Abstract
Decreasing coral cover on the Great Barrier Reef (GBR) may provide opportunities for rapid growth and expansion of other taxa. The bioeroding sponges Cliona spp. are strong competitors for space and may take advantage of coral bleaching, damage, and mortality. Benthic surveys of the inshore GBR (2005–2014) revealed that the percent cover of the most abundant bioeroding sponge species, Cliona orientalis, has not increased. However, considerable variation in C. orientalis cover, and change in cover over time, was evident between survey locations. We assessed whether biotic or environmental characteristics were associated with variation in C. orientalis distribution and abundance. The proportion of fine particles in the sediments was negatively associated with the presence-absence and the percent cover of C. orientalis, indicating that the sponge requires exposed habitat. The cover of corals and other sponges explained little variation in C. orientalis cover or distribution. The fastest increases in C. orientalis cover coincided with the lowest macroalgal cover and chlorophyll a concentration, highlighting the importance of macroalgal competition and local environmental conditions for this bioeroding sponge. Given the observed distribution and habitat preferences of C. orientalis, bioeroding sponges likely represent site-specific – rather than regional – threats to corals and reef accretion.
Similar content being viewed by others
Introduction
Loss of coral cover has led to dire predictions for the future of coral reef ecosystems1,2,3, including the Great Barrier Reef (GBR)4. A number of processes compromise coral health and the broader health of coral reefs, including increased sea surface temperatures, ocean acidification, pollution, cyclones, and crown of thorns starfish outbreaks4, 5. All of these stressors are predicted to intensify over coming decades, potentially shifting the coral reef benthic community from coral-dominated systems to those dominated by less-sensitive species6, 7. Some community changes have already been documented on coral reefs, including changes along acidification gradients at CO2 seeps8 and the octocoral and sponge dominance of shallow habitat of the Florida Keys, USA9, 10.
Changes to reef communities may reduce reef accretion, which represents the balance of calcification and consolidation with erosional processes11. Increased abundance of eroding organisms (bioeroders) or decreased abundance of calcifying organisms already suggest that some reefs are eroding rather than growing12, 13. Bioeroding sponges break down coral skeleton and other calcium carbonate structures, oyster shells, and cave walls. The sponges grow several mm to several cm into the coral skeleton and some species can quickly overgrow adjacent live coral tissue14. While sponges erode calcium carbonate at fast rates15,16,17, bioeroding sponges are patchily distributed, which currently limits their impact on regional carbonate budgets13, 18.
In some locations, bioeroding sponges (mostly Cliona spp.) have recently increased in abundance19,20,21,22. While these reports are largely restricted to single reefs, the rates of increase are notable: Cliona caribbaea cover doubled between 1979 and 1998 at one location in Belize19 and Cliona spp. abundance doubled between 1996 and 2001 in the Florida Keys, USA21. In addition, the abundance of Cliona orientalis more than doubled between 1998 and 2004 at one location in Queensland, Australia20. These changes gave rise to the hypothesis that the abundance of bioeroding sponges may be increasing over time, but the geographic extent and rate of these increases are largely unknown.
Several physiological and ecological hypotheses have been proposed to explain the observed increases in abundance of bioeroding sponges. Cliona is thought to be a robust sponge genus that is tolerant of disturbances and changing environmental conditions23,24,25,26 as well as benefitting from the poor water quality that can adversely affect corals21, 27. Based on the success of Cliona spp. in similar habitats, the inshore GBR was expected to be optimal habitat for bioeroding sponges where increases in cover may be occurring throughout the region20. However, poor water quality is also associated with low light conditions that may negatively impact growth of photo-symbiotic bioeroding sponges such as C. orientalis and C. varians 28, 29.
Increases in the abundance of bioeroding sponges will have implications for coral reefs in addition to the erosion of substratum19. Bioeroding sponges weaken reef substrata, produce carbonate sediments11, 30, 31, and are strong competitors against live corals19, 32,33,34,35,36,37, particularly following coral bleaching events38. However, the growth of Cliona spp. can be limited by macroalgae32, 39, suggesting that the composition of the reef community may influence the success of Cliona.
Given that sponge erosion is expected to accelerate as oceans become more acidic18, 25, 40, 41, there is a clear need to monitor bioeroding sponge populations42, 43. The most conspicuous bioeroding sponge on the GBR is Cliona orientalis but percent cover has only been reported for a single GBR site20, 43, 44. Here, we quantified the abundance and trajectory of C. orientalis cover on the inshore GBR over a 10-year period (2005–2014) to resolve whether environmental conditions are drivers of change in sponge abundance. Our sampling covers a wide geographic area to assess whether previous reports of increasing Cliona abundance represent a GBR-wide trend or site-specific responses20.
