Abstract
Rif1 is a conserved protein that plays essential roles in orchestrating DNA replication timing, controlling nuclear architecture, telomere length and DNA repair. However, the relationship between these different roles, as well as the molecular basis of Rif1 function is still unclear. The association of Rif1 with insoluble nuclear lamina has thus far hampered exhaustive characterization of the associated protein complexes. We devised a protocol that overcomes this problem, and were thus able to discover a number of novel Rif1 interactors, involved in chromatin metabolism and phosphorylation. Among them, we focus here on PP1. Data from different systems have suggested that Rif1-PP1 interaction is conserved and has important biological roles. Using mutagenesis, NMR, isothermal calorimetry and surface plasmon resonance we demonstrate that Rif1 is a high-affinity PP1 adaptor, able to out-compete the well-established PP1-inhibitor I2 in vitro. Our conclusions have important implications for understanding Rif1 diverse roles and the relationship between the biological processes controlled by Rif1.
Similar content being viewed by others
Introduction
Rif1 protein is conserved throughout evolution from yeast to mammals. It was initially identified in Saccharomyces cerevisiae as regulator of telomere length1,2,3,4, a function that however appears to be specific to yeast. In fact, no major telomeric role could be attributed to mammalian Rif15, which instead has been implicated in various aspects of DNA repair5,6,7,8,9,10,11,12. Our group and others have recently identified Rif1 as a key regulator of DNA replication timing, which is the first common function of this protein across species13,14,15,16,17. In addition, we showed recently that mouse Rif1 is involved in the organization of the three-dimensional (3D) contacts of replication timing domains at around the same time when the replication-timing program is established in G118. These data raise the question of whether Rif1-dependent control of replication timing is related to its architectural function. In fact, the molecular mechanism through which Rif1 mediates such diverse processes remains unclear.
The analysis of Rif1 protein structure provides only limited clues to its mode of action. Mammalian Rif1 contains two conserved regions that could potentially mediate protein-protein and protein-nucleic acid interactions: an N-terminal region that is predicted to comprise HEAT-type α-helical repeats (HEAT repeats) and a disordered tripartite C-terminal region, comprising CRI, CRII and CRIII subdomains (Supp. Fig. 1A). With the exception of CRIII, all these domains are conserved11, 19, 20. The HEAT repeats are required for mouse Rif1 recruitment to double strand breaks21 and the mammalian CRII is a functional DNA-binding domain that selectively binds to cruciform DNA20, 22. Fission yeast Rif1 was recently reported to bind G quadruplexes23, however, the DNA-binding domain is still to be localized. The budding yeast homolog of the mammalian Rif1 CRII-domain mediates the interaction with Rap1 and DDK15, and is also required for Rif1 tetramerization4. Mammalian Rif1 also multimerizes through its C-terminal domain, although a finer mapping of interacting regions has not been performed20.
Throughout evolution, Rif1 has maintained one or more motifs that potentially mediate the interaction with the Ser/Thr protein phosphatase 1 (PP1), such as the PP1 docking motif [K/R][V/I]xF and the conserved SILK sequence, an additional binding motif necessary for a subset of PP1-interacting proteins19, 24, 25. In budding yeast, Rif1 PP1 docking motifs are localized at the N-terminus of the protein. Their function has been validated through mutagenesis, demonstrating that Rif1 counteracts DDK activity on the replicative MCM4 helicase through its interaction with PP115, 26, 27. Also, human and Drosophila Rif1 homologs have been identified in PP1-associated complexes28,29,30. However, human and mouse Rif1s contain at least two different potential PP1-interacting peptides, canonical RVSF/SILK motifs in CRI and an additional KIAF motif in the HEAT repeats. These structural features indicate that the interaction of Rif1 with PP1 may represent a central aspect of Rif1 biological role throughout evolution. However, which of these mammalian Rif1 motifs mediates the binding to PP1, how and which biological function of Rif1 requires this association, remains to be determined.
To understand the molecular basis of Rif1 multiple functions, we sought to define the composition of cellular Rif1 complexes. Due to the lack of suitable antibodies and biochemical difficulties in isolating Rif1, no comprehensive study of the endogenous Rif1-associated complex has so far been published. The data available are in fact limited to soluble complexes20, 31. Given the association of at least half of Rif1 to insoluble nuclear structures13, 17, investigating its function is challenging. In proof-of-principle studies, DamID-based approaches have recently identified Rif1 in close proximity to LaminA/C32. Here, we characterize the protein complexes associated with endogenous Rif1 in mouse cells, including those found in the insoluble fraction. Among its interactors, we focused on protein phosphatase 1 (PP1), given the compelling evidence for a functional significance of this interaction. We have mapped the interaction surface in Rif1 CRI and have generated point mutations that effectively abolish it. The interaction is nanomolar affinity but does not mask the PP1 active site. Our data therefore classify Rif1 as a bona fide PP1 regulatory subunit.
Results
Rif1 associated protein complexes
The characterization of endogenous Rif1-associated complexes has been hampered by the lack of optimized antibodies and the subnuclear localization of this protein, which render it extremely challenging to isolate. In this respect, we showed that Rif1 is associated to the nuclear lamina13, 18 and binds the genome at regions of constitutive heterochromatin18. Such features notoriously confer high protein insolubility. Accordingly, conventional approaches for the isolation of Rif1 would require harsh conditions such as high detergent or salt concentrations that result in loss of molecular interactors. To circumvent these limitations, we have optimized a biochemical extraction protocol employing low detergent and physiological salt concentrations, thereby preserving interactions whilst achieving solubilisation of the nuclear lamina-associated fraction (Supp. Fig. 1B,C,D and E). In addition, the use of cells derived from our previously described Rif1FLAG-HA2 (Rif1FH) mouse line13 permitted Rif1 affinity purification using standardized antibodies for immunoprecipitation.
