Abstract
Due to the invasiveness nature of tissue biopsy, it is common that investigators cannot collect sufficient normal controls for comparison with diseased samples. We developed a pathway enrichment tool, DRFunc, to detect significantly disease-disrupted pathways by incorporating normal controls from other experiments. The method was validated using both microarray and RNA-seq expression data for different cancers. The high concordant differentially ranked (DR) gene pairs were identified between cases and controls from different independent datasets. The DR gene pairs were used in the DRFunc algorithm to detect significantly disrupted pathways in one-phenotype expression data by combing controls from other studies. The DRFunc algorithm was exemplified by the detection of significant pathways in glioblastoma samples. The algorithm can also be used to detect altered pathways in the datasets with weak expression signals, as shown by the analysis on the expression data of chemotherapy-treated breast cancer samples.
Similar content being viewed by others
Introduction
High-throughput biotechnologies such as microarrays and RNA sequencing (RNA_seq) are generating a large volume of genetic data. Such massive data have promoted the development of various pathway enrichment tools1, which can be divided into three categories: singular enrichment analysis (SEA), gene set enrichment analysis (GSEA) and modular enrichment analysis (MEA)2, 3. SEA usually calculates the enrichment p-value for a pathway based on a list of preselected differentially expressed genes (DEGs) using statistical methods such as Student’s t-test4, 5. GSEA identifies a significant pathway by determining whether the genes of the pathway are ranked at the top or the bottom among all the genes according to their expression differences between two phenotypes6. The enrichment calculation in MEA is similar to that in SEA, but the network topology information is integrated7. These pathway enrichment tools are effective in identifying disease-associated genes with important pathophysiologic roles.
Tissue biopsy is a conventional method to collect samples for cancer diagnosis, monitoring and pathologic analysis8. However, biopsy is frequently very difficult for patients with brain cancer or metastatic cancers9, 10, and more challenging for healthy controls. As a consequence, studies for such diseases typically include very few or even no normal controls11. This situation poses a serious challenge to the common pathway enrichment tools discussed above, as they all compare quantitative expression levels of pathway genes between two phenotypes2, 3. Hereafter we refer to a dataset consisting of samples with only one phenotype (disease) as a one-sided dataset. The control samples for the same disease available in other datasets cannot be incorporated into a one-sided dataset because the quantitative expression values are sensitive to the so-called batch effects between different experiments12, 13. Datasets from the Cancer Genome Atlas database (TCGA) database14 should also be considered as one-sided, since TCGA samples were derived from different institutions and processed in different batches. Therefore, the DEGs detected directly between tumor samples and normal controls from TCGA are questionable without appropriate batch adjustment15. However, batch adjustments may be biased if study groups are not evenly distributed across batches15.
To tackle the above problem, some studies have used the within-sample relative expression orderings (REOs) instead of the quantitative expression values for disease screening16, 17 and gene signaling network analysis18. We previously developed a tool, individPath, to identify patient-specific dysregulated pathways based on reversal REOs in an individual sample compared with the highly stable REOs identified from a large cohort of normal samples which were accumulated previously from various sources19. Compared with the algorithms based on the quantitative expression values, the REO-based algorithms have some unique advantages, including insensitive to batch effects, free of between-sample data normalization, reproducible across independent data17, 20 and reuse of accumulated data21, 22. Therefore, for a one-sided disease dataset, it is intuitive to compare the differences between the REOs in diseased samples and the REOs in control samples which may come from an independent dataset, to identify whether a pathway is altered by the disease or not.
We developed a tool, DRFunc, to identify the pathways which are significantly enriched with differential REOs of the pathway member genes. Using two independent microarray datasets for gastric cancer, lung cancer and breast cancer, respectively, we demonstrated that differential REOs between diseased samples and control samples were reproducible for independent datasets. These differential REOs were preserved even after the control or case samples were changed with the corresponding control or case samples from the other dataset for the same cancer. Using two RNA-seq datasets from TCGA, we showed that differential REOs identified from the sequence-based data are also highly reproducible in the array-based data. The usage of this tool was further exemplified by applying to a one-sided glioblastoma dataset to detect significantly altered pathways. For two expression datasets collected for patients with breast cancer receiving chemotherapy, DRFunc could detect significant pathways which were elusive for the traditional tools which depend on the pre-selected DEGs, in particular when few DEGs could be identified.