Results and Discussion
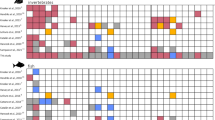
C. orientalis was present in at least three survey years at 16 of the 35 inshore GBR locations. Where present, C. orientalis occupied as much surface substratum (0.73% ± 0.97 SD) as all other sponges combined (0.56% ± 1.11 SD). Havannah Island had the highest average cover at 3.6% (Fig. 1A), although C. orientalis cover reached as high as 5% at Fitzroy Island and High Island in certain years (Fig. 2). C. orientalis percent cover was lower than previously reported from Orpheus Island (>6%)20, possibly due to a greater area surveyed or the untargeted design in the current study. When absences are included (i.e., zero cover), the average percent cover of C. orientalis on the inshore GBR was 0.14% (±0.51 SD), which is comparable to the average cover of C. delitrix in the Florida Keys, USA (~0.1%)45 and southeast Florida (~0.08%)46, but lower than C. delitrix cover in Colombia (~2%)47. Additional studies have assessed the abundance of bioeroding sponges by counting individual sponges27, 38, 48, 49, although it is challenging to reliably compare these measures of abundance with percent cover.
(A) Average percent cover of Cliona orientalis at the 16 sites used to measure changes over time. (B) Changes in Cliona orientalis cover per year. Changes in percent cover were estimated using linear regression and represent the average of 1–4 trends at each location. Dashed vertical lines indicate means and the solid vertical line indicates a value of zero.
C. orientalis occurred less frequently at locations with high accumulation of fine sediments. The model predicted a 50% probability of C. orientalis occurrence at 17% fine sediments, suggesting that even moderate accumulation of silt and clay sized particles prevents the establishment of C. orientalis (Fig. 3A). Furthermore, sites with large accumulations of fine sediments had low percent cover of C. orientalis (Fig. 3B). The amount of fine sediments distinguishes exposed and sheltered locations, as waves and currents resuspend fine particles and prevent accumulation50. Both suspended and deposited sediments can influence the composition of sponge communities51 and have negative physiological effects on sponges52, including reduced reproductive output53 and increased respiration54. The deposition of fine sediment may hinder filter-feeding or reduce the light available for photosynthesis55. The negative correlations observed between fine sediments and the distribution and abundance of C. orientalis suggest that sediments have negative physiological effects on C. orientalis, although these effects have not been demonstrated experimentally.
(A) Cliona orientalis was more likely to occur at sites with the lowest proportion of fine sediments (z = −2.5, P = 0.01; Wald test). The line represents the probability of occurrence using a binomial generalized linear model and shading represents the 95% confidence interval. The dashed line indicates the proportion of fine sediments (17%) with a 50% predicted probability of C. orientalis occurrence. Points represent presence-absence and the average fine sediment proportion for each location. (B) Cliona orientalis cover significantly decreased as a function of the proportion of fine particles in the sediment (z = −4.9, p < 0.01; Wald test). The line represents the predicted cover from a negative binomial generalized linear model and shading represents the 95% confidence interval. Points represent average cover and fine sediment proportion for each location.
As coral cover declines on the GBR4, changes in the cover of bioeroding taxa may dictate future reef growth2, 13, 18. In this study, the average change in C. orientalis percent cover was 0.03% yr−1 (±0.08 SD). Cover increased at 10 out of 16 locations (Fig. 1B), although only one trend was statistically significant (0.2% yr−1 at Fitzroy Island (East); t = 2.8, p < 0.05). C. orientalis cover exhibited non-linear patterns at some sites, possibly due to disturbances such as cyclones or outbreaks of crown of thorns starfish56, which altered community composition and potentially increased the detectability of C. orientalis. The rate of change in C. orientalis cover was similar to the rate of change in sponge cover at the same locations (0.03% yr−1 ± 0.10 SD), but slower than the changes in other benthic groups (Fig. 4). These time series indicate that cover of C. orientalis and other sponges has remained largely stable over the past decade on the inshore GBR despite changes to the reef community, such as a decline in octocoral cover (Fig. 4).