To gain further molecular insight into Rif1 function we employed a proteomic mass spectrometry approach to determine the composition of the Rif1-associated complexes from mouse embryonic stem cells (ESCs) expressing a heterozygote Rif1FH (Fig. 1A,B and Supp. Table 1A, total proteins). This approach has identified 96 nuclear interactors associated with Rif1 (Supp. Table 1C, above 1.5 nuclear). Consistent with the multiple aspects of nuclear organization and chromatin metabolism in which Rif1 has been implicated, the identified interactors were significantly enriched in chromatin organization and assembly and nuclear (organelle) organization (Fig. 1C). Within the top 15 Rif1 partners (Table 1) we found lamin B1, confirming our recent report18, and PP1 (highlighted in Fig. 1B and D). Interestingly, the proteins identified in the Rif1-associated complexes are also significantly enriched in known PP1 substrates and regulators (Fig. 1E). This observation, together with genetic evidence from yeast15, 26, 27 and Drosophila16, suggests that Rif1 may itself be a regulatory subunit of PP1. To explore this hypothesis, we have further characterized the Rif1-PP1 interaction in molecular detail.
Rif1-associated complexes are enriched for chromatin-related proteins and PP1 substrates/regulators. (A) Immunoprecipitation of Rif1-associated proteins from extracts obtained from ESCs homozygous for Rif1FH or Rif1+ alleles, visualized by Coomassie blue-stained SDS-PAGE. (B) Determination of Rif1 interactome using label-free quantitation pipeline MaxQuant49. For volcano plot, t-test was performed on data from 3 Rif1FH/+ and 2 Rif1+/+ ESC independent lines analysed in 2 independent experiments. t-test difference ratios were plotted against the negative logarithmic P-value of the t-test. Rif1, Lamin B1 and PP1 LFQs are indicated in red. (C) Top gene ontology (GO) terms enriched within nuclear proteins associated to Rif1FH, listed in supplemental table C (cut-off of enrichment over negative control 1.5). The analysis is a Panther overrepresentation test against the complete GO biological process annotation dataset. Bonferroni correction was applied. (D) PP1 interaction with Rif1 was confirmed by immunoprecipitation of Rif1-associated proteins from Rif1FH/+ ESCs and immunoblotting with anti-PP1 antibody. IN = input; FT = flow through; IP = immunoprecipitated. (E) List of the known PP1 substrates/regulators identified in the Rif1-associated complexes. The Rif1 interactome enrichment for PP1 substrates/regulators is statistically significant as evaluated by Fisher’s exact test (P-value: 0.00111).
Rif1 interacts with PP1 through canonical C-terminal RVSF and SILK motifs
PP1 has no intrinsic specificity for its substrates, but interacts via regulatory subunits that contain defined docking motifs. In addition, they are often intrinsically disordered proteins (IDPs) whose folding follows the interaction with PP1 itself. Several regulatory subunits are known to target PP1 to chromatin, including NIPP33, PNUTS34, RepoMan30 and Ki6735. Remarkably, recent mapping of PP1 genome-wide occupancy has shown very limited overlap between PP1 binding sites and the sites occupied by the known regulatory subunits, indicating that many more remain to be discovered36.
Sequence analysis suggests potential PP1 docking motifs in both CRI at the mouse Rif1 C-terminus (SILK and RVSF, residues 2128–2131 and 2150–2153) and within the N-terminal HEAT repeats (KIAF, residues 291–294) (Supp. Fig. 1A). To identify which of the motifs mediate a functional interaction, GST-Rif1 fragment fusion proteins were bound to glutathione beads (Supp. Fig. 1F and G) and incubated with an E. coli crude lysate containing recombinant hexahistidine-tagged PP1. PP1 co-precipitated with Rif1 CRI+II+III, CRI+II and CRI and did not bind fragments lacking CRI (CRII, CRIII, CRII+CRIII and HEAT repeats) (Fig. 2A). These results show that Rif1 CRI is a PP1-binding region. To measure the contribution of individual CRI residues to PP1 binding, we substituted every residue in the primary motif RVSF, and ILK residues in the additional binding motif SILK for alanine. Purified wild-type and mutant Rif1 CRI proteins were injected over immobilized recombinant purified α-isoform of mammalian PP1 in a surface plasmon resonance (SPR) assay. Analysis of binding curves revealed that the RVSF to RVSA substitution had the most pronounced effect on PP1 binding, resulting in less than 0.1% of the affinity of the wild-type interaction (Fig. 2B). The RVSF to RASF and SILK to SAAA substitutions resulted in approximately 20-fold decrease in affinity, suggesting a role of these residues in PP1 binding. Alanine substitution of the non-conserved serine residue in the canonical motif (RVSF to RVAF) had only a minor effect on PP1 binding. In contrast to the result on the longer CR1 construct (residues 2093–2190), a minimal synthetic peptide comprising only residues 2127–2154 of mouse Rif1 showed only 10% of binding affinity, indicating that residues in the natural protein beyond the core motifs may be involved in the interaction (Fig. 2B, Pept.). In conclusion, the single RVSF to RVSA substitution strongly inhibits PP1 binding. This can be further combined with the SAAA variant of the SILK motif to produce a Rif1 variant that does not detectably bind PP1 (Fig. 2B, SAAA+RVSA).
