts. P.L. and F.Y.M.F. prepared Figures. H.L. and Y.X.L. conceived and supervised the experiments. L.L.W. wrote the main manuscript text with contributions from all authors.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, L., Pang, S., Yao, Q. et al. Population-based study of effectiveness of neoadjuvant radiotherapy on survival in US rectal cancer patients according to age. Sci Rep 7, 3471 (2017). https://doi.org/10.1038/s41598-017-02992-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02992-7
This article is cited by
-
Effect of adjuvant chemotherapy on the oncological outcome of rectal cancer patients with pathological complete response
World Journal of Surgical Oncology (2024)
-
Prognostic Factors of Rectal Cancer in Southern Iran
Journal of Gastrointestinal Cancer (2022)
-
Cardiovascular diseases in survivors of childhood cancer
Cancer and Metastasis Reviews (2020)
-
Comparative survival analysis of preoperative and postoperative radiotherapy in stage II-III rectal cancer on the basis of long-term population data
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.