Few studies have reported the rate of change in percent cover of bioeroding sponges. Therefore, we estimated rates of change in cover of other Cliona spp. to provide context for the rates of change in C. orientalis cover measured in this study. The fastest estimated rate of change was for C. orientalis cover from 1998 to 2004 at Orpheus Island on the GBR (~0.9% yr−1)20. Slower rates of increase were reported from the Caribbean, where C. caribbaea cover increased ~0.14% yr−1 from 1979 to 1998 in Belize19, bioeroding sponge cover increased ~0.05% yr−1 from 2005 to 2009 in southwest Florida57 and C. delitrix cover changed <0.01% yr−1 from 2003 to 2009 in southeast Florida46. In contrast, C. delitrix cover decreased (−0.03% yr−1) in the Florida Keys45. The rate of change reported here (0.03% yr−1 for C. orientalis) is relatively low in the context of these estimates, but also encompassed a comparatively large number of survey locations. It is worth noting that many of the observations of increased cover of bioeroding sponges were initiated prior to 200119,20,21 and that subsequent studies have not observed increased cover38, 45, 46, 57.
Changes in C. orientalis cover are best explained by the abundance of macroalgae (Fig. 5, Table 1). Increases in C. orientalis cover occurred at locations with low macroalgal cover (t = −3.0, P = 0.01). However, these locations also had low average chlorophyll a concentration in the water (Fig. 5), which also significantly affected the change in C. orientalis cover (t = −2.4, P = 0.03). Therefore, the fastest increases in C. orientalis cover occurred at locations with a combination of low macroalgal cover and low chlorophyll concentrations, which were clustered near Cairns (Fig. 6). When analysed together, neither macroalgal cover nor chlorophyll concentration was significantly associated with change in C. orientalis cover (P > 0.05), likely due to the positive correlation between macroalgal cover and chlorophyll concentration (r = 0.56). While macroalgal cover explained 39% of the variation in change in C. orientalis cover (Table 1), macroalgal cover (or chlorophyll a) did not predict the distribution or abundance of C. orientalis (Table 1, Supplementary Figure 1).
The spatial distribution of changes in C. orientalis cover on the inshore GBR. Circles represent locations where change over time was measured and an * represent locations where C. orientalis was absent (not detected in at least 3 survey years). Blue circles indicate increases in cover; white circles indicate zero change in cover; and red circles indicate decreases in cover. The map was created using R statistical software (version 3.3.1; https://www.r-project.org) and the packages “ggplot2”69, “mapdata”70, and “oz”71.
These results suggest that macroalgae outcompete bioeroding sponges for space: all but one of the locations with increased C. orientalis cover had less than 10% macroalgal cover and all had less than 0.45 µg/L chlorophyll a (Fig. 5), a water quality threshold that separates reefs with low and high macroalgal abundance58. Previous work observed that macroalgal cover was negatively correlated with C. orientalis cover39 and macroalgae have also been reported to outcompete C. tenuis for substratum in the Caribbean32. In addition, several studies have observed that large colonies of bioeroding sponges occur where macroalgal cover is low32, 59. By extension, controls on macroalgal growth, such as fish and urchin herbivory39 as well as dissolved nutrient levels58, may indirectly affect the growth of bioeroding sponges.
The gradual increases in C. orientalis cover observed at multiple locations suggest that broader ecological changes may be responsible for increases in C. orientalis cover. Water quality is declining across the inshore GBR, driven by inputs of terrestrial nutrients that are delivered during seasonal flood events60, 61. Dissolved nutrient levels increase during floods61, which can lead to phytoplankton blooms and higher concentrations of organic material in the water62, which is a primary food for some Cliona species63. Nutrient levels likely increased over the survey period, as river flows were high, particularly during the middle of the study56, 64. At locations with high nutrient levels, additional nutrients would likely have benefited the already high macroalgal cover58. However, at locations with low nutrient levels and little cover of macroalgae, additional nutrients may have contributed to increases in C. orientalis cover (Fig. 5). Thus, increases in C. orientalis cover may reflect additional nutrient loads entering the GBR lagoon, but are restricted to locations where nutrient concentrations are insufficient to support high macroalgal cover.
While the response of C. orientalis to high nutrient levels has not been investigated experimentally, several other Cliona species exhibit positive associations with elevated nutrients, including C. delitrix and C. vastifica 21, 27, 47, 65. However, not all Cliona species respond the same way, as several exhibited either positive or negative responses to a chlorophyll a gradient in Mexico48. On the GBR, observation of higher abundance of bioeroding sponges on inshore versus offshore reefs suggests that bioeroding sponges benefit from high nutrient conditions66. The correlations reported here suggest that C. orientalis is affected by local environmental conditions, specifically fine sediments, dissolved nutrients (chlorophyll a), and macroalgal cover, but experimental evidence of how these conditions affect Cliona species is lacking.