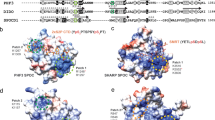
The Rif1 CRI region contains two canonical RVSF and SILK motifs interacting with PP1. (A) Beads loaded with GST-Rif1 fragments were incubated with cell lysates containing hexahistidine-PP1. PP1 retained by means of interaction with Rif1 was eluted and analysed by imunoblotting using antibodies against the hexahistidine tag. (B) The affinity of Rif1 CRI mutants and peptide for PP1 was determined by the SPR and expressed as percentage of wild-type. SAAA indicates the mutation of the residues within the SILK motif. (C) Analysis of Rif1 CRI interaction with PP1 by size exclusion chromatography. PP1 (light blue), Rif1 CRI (red), PP1/CRI at molar ratio 1:1 (magenta), PP1/CRI at molar ratio 1:3 (green) and PP1/CRI at molar ratio 2:1 (blue) were subjected to analytical gel-filtration. The Coomassie-stained gel shows recombinant PP1 and Rif1 CRI co-eluting from the column. mAU = milli Absorbance Unit. (D) Superposition of the 1H-15N HSQC spectra of 15N-labeled CRI (red) and 15N-labeled CRI in the presence of PP1 (blue). In the inset, assignments are shown for CRI for a selected region of the HSQC spectrum. (E) PP1-interacting region in mouse Rif1. Interacting residues identified by NMR analysis are highlighted in yellow, residues present in the synthetic peptide (Pept) are shown in bold, and residues subjected to mutagenesis are shown in red.
Previously, using the random library approach ESPRIT37 we identified a high-yielding soluble fragment encompassing mouse Rif1 CRI (residues 2093–2190)22. This protein exhibits behaviours characteristic of IDPs: it migrates at a higher-than-expected molecular mass on SDS-PAGE (15 kDa instead of 10.8 kDa, Fig. 2C), exhibits a higher Stokes radius by gel-filtration than standard globular proteins of similar molecular mass (elutes as 28 kDa, Fig. 2C), and is resistant to heat-induced aggregation22. The two-dimensional 1H-15N HSQC NMR spectrum of CRI shows very little chemical shift dispersion in the 1HN dimension, confirming that it is indeed intrinsically disordered (Fig. 2D and ref. 22). We used this fragment to perform interaction studies with PP1. Recombinantly expressed and purified PP1 (37.5 kDa) elutes from a gel-filtration column as a single peak with apparent molecular weight of ~33 kDa (Fig. 2C). In the presence of Rif1 CRI at the molar ratio 1:1, both the proteins were eluted in a single peak with apparent molecular weight of ~48 kDa. The excess of PP1 or CRI resulted in two peaks: the ~48 kDa peak corresponding to the protein complex and the other peak corresponding to the excess protein. These results indicate that Rif1 CRI binds to PP1 in a 1:1 ratio. Next, we compared spectra of 15N-labeled CRI recorded in the presence and absence of unlabelled PP1. A significant fraction of the resonances disappeared from the spectrum in the presence of PP1, while others retained their chemical shift values or shifted slightly (Fig. 2D and Supp. Fig. 1H). The former correspond to the residues interacting directly with PP1; following assignment of the CRI spectrum they were identified as residues 2115–2178, encompassing the SILK and RVSF motifs (Fig. 2E). Interestingly, the NMR signals of R2136, S2137, and Q2138 in the centre of this region were visible, although with reduced intensity, indicating that these residues stay sufficiently flexible in the CR1-PP1 complex to give rise to solution NMR signals. PP1 regulatory subunits are typically IDPs, further strengthening the conclusion that Rif1 is a PP1-regulatory subunit.
Rif1 CRI binds to PP1 with high affinity
The concentration of PP1 regulators in the cell is higher than that of PP1 itself, suggesting that formation of different complexes and their balance is driven by competition for a limited phosphatase pool38, 39. Therefore, different affinities of interactors for PP1 likely regulate the balance between different complexes. To measure the strength of the Rif1 CRI-PP1 interaction, we employed isothermal titration calorimetry (ITC) and SPR. ITC of PP1 and Rif1 CRI confirmed the 1:1 binding with KD = 22.8 ± 4.2 nM (∆H = - 23.8 ± 0.129 kcal/mol; ∆S = −42.4 ± 4.1 cal/mol/deg, Fig. 3A). Injections of various concentrations of CRI over PP1 immobilized on a CM5 sensor chip resulted in sensorgrams with a clear association phase followed by a slow dissociation phase (Fig. 3B). Binding curves were fitted using the simple Langmuir binding model 1:1 with KD = 9.7 ± 1.7 nM. The nanomolar affinity of Rif1 CRI to PP1 is close to the highest values reported for PP1 interactors PNUTS (KD = 8.7 nM)40 and spinophilin (KD = 9.3 nM)25, while the determined KD values for NIPP (73 nM)41 and GADD34 (62 nM)42 were significantly weaker.
Rif1 CRI mediates high-affinity binding to PP1. (A) ITC of PP1 and Rif1 CRI was used to quantify the affinity of the interaction (KD = 22.8 ± 4.2 nM). (B) Kinetic analysis of Rif1 CRI-PP1 binding by SPR. CRI at 0.625, 1.25, 2.5, 5, 10, 20, 40 nM was injected in duplicate over captured PP1. The raw data (thick black lines) are overlaid with a global fit to a 1:1 binding model. (KD = 9.7 ± 1.7 nM). (C) Dephosphorylation assay in presence of I2, Rif1 CRI and CRI+II. (D) Activation of I2 inhibited PP1 (PP1/I2 at molar ratio 1:3.75) by Rif1 fragments and Pept. PP1 activity was calculated from initial reaction rates (Vo) determined measuring optical absorbance of dephosphorylated pNP product.