Materials and Methods
Data source and data preprocessing
We collected 11 microarray datasets from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/), as shown in Table 1. All of the datasets were measured by the Affymetrix platforms. The raw data were preprocessed by the Robust Multi-array Analysis algorithm23. The SOURCE database24 was used for mapping CloneIDs to GeneIDs. From the Cancer Genome Atlas database (TCGA), two RNA-seq datasets were downloaded (see Table 1). The RNA-seq datasets were measured by the Illumina HiSeq platform. The raw data were normalized25 using the edgeR BioConductor package26.
Pathway databases
The gene ontology (GO), Kyoto encyclopedia of genes and genomes (KEGG) and the Molecular Signatures Database (MSigDB) were used for enrichment analysis in DRFunc. Taking the C2 gene sets of MSigDB as an example, 1330 canonical pathways (as of 16 February 2016) were download from the GSEA website. For a given dataset, all of the measured genes which were annotated in the 1330 pathways were considered as the background genes. In total, there were 8039, 6825 and 8548 genes for the GPL570, GPL96 and Illumina HiSeq2000 platforms, respectively.
Identification of differential REOs between two phenotypes
Given that the expression values of a gene pair (i, j) are denoted as (G i , G j ), R ij , which is 1 if G i > G j and 0 if G i < G j within one sample, is defined as the REO of the gene pair. If two genes have the same expression value, the pair is excluded from analysis. For a dataset with n cases and m controls, differential REOs are identified through the following steps. (1) Calculate the values of R ij (0 or 1) for all pairs in each sample. (2) Count the frequencies of the binary values (1 or 0) of R ij for each pair (i, j) in each phenotype. For example, there are n 1 samples with R ij = 1 and n 2 samples with R ij = 0 in the case group (n 1 + n 2 = n), and m 1 samples with R ij = 1 and m 2 samples with R ij = 0 in the control group (m 1 + m 2 = m). (3) Test the null hypothesis that the frequencies have no association with phenotype (case or control) using the Fisher’s exact test. (4) Select differentially ranked (DR) gene pairs. After the Fisher’s exact test is done for all the pairs, the p-values are corrected to control the false discovery rate (FDR)27. A gene pair is considered as a DR gene pair if the adjusted p-value is less than 5%. Furthermore, for a DR gene pair, there are two possible patterns. If n 1/n 2 > m 1/m 2, the pair is called as Pattern 1, otherwise it is called as Pattern 2.
Reproducibility of DR gene pairs
The binomial test is employed to evaluate the reproducibility between the two lists of DR gene pairs. If a gene pair has the same pattern of reversal REO in the two lists, this gene pair is considered as a concordant gene pair. If two lists of DR gene pairs have M common pairs, the probability of observing at least M 1 concordant gene pairs by chance is calculated by the following cumulative binomial distribution model,
where p 0 is the probability for a random gene pair to be a concordant gene pair by chance between two lists (here p 0 = 0.5 since there are only two mutual-exclusive outcomes, Pattern 1 or Pattern 2, of a DR gene pair). The concordant ratio of these two lists of DR gene pairs is defined as M 1/M. The two lists of DR gene pairs are considered significantly reproducible if P < 0.05.
Pathway enrichment analysis based on DR gene pairs
If k gene pairs are DR gene pairs from n background gene pairs, the probability of observing at least x DR gene pairs in a pathway with a total of m background gene pairs by chance is given by the cumulative hypergeometric distribution function as follows,
The number of the background gene pairs (n) is equal to N(N − 1)/2, where N represents the number of the background genes. The pathways significantly enriched with DR gene pairs were identified after multiple testing adjustments with FDR < 5%27.