Factors other than fine sediments, macroalgal cover, and chlorophyll a explained little variation in the cover or distribution of C. orientalis. The cover by other taxa (scleractinian corals, soft corals, sponges, macroalgae) did not influence the distribution or abundance of C. orientalis (Table 1; Supplementary Figure 1), suggesting that competition with these groups does not exclude C. orientalis from its habitat. Total carbon in the sediment explained some variation in C. orientalis abundance, but the effect was not significant in a model that included both total carbon and fine sediments (Table 1). Latitude explained little variation in C. orientalis abundance or distribution (Table 1) or in the environmental predictors (Supplementary Figure 2). Importantly however, processes affecting C. orientalis at small spatial scales were not accounted for. For example, whilst the presence-absence of C. orientalis varied between nearby locations (i.e., kilometres; Fig. 6), presence-absence also varied within locations (i.e., 250 m). Much of the unexplained variation in the distribution and cover of C. orientalis may be due to small-scale factors, such as the availability of hard substratum44.
Conclusion
Here, we present a large-scale monitoring effort to assess temporal changes in the abundance of the bioeroding sponge Cliona orientalis on the inshore GBR. Whilst Cliona abundance increased at 11 of 16 locations, increases in macroalgal cover and decreases in scleractinian and octocoral cover all outpaced changes in Cliona abundance. Low deposition of fine sediments was strongly associated with both the presence and abundance of C. orientalis, suggesting that the sponge requires exposed habitat. Increased cover of C. orientalis was only observed where mean chlorophyll a concentration was less than 0.45 µg/L and macroalgal cover was low, suggesting that C. orientalis can only increase in habitats where macroalgae are nutrient-limited. Experimental work that identifies the limiting environmental conditions (light, suspended sediment, nutrients) for C. orientalis is clearly warranted. Given the clumped distribution and strong association with local environmental conditions (e.g., sediment, macroalgae), bioeroding sponges such as C. orientalis likely represent site-specific – rather than regional – threats to coral health and reef accretion on the GBR.
Methods
Benthic surveys
Benthic cover was surveyed at 35 locations on the inshore GBR between 2005 and 2014 as part of the Inshore Water Quality and Coral Reef Monitoring program at the Australian Institute of Marine Science56. Briefly, at each location, two sites and two depths (2 and 5 m) were surveyed using five, fixed, 20 m transects. Every 0.5 m along each transect, photographs were taken of the benthos, which were used to determine presence-absence, percent cover, and change in percent cover. Survey data were pooled across sites and depths to relate to environmental variables measured at each location.
Percent cover was measured from digital photographs of the benthos. Five markers were overlaid onto each photograph and percent cover was calculated as the proportion of points occupied by each taxon. Percent cover of C. orientalis (encrusting ß form), other sponges, scleractinian corals, octocorals, and macroalgae was calculated for each of the four within-location survey sites. The influence of other benthic taxa on C. orientalis cover was explored using biplots of cover at each within-location site.
Trends in cover were analysed for each within-location site where C. orientalis was detected in at least three survey years. Trends were estimated for each location separately as the locations were surveyed at different frequencies over the course of the study. Change in percent cover was estimated for each within-location site using linear regression. Thus, change in percent cover represents the average of the within-location sites (1–4) where C. orientalis was detected. Analysis of presence-absence of C. orientalis at each location followed the same criterion as change in percent cover, whereby C. orientalis was considered present if it occurred in at least three survey years at any of the sites.
Environmental variables
Survey data were related to environmental variables collected at the location scale (not sites or transects). Water quality was assessed using satellite-derived data from the eReefs Marine Water Quality Dashboard (http://ereefs.org.au/ereefs), including chlorophyll a concentration, coloured dissolved organic matter, and non-algal particulates (1 km resolution). The data nearest each survey location were analysed for each survey year. Sediment was collected from the 5 m survey sites and the proportion of fine particles, carbon content, and nitrogen content in the sediment were measured as described in56, with average values compared to C. orientalis cover. Fine particles in the sediments were defined as all particles smaller than 63 µm and expressed as a proportion of the total sediment67.