Binding of regulatory proteins to the RVxF interaction site, which is approximately 20 Å from the PP1 catalytic site, does not alter the PP1 catalytic function; however, a number of PP1 regulators can inhibit phosphatase activity43. The best-characterized inhibitory regulator is Inhibitor 2 (I2), which has three PP1 docking sites: RVxF, SILK and IDoHA. The latter binds as an α-helix, and blocks phosphatase activity by covering the catalytic site44. To analyse the effect of interaction on PP1 activity we measured phosphatase activity of PP1 in the presence of various concentrations of Rif1 CRI and CRI+II using I2 for comparison. Rif1 fragments had no effect on pNPP substrate hydrolysis by PP1, while I2 showed potent inhibition at nanomolar concentrations (Fig. 3C). This indicates that Rif1-C does not block PP1 activity by covering the active site. Moreover, addition of recombinant Rif1 fragments to PP1 in presence of I2 fully restored PP1 activity (Fig. 3D). A synthetic peptide with lower affinity to PP1 than Rif1 CRI also activated PP1 (Fig. 3D); however, much higher concentrations of the peptide were needed, consistent with the lower affinity measured by SPR (Fig. 2B). These data suggest that Rif1-C, with its nanomolar affinity to PP1, may be able to compete effectively for binding to PP1 in the presence of other PP1 regulators.
Discussion
Our data classify Rif1 as a bona fide regulatory subunit for PP1. The significant enrichment for chromatin-related proteins in the Rif1-associated complexes represents an important step towards the understanding of its multiple related functions. We have recently described the involvement of Rif1 in organizing nuclear architecture in G118 and its function function as global regulator of replication timing. Experimental evidence had already connected these two processes, suggesting a role for nuclear 3D organization in the establishment of replication timing. Our results demonstrate that Rif1 links these two processes, yet the underlying molecular mechanism remains to be determined. Rif1 may act as a hub, controlling in parallel the two processes through de-phosphorylation of different substrates independently responsible for the control of nuclear architecture and replication timing. Alternatively, Rif1 could control replication timing at two levels, firstly by determining 3D chromatin contacts during G1 and influencing the timing of origin firing, possibly by regulating their affinity for limiting replication factors. Secondly, in S-phase, Rif1 may also mediate de-phosphorylation of proteins that control activation of origins of replication. In this case, the architectural function could be independent of the Rif1-PP1 interaction, potentially making the Rif1 mutants defective for PP1 binding described here a very useful separation-of-function allele.
Eukaryotic genomes encode a very limited number of phosphatases (about 40 Ser/Thr phosphatases in humans), intended to counteract the function of approximately 420 Ser/Thr kinases45. Consequently, each phosphatase is involved in a wide range of processes, where specificity is ensured by the interaction with regulatory subunits. Therefore, these represent the key to dissection of the individual roles of de-phosphorylation in different pathways. By inactivating a specific regulatory subunit, or inhibiting its interaction with phosphatase partners, it will be possible to interfere with a restricted subset of the phosphatase-mediated functions. In a first step towards this general approach, we believe that a comprehensive identification of PP1 substrates whose dephosphorylation depends upon Rif1, and characterization of these interactions at the molecular level, will elucidate the role of PP1 in the regulation of individual pathways. To further complicate the understanding of its functions, PP1 is present in three different isoforms, α, β/δ and γ1. Although more than 89% identical, these isoforms show distinct nuclear distribution46, 47, likely an important aspect of their functional specificity. Interestingly, previous work had identified that Rif1 preferentially associated to PP1α over PP1γ30. The data we present here confirm this finding, as we did not identify PP1γ as part of the Rif1-associated complex. Moreover, PP1α and Rif1 share a similar subnuclear distribution, being both associated to the insoluble nuclear structural components, unlike PP1γ46, 47. In the future, it will be interesting to understand the relationship between Rif1 and PP1 subnuclear distributions and how this contributes to substrate specificity.
Methods
ESCs chromatin isolation and Rif1 solubilisation test
Cell fractionation and solubilisation of chromatin-bound proteins by salt extraction was performed as described in ref. 48. For nuclease-mediated solubilisation of chromatin-bound proteins 2 × 106 ESCs were used for each reaction. MNase digest: chromatin was incubated in MNase buffer with 1 U MNase (Sigma N3755)/100 μl reaction volume at 28 °C for 1,1.5 and 2 h. The reaction was stopped with 5 μl 200 mM EGTA. DNAse I digest: chromatin was incubated in DNAse buffer with 50 U DNAse-I (Roche, 04536282001)/100 μl reaction volume for 2 h at 37 °C. The reaction stopped with 5 μl 0.5 M EDTA. RNAse A digest: chromatin was incubated in RNase buffer with 2 μg RNase A (Sigma, R5250)/100 μl reaction volume for 2 h at 37 °C. The reaction was stopped by putting samples on ice. Double RNAse+DNAse digest: chromatin was incubated with 2 μg RNase A+50 U DNAse-I/100 μl reaction volume for 2 h at 37 °C. The reaction was stopped with 5 μl 0.5 M EDTA on ice. Benzonase digest: chromatin was incubated in 50 U Benzonase (Sigma, E1014)/100 μl reaction volume for 2 h at 37 °C. The reaction was stopped with 5 μl 0.5 M EDTA.