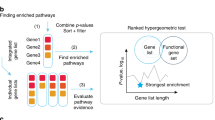
Figure 1 shows the flowchart of DRFunc. The identification of DR gene pairs and detection of significant pathways were implemented in an open-source R package which is available at https://github.com/keyougu/DRFunc.git.
Results
Reproducible DR gene pairs identified between tumor and normal samples
The datasets of gastric cancer, lung cancer and ER− breast cancer which have large sample size were first used to test whether DR gene pairs could be reproducibly identified in different subsets of the same parent dataset. For each dataset, the tumor samples and control samples were randomly divided into two subsets with approximately equal sample size respectively. For example, the 38 tumor samples in GC38-31 were divided into two groups with 19 samples each, while the 31 normal samples were divided into two groups with 15 samples and 16 samples respectively. They formed two subsets, one with 19 tumor samples and 15 normal samples and the other with 19 tumor samples and 16 normal samples. From these two subsets, DR gene pairs were identified and compared. This procedure was repeated 100 times. The result showed that the identified DR gene pairs were highly reproducible, with an average concordant ratio of 99.99% for the dataset of GC38-31 (see Table 2). Similar results were observed for LC91-65 and BC34-17 ER (see Table 2). These results show that the identified DR gene pairs are highly reproducibly within one dataset.
Next, the reproducibility was analyzed for the DR gene pairs identified from different experimental datasets for the same cancer. As shown in Table 3, in the dataset GC12-15, 249,379 DR gene pairs were identified between gastric tumor samples and normal controls, among which 75.67% were also detected as DR gene pairs in dataset GC38-31. Among the overlapped DR gene pairs, 99.97% showed the concordant REOs in the two gastric datasets, which could not happen by random chance (p < 2.2 × 10−16, binomial test). Similar result was observed in the two datasets for lung cancer. In the dataset LC60-60, the one with smaller sample size of the two datasets, 75.18% of the detected DR gene pairs were also identified in the dataset LC91-45 which has larger sample size than LC60-60, and 98.39% of the overlapped DR gene pairs had the concordant REOs in the two datasets, which could not happen by random chance (p < 2.2 × 10−16, binomial test). In the two datasets for ER− breast cancer, the concordant ratio was 99.84%. These results indicate that extensive disruptions of gene REOs existed in tumor samples and such disrupted REOs were reproducible in different datasets. The number of genes in each DR gene pair list were provided in Supplementary file, Table S1.
A further test on reproducibility was carried out to exchange the case and/or control samples between two datasets for the same cancer type. The DR gene pairs identified from the newly exchanged datasets were compared with the DR gene pairs identified from the original datasets. As shown in Table 4, 3,870,438 DR gene pairs were identified in the merged dataset GC12-31 by integrating the normal samples from GC38-31 and the tumor samples from GC12-15, among which 163,670 were included in the DR gene pairs identified from the original dataset GC12-15. Similarly, 4,523,783 DR gene pairs were identified in the merged dataset GC38-15, among which 1,560,772 were found in the original dataset GC38-31. With only the control samples exchanged, the concordant ratios of DR gene pairs between the new datasets and their respective original datasets were 99.19% and 92.41% (Table 4), which were comparable to the concordant ratio between the two original datasets (99.97%) and could not happen by random chance (p < 2.2 × 10−16, binomial test). For lung cancer, the concordant ratios between the control-exchanged datasets and the original datasets were 95.41% and 95.19% respectively, which were also comparable to the concordant ratio between the two original datasets (98.39%). For the two control-exchanged datasets for ER− breast cancer, the concordant ratios were 98.88% and 97.47% respectively, also comparable to the concordant ratio between the two original datasets (99.84%). The detected DR gene pairs were also highly reproducible in the case-exchanged datasets: the minimum concordant ratio was as high as 97.06% (see Supplementary file, Table S2). These analyses further indicate that differential REOs for a specific tumor type could be reproducibly detected from independent datasets of different sources. Therefore, when focusing on the REOs of genes, tumor samples and normal samples measured by different studies can be directly compared.