Data analysis
Exploratory plots were prepared to identify correlations amongst the environmental predictors and to compare the effects of different benthic taxa on C. orientalis cover. Note that only fine sediment, chlorophyll a, and total C in sediment were included in the model, as other environmental variables were strongly correlated with either the proportion of fine sediments or chlorophyll a (all r > 0.7). Uncorrelated environmental variables were used to predict the presence-absence of C. orientalis (generalized linear model (GLM) with binomial errors and logit link), the percent cover of C. orientalis (GLM with negative binomial errors and log link), and changes in C. orientalis cover per year (linear model). Latitude was used to account for the spatial relationships among locations. Model fit was evaluated by plotting residual and fitted values. For generalized linear models, model fit was also evaluated using the chi-square probability of the residual deviance and residual degrees of freedom and by comparing observed and simulated residuals from each model.
Three models were used to assess whether other taxa, the environment, or geography explained patterns in C. orientalis distribution. Models were compared using AIC and R2 values. For the GLM, R2 was calculated as the deviance ratio of models with and without predictors. The most parsimonious model was identified as the model that maximized explanatory power with the fewest predictors.
Analyses were conducted in R statistical software68. The map in Fig. 6 was produced using R statistical software and the packages, “ggplot2”69, “mapdata”70, and “oz”71 packages.
References
Hoegh-Guldberg, O. Coral bleaching, climate change and the future of the world’s coral reefs. Mar Freshwater Res 50, 839–866, doi:10.1071/MF99078 (1999).
Kennedy, E. V. et al. Avoiding Coral Reef Functional Collapse Requires Local and Global Action. Curr Biol 23, 912–918, doi:10.1016/j.cub.2013.04.020 (2013).
Pandolfi, J. M., Connolly, S. R., Marshall, D. J. & Cohen, A. L. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422, doi:10.1126/science.1204794 (2011).
De’ath, G., Fabricius, K. E., Sweatman, H. & Puotinen, M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci USA 109, 17995–17999, doi:10.1073/pnas.1208909109 (2012).
Coral Reefs: An Ecosystem in Transition (ed. Dubinsky, Z. and Stambler, N.) (Springer Science & Business Media, 2010).
Hughes, T. P., Graham, N. A. J., Jackson, J. B. C., Mumby, P. J. & Steneck, R. S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evolut 25, 633–642, doi:10.1016/j.tree.2010.07.011 (2010).
Bell, J. J., Davy, S. K., Jones, T., Taylor, M. W. & Webster, N. S. Could some coral reefs become sponge reefs as our climate changes? Global Change Biol 19, 2613–2624, doi:10.1111/gcb.2013.19.issue-9 (2013).
Fabricius, K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Change 1, 165–169, doi:10.1038/nclimate1122 (2011).
Loh, T.-L., McMurray, S. E., Henkel, T. P., Vicente, J. & Pawlik, J. R. Indirect effects of overfishing on Caribbean reefs: sponges overgrow reef-building corals. PeerJ 3, e901, doi:10.7717/peerj.901 (2015).
Ruzicka, R. R. et al. Temporal changes in benthic assemblages on Florida Keys reefs 11 years after the 1997/1998 El Niño. Mar Ecol Prog Ser 489, 125–141, doi:10.3354/meps10427 (2013).
Glynn, P. W. Bioerosion and coral reef growth: a dynamic balance in life and death of coral reefs In Coral Reefs in the Anthropocene (ed. Birkeland, C.) 68–95 (Springer Netherlands, 2015).
Perry, C. T. et al. Caribbean-wide decline in carbonate production threatens coral reef growth. Nat Comms 4, 1402, doi:10.1038/ncomms2409 (2013).
Perry, C. T. et al. Changing dynamics of Caribbean reef carbonate budgets: emergence of reef bioeroders as critical controls on present and future reef growth potential. Proc. R. Soc. B 281, 20142018–20142018, doi:10.1098/rspb.2014.2018 (2014).
Schönberg, C. H. L. Substrate effects on the bioeroding demosponge Cliona orientalis. 2. Substrate colonisation and tissue growth. Mar Ecol-P S Z N I 24, 59–74, doi:10.1046/j.1439-0485.2003.03812.x (2003).
Neumann, A. C. Observations on coastal erosion in Bermuda and measurements of the boring rate of the sponge Cliona lampa. Limnol Oceanogr 11, 92–108, doi:10.4319/lo.1966.11.1.0092 (1966).
Acker, K. L. & Risk, M. J. Substrate destruction and sediment production by the boring sponge Cliona caribbaea on Grand Cayman Island. J Sediment Res 55, 705–711 (1985).
Rützler, K. The role of burrowing sponges in bioerosion. Oecologia 19, 203–216, doi:10.1007/BF00345306 (1975).