After nuclease digestion samples were divided in half for DNA or protein analysis. DNA samples were subjected to Proteinase K digestion (Proteinase K, Sigma P6556 1 h 37 °C) followed by DNA extraction by standard phenol-chloroform procedure and loaded onto a 0.7% agarose gel for electrophoresis. Laemmli sample buffer was added to the protein samples, to a final concentration of 1X, samples were boiled according to standard procedures and used for western blotting.
Protein extract preparation
109 Rif1FH/+ and Rif1+/+ mouse ESCs were harvested and resuspended in cold Hypotonic buffer (25 mM Tris HCl pH 7.4, 50 mM KCl, 2 mM MgCl2, 1 mM EDTA, freshly added Protease Inhibitors) at 20 000 cells/μl and incubated 20 min to eliminate the cytoplasmic fraction. A total of 3 Rif1FH/+ and 2 Rif1+/+ cell lines were used in 2 independent experiments. Samples were centrifuged 15 min at 1 500 g, 4 °C and supernatant was discarded. Nuclei were washed twice in cold Hypotonic buffer and resuspended in cold Benzonase buffer (50 mM Tris HCl pH 8.0, 100 mM NaCl, 1.5 mM MgCl2, 10% Glycerol freshly added Protease inhibitors) at 20 000 cells/μl. Samples were subjected to 3 cycles of snap freezing and thawing. Fifty U/ml of Benzonase (Sigma, E1014) was added to the thawed extracts and incubated at RT for 25 min. Triton X-100 was added to a final concentration of 0.2% and incubated on ice for 10 min. Samples were centrifuged for 20 min at 16 000 g to remove insoluble debris. Supernatants were used for immunoprecipitation (IP). One percent of the extract was used as Input and not subjected to IP.
Immunoprecipitation (IP) of Rif1–associated complex
Rif1FH and interacting factors were immunoprecipitated using Anti-Flag M2 affinity gel (Sigma, A2220) according to the manufacturer’s instructions. Protein extracts prepared as described above were pre-cleared with Sepharose beads (Sigma, 4B200) for 1 h at 4 °C. Two millilitres of anti-Flag resin was used for 50 ml extract and IP was performed overnight at 4 °C. Following incubation, beads were collected at 2 000 g for 5 min, 1% of the supernatant was retained as flowthrough and the rest was discarded. Beads were subjected to 3 washes for 5 min at 4 °C in cold IP buffer (50 mM Tris HCl pH 8.0, 150 mM NaCl, 1.5 mM MgCl2, 10% Glycerol, 0.1% Triton X-100 with freshly added Protease inhibitors). Rif1FH was eluted by competition with Flag-peptide (Sigma, F4799) through 3 consecutive steps of incubation of the beads with 1 packed volume of Flag-peptide diluted in IP-buffer, for 1 h at 4 °C under rotation. Eluates were concentrated and excessive Flag-peptide was removed using centrifugal filter units (Amicon, Ultra 4; UFC801008) according to the manufacturer’s instructions. Concentrated filtrate was then supplemented with 4X Laemmli loading buffer boiled for 5 min and loaded onto a Tris-glycine 4–20% gradient gel (Life Technologies, EC6028BOX). The gel was run by electrophoresis to one third of its total length and then stained with Coomassie brilliant blue.
Coomassie gel for mass spectrometry
Gel was fixed for 1 h at RT in 45% methanol and 10% acetic acid and rinsed twice with double-distilled H2O (ddH2O). Staining was performed with Coomassie blue solution (3 g/l Coomassie Brilliant blue G-250, Thermo 20279, in 45% methanol, 10% acetic acid) for up to 3 h at room temperature. Following two brief rinses in ddH2O, the gel was de-stained overnight in 45% methanol and 10% acetic acid. Finally, the gel was rinsed twice with ddH2O and stored in 1% acetic acid. After whole lane tryptic digest, mass spectrometry was performed on a Thermo Orbitrap Velos Pro at the Proteomics Core Facility of EMBL Heidelberg.
Rif1 interactome enrichment analysis
Mass-spec raw data were analysed as described in ref. 49. In brief, samples were analysed in duplicates (Rif1+/+) or triplicates (Rif1FH/+) and processed using MaxQuant LFQ 1.5.3.8. A student t-test was performed on data pre-processed with Perseus 1.5.3.2, and p-values were plotted against t-test ratio. Proteins enriched above 1.5 fold were considered as significant interactors. Known human PP1 regulators43 and substrates50, 51 were extracted, combined and subsequently mapped to the corresponding mouse orthologous proteins with BioMart52. Two hundred and eight mouse PP1 regulators/substrates were thus compiled. A Fisher’s exact test (two-sided) was used to check the mouse Rif1 interactome enrichment for PP1 regulators/substrates, and the whole mouse proteome (downloaded on 03.30.2016 from Ensembl, http://www.ensembl.org/) was used as the background set.