Performance of DRFunc in detecting significant pathways
Significant pathways were detected from the 1330 MSigDB C2 collection by employing the cumulative hypergeometric distribution test implemented in DRFunc. With FDR < 5%, 73 and 239 pathways were detected, respectively, to be significantly enriched with the DR gene pairs identified from GC12-15 and GC38-31. For lung cancer, 255 and 380 pathways were detected in LC60-60 and LC91-65, respectively. For ER− breast cancer, 363 and 366 pathways were detected for BC12-27 ER and BC34-17 ER, respectively. The overlapped pathways were shown in Fig. 2. The pathway names were listed in Supplementary file. Notably, there were 17 pathways commonly detected in the six datasets for the three caner types, including the ECM receptor interaction, focal adhesion pathways in KEGG, the RB1 and integrin related pathways in PID. There were 290 pathways commonly detected for at least two cancer types, indicating that different cancers may have many similar enriched pathways. These results suggest that the REOs of genes in many pathways were significantly disrupted under cancerous conditions, and DRFunc could capture such disruptions.
With FDR < 5%, 183 and 307 significant pathways were detected, respectively, in the control-exchanged datasets GC12-31 and GC38-15 for gastric cancer. In the two control-exchanged datasets for lung cancer, 440 and 383 pathways were detected, respectively, and in the two control-exchanged datasets for ER− breast cancer, 428 and 529 pathways were detected, respectively. Figure 3 show the number of overlapped significant pathways detected for the original datasets and for the control-exchanged datasets. This indicate that integration of cancer samples and control samples from different datasets is feasible using the DR gene pairs in order to detect significant pathways.
One RNA-seq dataset with 125 adenocarcinoma samples and 37 normal controls from 20 batches, denoted as LUAD125-37 were downloaded from TCGA (see Table 1). By applying DRFunc, 7,661,296 DR gene pairs were identified and they were significantly enriched in 530 pathways. These results were compared with those obtained from the microarray datasets. There were 2,656,494 overlapped gene pairs between the 5,035,285 DR gene pairs identified in LC60-60 and the 7,661,296 DR gene pairs identified in LUAD125-37. Among these overlapped DR gene pairs, 97.48% showed the concordant REOs between the microarray-based and sequence-based results, which could not happen by random chance (p < 2.2 × 10−16, binomial test). Among the 255 pathways detected for LC60-60, 215 pathways were also detected for LUAD125-37. There were 3,611,571 pairs overlapped between the DR gene pairs identified in LC91-65 and in LUAD125-37 with a concordant ratio of 95.58% (p < 2.2 × 10−16, binomial test). Of the 380 pathways detected in LC91-65 291 were also detected in the sequence-based dataset LUAD125-37. Similar results were observed for colorectal cancer, for which one microarray dataset and one RNA-seq dataset were collected (see Table 1). The concordant ratio of the DR gene pairs between CRC32-32 and COAD285-41 was as high as 97.81%. Of the 283 significant pathways detected in CRC32-32, 205 were also detected in COAD285-41. These results suggest that differential REOs between case and control samples identified by DRFunc had cross-platform reproducibility.
To address whether pathways detected by using DRFunc is robust, random experiments were performed by adding different proportions of arbitrarily chosen gene pairs from the background into the real DR gene pairs identified between cases and controls in each dataset. The result showed that the pathways detected by DRFunc were robust (see Supplementary file). This conclusion is consistent with the viewpoint that functional categories are robust to different levels of noises28.