Enochs, I. C. et al. Ocean acidification enhances the bioerosion of a common coral reef sponge: implications for the persistence of the Florida Reef Tract. Bull Mar Sci 91, 271–290, doi:10.5343/bms.2014.1045 (2015).
Rützler, K. Impact of Crustose Clionid Sponges on Caribbean Reef Corals. Acta Geol Hisp 37, 61–72 (2002).
Schönberg, C. H. L. & Ortiz, J. C. Is sponge bioerosion increasing? Proceedings of 11th International Coral Reef Symp 7–11 (2008).
Ward-Paige, C. A., Risk, M. J., Sherwood, O. A. & Jaap, W. C. Clionid sponge surveys on the Florida Reef Tract suggest land-based nutrient inputs. Marine Poll Bull 51, 570–579, doi:10.1016/j.marpolbul.2005.04.006 (2005).
Kelmo, F., Bell, J. J. & Attrill, M. J. Tolerance of sponge assemblages to temperature anomalies: resilience and proliferation of sponges following the 1997-8 El-Niño southern oscillation. Plos One 8, e76441, doi:10.1371/journal.pone.0076441 (2013).
Schönberg, C. H. L., Suwa, R., Hidaka, M. & Loh, W. K. W. Sponge and coral zooxanthellae in heat and light: preliminary results of photochemical efficiency monitored with pulse amplitude modulated fluorometry. Mar Ecol 29, 247–258, doi:10.1111/mae.2008.29.issue-2 (2008).
Vicente, V. P. Response of sponges with autotrophic endosymbionts during the coral-bleaching episode in Puerto Rico. Coral Reefs 8, 199–202, doi:10.1007/BF00265011 (1990).
Fang, J. K. H. et al. Sponge biomass and bioerosion rates increase under ocean warming and acidification. Global Change Biol 19, 3581–3591, doi:10.1111/gcb.12334 (2013).
Stubler, A. D., Furman, B. T. & Peterson, B. J. Effects of pCO2 on the interaction between an excavating sponge, Cliona varians, and a hermatypic coral, Porites furcata. Mar Biol 161, 1851–1859, doi:10.1111/gcb.13002 (2014).
Holmes, K. E. Effects of eutrophication on bioeroding sponge communities with the description of new West Indian sponges, Cliona spp. (Porifera: Hadromerida: Clionidae). Invertebr Biol 119, 125–138, doi:10.1111/j.1744-7410.2000.tb00001.x (2000).
Schönberg, C. H. L. Growth and erosion of the zooxanthellate Australian bioeroding sponge. Proceedings of the 11th International Coral Reef Symposium 168–174 (2006).
Hill, M. S. Symbiotic zooxanthellae enhance boring and growth rates of the tropical sponge Anthosigmella varians forma varians. Mar Biol 125, 649–654, doi:10.1007/BF00349246 (1996).
Carballo, J. L., Beltrán, H. O., Yáñez, B., Bautista Guerrero, E. & Nava-Bravo, H. Assessment of the distribution of sponge chips in the sediment of East Pacific Ocean reefs. Mar Ecol 1, 208–11, doi:10.1111/maec.12390 (2016).
Hutchings, P. A. Biological destruction of coral reefs. Coral Reefs 4, 239–252, doi:10.1007/BF00298083 (1986).
González-Rivero, M., Ferrari, R., Schönberg, C. H. L. & Mumby, P. J. Impacts of macroalgal competition and parrotfish predation on the growth of a common bioeroding sponge. Mar Ecol Prog Ser 444, 133–142, doi:10.3354/meps09424 (2012).
Márquez, J. C. & Zea, S. Is competition for space between the encrusting excavating sponge Cliona tenuis and corals influenced by higher-than-normal temperatures? Bol. Invest. Mar. Cost 35, 259–265 (2006).
Lopez-Victoria, M. & Zea, S. Storm-mediated coral colonization by an excavating Caribbean sponge. Clim. Res 26, 251–256, doi:10.3354/cr026251 (2004).
Halperin, A. A., Chaves-Fonnegra, A. & Gilliam, D. S. Effects of excavating-sponge removal on coral growth. J Mar Biol Assoc UK 96, 473–479, doi:10.1017/S0025315415001228 (2015).
Schönberg, C. H. L. & Wilkinson, C. R. Induced colonization of corals by a clionid bioeroding sponge. Coral Reefs 20, 69–76, doi:10.1007/s003380100143 (2001).