Protein expression and purification
The Rif1 protein fragment CRI (2093–2190) was purified as described previously22. Briefly, hexahistidine-tagged Rif1 CRI was purified on TALON (Clontech) beads, and the tag was cleaved by TEV protease. Mutations were introduced using the QuikChange kit (Stratagene). The gene coding for the α-isoform of PP1 (residues 1–330) fused with an N-terminal TEV cleavable hexahistidine tag was synthesized by GenScript and subcloned into pMAL-c2G under control of the Ptac promoter. PP1 was expressed in E. coli BL21 AI (RIL) cells grown in LB medium containing 0.5 mM MnCl2 until OD595 0.6, followed by 1 mM IPTG induction for 20 h at 25 °C. The pellet was resuspended in buffer containing 50 mM Tris-HCl (pH 7.5), 300 mM NaCl, 1 mM MnCl2 in the presence of protease inhibitor cocktail (Roche Applied Science), and disrupted by a microfluidizer. The cleared extract was loaded on a TALON resin and purified according to the manufacturer’s recommendations. The eluted protein was incubated at 4 °C with TEV protease overnight, dialysed against buffer A (20 mM Tris-HCl (pH 7.5), 200 mM NaCl, 1 mM MnCl2, 2 mM DTT, 10% Glycerol), and loaded on a heparin Sepharose column (GE Healthcare). Peak fractions collected after elution by a NaCl gradient were dialysed against the buffer A and stored at −80 °C. Inhibitor 2 was purchased from New England Biolabs.
Co-precipitation assay
The Rif1 fragments CRI (2093–2190), CRII (2226–2340), CRIII (2340–2418), CRI+II (2090–2316), CRII+III (2226–2418) and CRI+II+III (2093–2418) were subcloned into a pGEX-06-P-1 GST expression vector between the BamHI and NotI sites. All the constructs were sequence verified. GST-tagged proteins were expressed in E. coli BL21 AI (RIL) cells grown in LB medium until OD595 0.6, followed by 1 mM IPTG induction for 20 h at 20 °C. The GST-tagged HEAT repeat region (residues 1–966) was expressed in insect cells using the MultiBac system53. The cells were resuspended in PBS containing 5 mM DTT and protease inhibitor cocktail, and sonicated. The cleared extracts were incubated with Glutathione Sepharose 4 Fast Flow (GE Healthcare) beads for 2 h with rocking at 4 °C. The beads were washed two times with PBS. The extract containing hexahistidine-tagged PP1 was prepared as described above, pre-cleared on glutathione sepharose beads, and incubated with beads saturated with the Rif1 fragments overnight, rocking at 4 °C. The beads were then washed 4 times with buffer containing 50 mM phosphate (pH 7.4), 500 mM NaCl and 5 mM DTT. SDS loading buffer was then added to the beads, after which they were boiled at 95 °C for 5 min. The samples were loaded on a 15% SDS-PAGE gel, followed by a transfer on a nitrocellulose membrane. Detection was performed using a primary antibody against the hexahistidine tag with corresponding Alexa532 secondary antibody (Life Technologies). Membranes were imaged using a Typhoon Trio imager (GE Healthcare).
Analytical gel fitration
Chromatography was performed on a Superdex 75 column in buffer containing 20 mM Tris-HCl (pH 7.5), 200 mM NaCl and 2 mM DTT, 1 mM MnCl2. The molecular weight values were calculated by interpolation from the standard curve obtained using a set of standard proteins.
Isothermal titration calorimetry
Rif1 CRI and PP1 were dialyzed overnight into a buffer containing 20 mM Tris-HCl (pH 7.5), 200 mM NaCl, 1 mM MnCl2, 5 mM β-Mercaptoethanol, 10% Glycerol. CRI (140 μM) was titrated into PP1 (18 μM) using an iTC200 micro-calorimeter (Microcal) at 25 °C.
Surface plasmon resonance
Measurements were conducted on a Biacore 3000 (GE Healthcare). PP1 was diluted to 30 ng/µL in 10 mM acetate (pH 5.5) buffer containing 1 mM DTT and 2 mM MnCl2, and covalently coupled to a CM5 sensor chip CM5 following the manufacturer’s protocol. Rif1 CRI and CRI mutants, were diluted with running buffer (10 mM Tris-HCl (pH 7.5), 250 mM NaCl, 1 mM DTT, 2 mM MnCl2, 0.005% Tween 20). Samples were injected over the PP1 and control instead of reference surfaces at a flow rate of 20 µl/min. Non-specific binding was controlled by subtracting the signal obtained on the control surface. Regeneration of the surface was achieved by a short injection of 2 M MgCl2. Each binding experiment was repeated twice. Kinetic parameters were determined by curve fitting with BIAevaluation software (GE Healthcare) using a simple 1:1 binding model.
Nuclear magnetic resonance
The Rif1 CRI fragment was 15N or 15N/13C labelled by growing cells in M9 minimal medium containing [15N]ammonium chloride and [13C6]glucose as a sole nitrogen/carbon source. The protein was concentrated to 100–330 µM in an NMR buffer (50 mM sodium phosphate, 200 mM NaCl, 1 mM DTT, pH 6.8). To analyse the interactions, the complex of PP1 and 15N or 15N/13C labelled CRI was purified on a Superdex 75 gel-filtration column (GE Healthcare) in the NMR buffer and concentrated. NMR measurements were performed at 10 °C on Varian spectrometers operating at a 1H frequency of 600 or 800 MHz. The 1H-15N heteronuclear single quantum coherence (HSQC) spectrum of CRI was assigned using a set of triple resonance experiments: HNCO, intra-residue HN(CA)CO, HN(CO)CA, intra-residue HNCA, HN(COCA)CB and intra-residue HNCACB54.
Dephosphorylation reactions
40 nM PP1 was premixed with interacting protein(s) in assay buffer (50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 1 mM MnCl2, 2 mM DTT, 0.1 mg/ml BSA) and incubated at room temperature for 1 h. Dephosphorylation reactions were initiated by addition of 1 mM pNPP. Optical absorbance of dephosphorylated pNP product (405 nm) was measured at 90 s intervals for 90 min to determine initial reaction rates (Vo).
References
Hardy, C. F., Sussel, L. & Shore, D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes & development 6, 801–814 (1992).