Application of DRFunc to one-sided GBM data
To demonstrate the applicability of DRFunc in one-sided data, two datasets, GBM70-0 with 70 samples of primary GBM samples10 and GBM34-13 with 34 primary GBM samples and 13 normal brain tissue samples29, were collected. By integrating the GBM samples in GBM70-0 and the normal samples in GBM34-13 (denoted as integrated GBM70-13 dataset) 5,756,553 DR gene pairs were identified. In the dataset GBM34-13 itself, 3,659,102 DR gene pairs were identified, among which 80.84% overlapped with the former DR gene pairs. In particular, 99.85% of the overlapped gene pairs had the concordant REOs in the two groups of GBM patients. With FDR < 5%, 363 pathways were detected to be significantly enriched with the DR gene pairs identified in the integrated GBM70-13 dataset. Meanwhile, 324 pathways were identified in GBM34-13, among which 266 were also detected in the integrated GBM70-13 dataset. They were listed in the supplementary material. As the 1330 MSigDB C2 pathways integrated several online pathway databases with redundancy, the number of the detected pathways was also showed in Fig. 4, grouped by the pathway database source. Many of the pathways were found to be associated with GBM in literature, including the BioCarta EGF pathway and MTOR pathway30, the KEGG P53 signaling pathway and the PID TGF-β and Ras signaling pathway31.
Since the number of GBM samples in GBM70-0 was approximately twice of that in GBM34-13, we performed resampling experiments to evaluate the effect of sample size. A subset of 34 GBM samples were randomly extracted from GBM70-0 and integrated with the normal samples from GBM34-13 for DR gene pair identification and significant pathway detection. This random experiment was repeated 100 times. The average number of DR gene pairs identified in the resampling experiments was 5,046,700, and the average concordant ratio was 99.91% with the 3,659,102 DR gene pairs identified in GBM34-13. The average number of significant pathways detected in the 100 resampling experiments was 374.80, and the average of overlapped pathways were 271.40 with the 324 pathways detected in GBM34-13. These results suggest that DRFunc could be used in one-sided data to detect the underlying dysregulated and disease associated biological pathways.
The DRFunc algorithm was compared with two pathway analysis algorithms, SEA5 and GSEA6. As no controls were collected in GBM70-0, the traditional SEA analysis could not be applied5. In GBM34-13, 8,731 DEGs were identified (FDR < 5%, Student’s t-test). Using SEA, these DEGs were significantly enriched in 41 MSigDB pathways5, much fewer than the number of pathways detected by DRFunc in GBM34-13. Notably, the above mentioned EGR, MTOR, P53, TGF-β and Ras signaling pathways detected by DRFunc were not included in these 41 pathways. In contrast, 32 of these 41 pathways were also detected by DRFunc. When using GSEA, even with FDR < 25%, no significant pathways were detected in GBM34-13. These results suggest that the rank-based tool DRFunc could identify much more biologically meaningful pathways than the traditional enrichment analysis.
DRFunc can detect pathways with only a few DEGs, since a dysregulated gene with a large change in quantitative expression level may result in many DR gene pairs. For example, the BioCarta EIF4 pathway, which mainly describes the regulation of eIF4E and p70 S6 kinase, contained 24 genes measured in the GBM34-13 dataset, among which only nine genes were identified as DEGs using Student’s t-test. The percentage of DEGs was only 37.50% in this pathway, while the percentage of DEGs was 47.54% in the background. Thus this pathway was not detected as significant by SEA. In contrast, these 24 genes formed 276 gene pairs, among which 53 were identified as DR gene pairs in GBM34-13. Therefore the pathway was detected to be significant by DRFunc. It has been reported that the overexpression of eIF4E could cause oncogenic transformation and elevated eIF4E protein levels were found in many human cancers including GBM32, 33. Interestingly, PRKCB in this pathway involved in 20 DR gene pairs, and its average expression level was higher than the expression levels of all its 20 partner genes in the normal samples but became lower than the expression levels of all its 20 partner genes in the GBM samples. That is to say that PRKCB was down-regulated greatly in GBM. This was consistent with the expression level changes as observed in GBM34-13 and literature results reported for GBM34 as well as for other cancer types35. Similarly, WIF1 in KEGG WNT signaling pathway was found to be down-regulated greatly in GBM by comparing its expression level with those of 127 partner genes. This was consistent with the result reported previously36. These two examples suggest that such strongly dysregulated genes could lead to a high appearance frequency in DR gene pairs and make the associated pathways detectable by DRFunc.