Chaves-Fonnegra, A. & Zea, S. Observations on reef coral undermining by the Caribbean excavating sponge Cliona delitrix (Demospongiae, Hadromerida) In Porifera Research: Biodiversity, Innovation and Sustainability (ed. Custódio, M.R.) 247–254 (2007).
Carballo, J. L., Bautista, E., Nava, H., Cruz-Barraza, J. A. & Chávez, J. A. Boring sponges, an increasing threat for coral reefs affected by bleaching events. Ecol Evol 3, 872–886, doi:10.1002/ece3.452 (2013).
Cebrian, E. Grazing on coral reefs facilitates growth of the excavating sponge Cliona orientalis (Clionaidae, Hadromerida). Mar Ecol 31, 533–538, doi:10.1111/mae.2010.31.issue-4 (2010).
Wisshak, M., Schönberg, C. H. L., Form, A. & Freiwald, A. Sponge bioerosion accelerated by ocean acidification across species and latitudes? Helgol Mar Res 68, 253–262, doi:10.1007/s10152-014-0385-4 (2014).
Stubler, A. D., Furman, B. T. & Peterson, B. J. Sponge erosion under acidification and warming scenarios: differential impacts on living and dead coral. Global Change Biol 21, 4006–4020, doi:10.1111/gcb.13002 (2015).
Murphy, G. N., Perry, C. T., Chin, P. & McCoy, C. New approaches to quantifying bioerosion by endolithic sponge populations: applications to the coral reefs of Grand Cayman. Coral Reefs 35, 1109–1121, doi:10.1007/s00338-016-1442-z (2016).
Schönberg, C. H. L. Monitoring bioeroding sponges: using rubble, quadrat, or intercept surveys? Biol Bull 228, 137–155, doi:10.1086/BBLv228n2p137 (2015).
Schönberg, C. H. L. Small-scale distribution of Great Barrier reef bioeroding sponges in shallow water. Ophelia 55, 39–54, doi:10.1080/00785236.2001.10409472 (2001).
Ruzicka, R. et al. CREMP 2009 Final Report (Fish & Wildlife Research Institute/Florida Fish & Wildlife Conservation Commission, 2010).
Gilliam, D. S. Southeast Florida Coral Reef evaluation and monitoring project 2009: Year 7 Final Report (Florida DEP report# RM085, Miami Beach, FL, 2010).
Chaves-Fonnegra, A., Zea, S. & Gómez, M. L. Abundance of the excavating sponge Cliona delitrix in relation to sewage discharge at San Andrés Island, SW Caribbean, Colombia. Bol. Invest. Mar. Cost 36, 63–78, doi:10.1007/s10886-008-9565-5 (2007).
Nava, H., Ramírez-Herrera, M. T., Figueroa-Camacho, A. G. & Villegas-Sanchez, B. M. Habitat characteristics and environmental factors related to boring sponge assemblages on coral reefs near populated coastal areas on the Mexican Eastern Pacific coast. Mar Biodiv 44, 45–54, doi:10.1007/s12526-013-0182-3 (2014).
Bautista Guerrero, E., Carballo, J. L. & Maldonado, M. Abundance and reproductive patterns of the excavating sponge Cliona vermifera: a threat to Pacific coral reefs? Coral Reefs 33, 259–266, doi:10.1007/s00338-013-1094-1 (2013).
Wolanski, E., Fabricius, K., Spagnol, S. & Brinkman, R. Fine sediment budget on an inner-shelf coral-fringed island, Great Barrier Reef of Australia. Estuar Coast Shelf Sci 65, 153–158, doi:10.1016/j.ecss.2005.06.003 (2005).
Knapp, I. S. S. et al. Restriction of sponges to an atoll lagoon as a result of reduced environmental quality. Marine Poll Bull 66, 209–220, doi:10.1016/j.marpolbul.2012.08.017 (2013).
Bell, J. J. et al. Sediment impacts on marine sponges. Marine Poll Bull 94, 5–13, doi:10.1016/j.marpolbul.2015.03.030 (2015).
Whalan, S., Battershill, C. N. & de Nys, R. Variability in reproductive output across a water quality gradient for a tropical marine sponge. Mar Biol 153, 163–169, doi:10.1007/s10126-010-9300-4 (2007).
Bannister, R. J., Battershill, C. N. & de Nys, R. Suspended sediment grain size and mineralogy across the continental shelf of the Great Barrier Reef: Impacts on the physiology of a coral reef sponge. Cont Shelf Res 32, 86–95, doi:10.1016/j.csr.2011.10.018 (2012).