Teixeira, M. T., Arneric, M., Sperisen, P. & Lingner, J. Telomere length homeostasis is achieved via a switch between telomerase- extendible and -nonextendible states. Cell 117, 323–335 (2004).
Gallardo, F. et al. Live cell imaging of telomerase RNA dynamics reveals cell cycle-dependent clustering of telomerase at elongating telomeres. Molecular cell 44, 819–827 (2011).
Shi, T. et al. Rif1 and Rif2 shape telomere function and architecture through multivalent Rap1 interactions. Cell 153, 1340–1353 (2013).
Buonomo, S. B., Wu, Y., Ferguson, D. & de Lange, T. Mammalian Rif1 contributes to replication stress survival and homology-directed repair. The Journal of cell biology 187, 385–398 (2009).
Chapman, J. R. et al. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Molecular cell 49, 858–871 (2013).
Daley, J. M. & Sung, P. RIF1 in DNA break repair pathway choice. Molecular cell 49, 840–841 (2013).
Di Virgilio, M. et al. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science New York, N.Y. 339, 711–715 (2013).
Feng, L., Fong, K. W., Wang, J., Wang, W. & Chen, J. RIF1 counteracts BRCA1-mediated end resection during DNA repair. The Journal of biological chemistry 288, 11135–11143 (2013).
Martina, M., Bonetti, D., Villa, M., Lucchini, G. & Longhese, M.P. Saccharomyces cerevisiae Rif1 cooperates with MRX-Sae2 in promoting DNA-end resection. EMBO Rep (2014).
Silverman, J., Takai, H., Buonomo, S. B., Eisenhaber, F. & de Lange, T. Human Rif1, ortholog of a yeast telomeric protein, is regulated by ATM and 53BP1 and functions in the S-phase checkpoint. Genes & development 18, 2108–2119 (2004).
Zimmermann, M., Lottersberger, F., Buonomo, S. B., Sfeir, A. & de Lange, T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science, N.Y. 339, 700–704 (2013).
Cornacchia, D. et al. Mouse Rif1 is a key regulator of the replication-timing programme in mammalian cells. The EMBO journal 31, 3678–3690 (2012).
Hayano, M. et al. Rif1 is a global regulator of timing of replication origin firing in fission yeast. Genes & development 26, 137–150 (2012).
Hiraga, S. et al. Rif1 controls DNA replication by directing Protein Phosphatase 1 to reverse Cdc7-mediated phosphorylation of the MCM complex. Genes & development 28, 372–383 (2014).
Sreesankar, E., Bharathi, V., Mishra, R. K. & Mishra, K. Drosophila Rif1 is an essential gene and controls late developmental events by direct interaction with PP1-87B. Sci Rep 5, 10679 (2015).
Yamazaki, S. et al. Rif1 regulates the replication timing domains on the human genome. The EMBO journal 31, 3667–3677 (2012).
Foti, R. et al. Nuclear Architecture Organized by Rif1 Underpins the Replication-Timing Program. Molecular cell 61, 260–273 (2016).
Sreesankar, E., Senthilkumar, R., Bharathi, V., Mishra, R. K. & Mishra, K. Functional diversification of yeast telomere associated protein, Rif1, in higher eukaryotes. BMC Genomics 13, 255 (2012).
Xu, D. et al. Rif1 provides a new DNA-binding interface for the Bloom syndrome complex to maintain normal replication. The EMBO journal 29, 3140–3155 (2010).
Escribano-Diaz, C. et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Molecular cell 49, 872–883 (2013).
Sukackaite, R. et al. Structural and Biophysical Characterization of Murine Rif1 C Terminus Reveals High Specificity for DNA Cruciform Structures. The Journal of biological chemistry (2014).
Kanoh, Y. et al. Rif1 binds to G quadruplexes and suppresses replication over long distances. Nature structural & molecular biology 22, 889–897 (2015).
Hendrickx, A. et al. Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem Biol 16, 365–371 (2009).
Bollen, M., Peti, W., Ragusa, M. J. & Beullens, M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 35, 450–458 (2010).
Dave, A., Cooley, C., Garg, M. & Bianchi, A. Protein Phosphatase 1 Recruitment by Rif1 Regulates DNA Replication Origin Firing by Counteracting DDK Activity. Cell Rep 7, 53–61 (2014).
Mattarocci, S. et al. Rif1 Controls DNA Replication Timing in Yeast through the PP1 Phosphatase Glc7. Cell Rep 7, 62–69 (2014).
Guruharsha, K. G. et al. A protein complex network of Drosophila melanogaster. Cell 147, 690–703 (2011).
Moorhead, G. B. et al. Displacement affinity chromatography of protein phosphatase one (PP1) complexes. BMC Biochem 9, 28 (2008).
Trinkle-Mulcahy, L. et al. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. The Journal of cell biology 172, 679–692 (2006).
Zhang, H. et al. A cell cycle-dependent BRCA1-UHRF1 cascade regulates DNA double-strand break repair pathway choice. Nat Commun 7, 10201 (2016).
Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. The Journal of cell biology 196, 801–810 (2012).
Jagiello, I., Beullens, M., Stalmans, W. & Bollen, M. Subunit structure and regulation of protein phosphatase-1 in rat liver nuclei. The Journal of biological chemistry 270, 17257–17263 (1995).
Allen, P. B., Kwon, Y. G., Nairn, A. C. & Greengard, P. Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. The Journal of biological chemistry 273, 4089–4095 (1998).
Booth, D. G. et al. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. Elife 3, e01641 (2014).