Application of DRFunc to preoperative chemotherapy response data of breast cancer
A pathway with only a few DEGs cannot be detected by SEA but it may be significantly enriched with DR gene pairs. This hinted us that DRFunc might be able to capture functional disruptions in data with weak expression signals. Breast cancer patients with the pathological complete response (pCR) have a favorable prognosis compared to patients with residual disease (RD) and our previous analysis has shown that expression differences between these two conditions could be weak37. Two gene expression datasets were collected for preoperative chemotherapy response of breast cancer (see Table 1) to test whether DRFunc could identify such weak expression signals. Using Student’s t-test with FDR < 5%, one gene was identified as DEG between 61 RD patients and 19 pCR patients in the dataset BC61-19 Response, indicating that expression differences between these two conditions were small. Because there was only one DEG, it was unable to detect significantly dysregulated pathways using SEA. However, using DRFunc, 9,569 DR gene pairs were identified with FDR < 5%, which significantly enriched in 38 MSigDB pathways (Supplementary file). In BC68-46 Response, 321 genes were identified as DEGs between 68 RD patients and 46 pCR patients, significantly enriched in 28 MSigDB pathways as detected by SEA. With FDR < 5%, DRFunc identified 90,561 DR gene pairs, which significantly enriched in 84 MSigDB pathways (Supplementary file). When using GSEA, with FDR < 5%, no significant pathways were detected in either of the two datasets. These results suggested that the rank-based algorithm DRFunc could identify more biological pathways than the traditional enrichment analysis, especially when the expression differences were not significant.
Discussion
Gene expression profiling for only one phenotype is frequently seen in experimental design when sampling of normal control tissues is difficult due to the invasive nature of biopsy9. For such one-sided data, current functional enrichment analysis tools which focus on quantitative expression differences between two phenotypes have difficulty in finding phenotype-related functional pathways. The within-sample REOs have been found robust against systematic batch effects and transferable among independent datasets which enables the reuse of accumulated samples19, 21, 38. In the present work, we proposed an REO-based algorithm DRFunc, which could robustly identify the underlying disturbed pathways from such one-sided dataset by integrating control samples of the same tissue measured by other independent experiments.
Our analyses showed that the DR gene pairs identified by DRFunc for gastric cancer, lung cancer and ER− breast cancer were highly reproducible among independent datasets and among datasets with case-control samples integrated from different studies. The comparison between microarray-based and sequence-based data for lung cancer and colorectal cancer also suggested the high cross-platform reproducibility of DR gene pairs identified by DRFunc. Such consistent DR gene pairs were previously observed among datasets generated by different microarray platforms39.
The power of DRFunc may be influenced by the sample size in detecting DR gene pairs. For example, with FDR < 5%, 249,379 DR gene pairs were identified from the smaller-size dataset of GC12-15, which was less than ten-fold of the number of DR gene pairs (3,060,113) identified from the larger-size dataset of GC38-31 (Table 3). The insufficient sample size for any of the datasets will reduce the number of overlapped DR gene pairs40. Although the numbers of DR gene pairs in GC12-31 and GC38-15 were almost the same, the overlapped DR gene pairs between GC12-31 and GC12-15 was ten-fold less than the number of overlapped DR gene pairs between GC38-15 and GC38-31 (Table 3). The reduced power of DR gene pair identification will ultimately reduce the power of significant pathway detection. As shown in Fig. 3, only 73 pathways were significantly enriched with the DR gene pairs identified in GC12-15, whereas 239 significant pathways were detected in GC38-31.
Some DR gene pairs may not overlap between different experiments. This is probably due to the fact that an experiment cannot capture all disease-associated differential signals, thus different experiments for the same disease may capture only partial DEGs each40. For example, among the top 100 genes with the highest appearance frequencies in the DR gene pairs identified only in GC38-31, not in GC12-15, 65 were identified as DEGs in GC38-31, not in GC12-15 (Student’s t-test, FDR < 5%). Non-overlapped DEGs would result in non-overlapped DR gene pairs between different experiments.
Due to the above mentioned reasons, ultimately, some significant pathways cannot overlap between different datasets for the same disease. The problem of pathway overlaps has been discussed, and it has been suggested that the significant pathways could be rather functionally similar by reducing their corresponding statistical significance levels5, 41.