Fabricius, K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Marine Poll Bull 50, 125–146, doi:10.1016/j.marpolbul.2004.11.028 (2005).
Thompson, A. et al. Marine Monitoring Program. Annual Report for inshore coral reef monitoring: 2014 to 2015. Report for the Great Barrier Reef Marine Park Authority 1–133 (Australian Institute of Marine Science, Townsville, 2016).
Makowski, C. & Keyes, P. Using the Benthic Ecological Assessment for Marginal Reefs (BEAMR) Method to Quantify Nearshore Reef Conditions in the Southeast Gulf of Mexico. J Coastal Res 27, 428–440, doi:10.2112/JCOASTRES-D-10-00074.1 (2011).
De’ath, G. & Fabricius, K. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol Appl 20, 840–850, doi:10.1890/08-2023.1 (2010).
Lopez-Victoria, M. & Zea, S. Current trends of space occupation by encrusting excavating sponges on Colombian coral reefs. Mar Ecol 26, 33–41, doi:10.1111/mae.2005.26.issue-1 (2005).
Schaffelke, B., Carleton, J., Skuza, M., Zagorskis, I. & Furnas, M. J. Water quality in the inshore Great Barrier Reef lagoon: Implications for long-term monitoring and management. Marine Poll Bull 65, 249–260, doi:10.1016/j.marpolbul.2011.10.031 (2012).
Brodie, J. E., Brodie, J. E., Devlin, M., Haynes, D. & Waterhouse, J. Assessment of the eutrophication status of the Great Barrier Reef lagoon (Australia). Biogeochemistry 106, 281–302, doi:10.1007/s10533-010-9542-2 (2011).
Furnas, M. J., Mitchell, A., Skuza, M., Brodie, J. E. & Brodie, J. In the other 90%: phytoplankton responses to enhanced nutrient availability in the Great Barrier Reef Lagoon. Marine Poll Bull 51, 253–265, doi:10.1016/j.marpolbul.2004.11.010 (2005).
Mueller, B. et al. Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC). Plos One 9, e90152, doi:10.1371/journal.pone.0090152 (2014).
Fabricius, K. E., Logan, M., Weeks, S. J., Lewis, S. E. & Brodie, J. E. Changes in water clarity in response to river discharges on the Great Barrier Reef continental shelf: 2002–2013. Estuar Coast Shelf Sci 173, A1–A15, doi:10.1016/j.ecss.2016.03.001 (2016).
Rose, C. S. & Risk, M. J. Increase in Cliona delitrix infestation of Montastrea cavernosa heads on an organically polluted portion of the Grand Cayman fringing reef. Mar Ecol 6, 343–363 (1985).
Sammarco, P. & Risk, M. Large-scale patterns in internal bioerosion of Porites: cross continental shelf trends on the GBR. Mar Ecol Prog Ser 59, 145–156, doi:10.3354/meps059145 (1990).
Cooper, T. F., Uthicke, S., Humphrey, C. & Fabricius, K. E. Gradients in water column nutrients, sediment parameters, irradiance and coral reef development in the Whitsunday Region, central Great Barrier Reef. Estuar Coast Shelf Sci 74, 458–470, doi:10.1016/j.marpolbul.2011.09.004 (2007).
R core team. R: A Language and Environment for Statistical Computing (version 3.3.1) (R Foundation for Statistical Computing, Vienna, Austria, 2016).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (version 2.2.1.9000) (Springer-Verlag, 2016).
Becker, R. A., Wilks, A. R. & Brownrigg, R. mapdata: extra map databases (version 2.2–6) (2016).
Venables, B. & Hornik, K. Plot the Australian coastline and states (version 1.0–20) (2015).
Acknowledgements
We would like to thank the AIMS Long term monitoring team and the volunteers who conducted field surveys as well as P. Menendez and M. Logan who provided statistical advice and R code. N.S.W. was funded by an Australian Research Council Future Fellowship FT120100480.
Author information
Authors and Affiliations
Contributions
B.R., M.H., S.W., N.W., and A.T. designed the study. A.T. collected the data. B.R. analysed the data. B.R., M.H., S.W., N.W., and A.T. contributed to writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramsby, B.D., Hoogenboom, M.O., Whalan, S. et al. A decadal analysis of bioeroding sponge cover on the inshore Great Barrier Reef. Sci Rep 7, 2706 (2017). https://doi.org/10.1038/s41598-017-02196-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02196-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.