Verheyen, T. et al. Genome-wide promoter binding profiling of protein phosphatase-1 and its major nuclear targeting subunits. Nucleic acids research 43, 5771–5784 (2015).
Yumerefendi, H., Tarendeau, F., Mas, P. J. & Hart, D. J. ESPRIT: an automated, library-based method for mapping and soluble expression of protein domains from challenging targets. Journal of structural biology 172, 66–74 (2010).
Beck, M. et al. The quantitative proteome of a human cell line. Mol Syst Biol 7, 549 (2011).
Nagaraj, N. et al. Deep proteome and transcriptome mapping of a human cancer cell line. Mol Syst Biol 7, 548 (2011).
Choy, M. S. et al. Understanding the antagonism of retinoblastoma protein dephosphorylation by PNUTS provides insights into the PP1 regulatory code. Proceedings of the National Academy of Sciences of the United States of America 111, 4097–4102 (2014).
O’Connell, N. et al. The molecular basis for substrate specificity of the nuclear NIPP1:PP1 holoenzyme. Structure 20, 1746–1756 (2012).
Choy, M. S. et al. Structural and Functional Analysis of the GADD34:PP1 eIF2alpha Phosphatase. Cell Rep 11, 1885–1891 (2015).
Heroes, E. et al. The PP1 binding code: a molecular-lego strategy that governs specificity. FEBS J 280, 584–595 (2013).
Hurley, T. D. et al. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. The Journal of biological chemistry 282, 28874–28883 (2007).
Choy, M. S., Page, R. & Peti, W. Regulation of protein phosphatase 1 by intrinsically disordered proteins. Biochem Soc Trans 40, 969–974 (2012).
Andreassen, P. R., Lacroix, F. B., Villa-Moruzzi, E. & Margolis, R. L. Differential subcellular localization of protein phosphatase-1 alpha, gamma1, and delta isoforms during both interphase and mitosis in mammalian cells. The Journal of cell biology 141, 1207–1215 (1998).
Trinkle-Mulcahy, L., Sleeman, J. E. & Lamond, A. I. Dynamic targeting of protein phosphatase 1 within the nuclei of living mammalian cells. Journal of cell science 114, 4219–4228 (2001).
Mendez, J. & Stillman, B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Molecular and cellular biology 20, 8602–8612 (2000).
Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics 13, 2513–2526 (2014).
Duan, G., Li, X. & Kohn, M. The human DEPhOsphorylation database DEPOD: a 2015 update. Nucleic acids research 43, D531–535 (2015).
Li, X., Wilmanns, M., Thornton, J. & Kohn, M. Elucidating human phosphatase-substrate networks. Sci Signal 6 (2013).
Kasprzyk, A. BioMart: driving a paradigm change in biological data management. Database (Oxford) 2011 (2011).
Trowitzsch, S., Bieniossek, C., Nie, Y., Garzoni, F. & Berger, I. New baculovirus expression tools for recombinant protein complex production. Journal of structural biology 172, 45–54 (2010).
Lescop, E. & Brutscher, B. Hyperdimensional protein NMR spectroscopy in peptide-sequence space. J Am Chem Soc 129, 11916–11917 (2007).
Acknowledgements
For mass spectrometry we thank Jeroen Krijgsveld and Stefan Leicht from the EMBL Heidelberg proteomics facility. R.S was an EMBL Interdisciplinary Postdoc (EIPOD) under Marie Curie Actions COFUND [229597]. This work was supported by the French AgenceNationale de la Recherche through ANR JCJC NMRSignal (M.R.J.); ANR ComplexDynamics (M.B); and TGIR-RMN-THC FR3050 CNRS. This work used the platforms of the Grenoble Instruct center (ISBG; UMS 3518 CNRS-CEA-UJF-EMBL) with support from FRISBI (ANR-10-INSB-05-02) and GRAL (ANR-10-LABX-49-01) within the Grenoble Partnership for Structural Biology (PSB). E.E. was supported by the Wenner-Gren Foundations. Research performed by T.A. is supported by Wellcome Trust Principal Research Fellowship funding to Robin Allshire [095021/Z/10/Z] and core funding to the Wellcome Trust Centre for Cell Biology [092076/Z/10/Z].
Author information
Authors and Affiliations
Contributions
R.S. performed all the in vitro experiments; D.C. derived Rif1FH/+ embryonic stem cells, devised the solubilisation protocol and performed the immunoprecipitation and mass spectrometry analysis; E.E. helped with some experiments; D.H. and S.B. designed experiments and co-supervised the project; M.R.J. and M.B. performed NMR experiments and data analyses, P.M. performed molecular biology and protein purification; G.D. and M.K. analysed the list of Rif1-interacting proteins; T.A. analysed the mass spectrometry data.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sukackaite, R., Cornacchia, D., Jensen, M. et al. Mouse Rif1 is a regulatory subunit of protein phosphatase 1 (PP1). Sci Rep 7, 2119 (2017). https://doi.org/10.1038/s41598-017-01910-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01910-1
This article is cited by
-
MBD1 protects replication fork stability by recruiting PARP1 and controlling transcription-replication conflicts
Cancer Gene Therapy (2024)
-
Rif1 restrains the rate of replication origin firing in Xenopus laevis
Communications Biology (2023)
-
Nuclear organisation and replication timing are coupled through RIF1–PP1 interaction
Nature Communications (2021)
-
Heterochromatin replication goes hand in hand with telomere protection
Nature Structural & Molecular Biology (2020)
-
The impact of transcription-mediated replication stress on genome instability and human disease
Genome Instability & Disease (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.