It has been reported that many confounding factors such as gender and ethnicity may lead to gene expression differences among individuals42,43,44,45. Therefore, the two datasets (LC91-65 and LC60-60) for lung cancer with larger sample sizes were used to evaluate whether heterogeneous gene expression exists among normal samples. Information on the normal samples of the two lung cancer datasets was available in Supplementary file, Table S3. The normal samples in LC91-65 were obtained from 41 males, 11 females and 13 samples without gender information. Comparing the gene expression profiles of the 41 males and 11 females, only 0.03% of the background gene pairs could be identified as DR gene pairs. However, when comparing the 11 normal female samples of Caucasian in LC91-65 to the 60 normal female samples of Chinese in LC60-60, about 3.50% of the background gene pairs were found as DR gene pairs. This result indicates that ethnicity might be a confounding factor, which might introduce some disease-irrelevant DR gene pairs. Consequently, when applying DRFunc to detect DR gene pairs for significant pathway detection, some disease-irrelevant pathways may creep in.
In spite of this, comparing to the traditional pathway enrichment analysis methods based on quantitative gene expression levels, which have limited usage with one-sided data, DRFunc has superiority in providing candidate pathways. To evaluate whether a significant pathway detected by DRFunc have specific biological implications or not, it is required to generate some biological hypotheses for wet lab experiment (such as Q-PCR) validation5. In this paper, we firstly showed that, in the one-sided GBM data, 266 of the 363 pathways detected in GBM70-13 could be reproducibly detected in the other dataset GBM34-13, which shares the same normal samples. Then, in the two application examples, besides the pathways already discussed in the Result section, we have additionally found evidence from published literature for the top 10 most significant pathways to support their association with the corresponding phenotype (Supplementary file). Further, to show that the significant pathways detected by DRFunc could not be detected if no phenotype differences exist, we have additionally performed random experiments by randomly reassigning labels to the disease and normal samples. By independently permuting the 70 GBM samples from GBM70-0 and 13 normal samples from GBM34-13 for 100 times, only 10.70 significant pathways were detected on average. When applying the same randomization procedure to BC68-46 Response dataset, only 7.02 pathways were detected on average. All these results support the ability of DRFunc in providing candidate disease-associated significant pathways using gene expression data even the one-sided data. Finally, if only a limited (or insufficient) number of normal control samples for a tissue were obtained in a study, normal samples from other independent datasets should be integrated for DR gene pair identification to reduce disease-irrelevant DR gene pairs introduced by population variations.
In conclusion, through detection of DR gene pairs between diseased samples and normal controls collected from different experiments, disease-relevant pathways can be identified, which provide functional insights into the disease mechanism. The usage of the DR gene pairs instead of the DEGs enables us to make adequate use of the large one-sided disease samples and the samples with weak expression signals available in public data archives. This may facilitate many downstream analyses such as survival prediction. Our algorithm also provides a new tool for comparing transcriptional expression profiling of genes between two groups of samples from the same or different experiments.
References
Emmert-Streib, F. & Glazko, G. V. Pathway analysis of expression data: deciphering functional building blocks of complex diseases. PLoS Comput Biol 7, e1002053, doi:10.1371/journal.pcbi.1002053 (2011).
Khatri, P., Sirota, M. & Butte, A. J. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol 8, e1002375, doi:10.1371/journal.pcbi.1002375 (2012).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37, 1–13, doi:10.1093/nar/gkn923 (2009).
Huang, D. W. et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 35, W169–175, doi:10.1093/nar/gkm415 (2007).
Hong, G., Zhang, W., Li, H., Shen, X. & Guo, Z. Separate enrichment analysis of pathways for up- and downregulated genes. Journal of the Royal Society, Interface 11, 20130950–20130950, doi:10.1098/rsif.2013.0950 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 102, 15545–15550, doi:10.1073/pnas.0506580102 (2005).
Tarca, A. L. et al. A novel signaling pathway impact analysis. Bioinformatics 25, 75–82, doi:10.1093/bioinformatics/btn577 (2009).
Hyun, K. A., Kim, J., Gwak, H. & Jung, H. I. Isolation and enrichment of circulating biomarkers for cancer screening, detection, and diagnostics. The Analyst 141, 382–92, doi:10.1039/c5an01762a (2015).
Heitzer, E. et al. Tumor-associated copy number changes in the circulation of patients with prostate cancer identified through whole-genome sequencing. Genome medicine 5, 30, doi:10.1186/gm434 (2013).
Reifenberger, G. et al. Molecular characterization of long-term survivors of glioblastoma using genome- and transcriptome-wide profiling. International journal of cancer. Journal international du cancer 135, 1822–1831, doi:10.1002/ijc.28836 (2014).
Ein-Dor, L., Zuk, O. & Domany, E. Thousands of samples are needed to generate a robust gene list for predicting outcome in cancer. Proceedings of the National Academy of Sciences of the United States of America 103, 5923–5928, doi:10.1073/pnas.0601231103 (2006).
Leek, J. T. et al. Tackling the widespread and critical impact of batch effects in high-throughput data. Nature reviews. Genetics 11, 733–739, doi:10.1038/nrg2825 (2010).
Rung, J. & Brazma, A. Reuse of public genome-wide gene expression data. Nature reviews. Genetics 14, 89–99, doi:10.1038/nrg3394 (2013).
Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068, 10.1038/nature07385 (2008).
Nygaard, V., Rodland, E. A. & Hovig, E. Methods that remove batch effects while retaining group differences may lead to exaggerated confidence in downstream analyses. Biostatistics 17, 29–39, doi:10.1093/biostatistics/kxv027 (2016).
Geman, D., d’Avignon, C., Naiman, D. Q. & Winslow, R. L. Classifying gene expression profiles from pairwise mRNA comparisons. Statistical applications in genetics and molecular biology 3, 1–19, doi:10.2202/1544-6115.1071 (2004). Article19.
Tan, A. C., Naiman, D. Q., Xu, L., Winslow, R. L. & Geman, D. Simple decision rules for classifying human cancers from gene expression profiles. Bioinformatics 21, 3896–3904, doi:10.1093/bioinformatics/bti631 (2005).
Eddy, J. A., Hood, L., Price, N. D. & Geman, D. Identifying tightly regulated and variably expressed networks by Differential Rank Conservation (DIRAC). PLoS Comput Biol 6, e1000792, doi:10.1371/journal.pcbi.1000792 (2010).
Wang, H. et al. Individualized identification of disease-associated pathways with disrupted coordination of gene expression. Briefings in bioinformatics 17, 78–87, doi:10.1093/bib/bbv030 (2015).
Afsari, B., Fertig, E. J., Geman, D. & Marchionni, L. switchBox: an R package for k-Top Scoring Pairs classifier development. Bioinformatics 31, 273–274, doi:10.1093/bioinformatics/btu622 (2015).
Tyack, P. L., Calambokidis, J., Friedlaender, A., Goldbogen, J. & Southall, B. Formal Comment on Schorr, G. S., Falcone, E. A., Moretti, D. J., Andrews, R. D. (2014) First Long-Term Behavioral Records from Cuvier’s Beaked Whales (Ziphius cavirostris) Reveal Record-Breaking Dives. PLoS ONE 9(3), e92633, doi:10.1371/journal.pone.0092633. PLoS One 10, e0142287, doi:10.1371/journal.pone.0142287 (2015).
Dembele, D. & Kastner, P. Fold change rank ordering statistics: a new method for detecting differentially expressed genes. BMC bioinformatics 15, 14, doi:10.1186/1471-2105-15-14 (2014).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264, doi:10.1093/biostatistics/4.2.249 (2003).
Diehn, M. et al. SOURCE: a unified genomic resource of functional annotations, ontologies, and gene expression data. Nucleic Acids Res 31, 219–223, doi:10.1093/nar/gkg014 (2003).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11, R25, doi:10.1186/gb-2010-11-3-r25 (2010).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140, doi:10.1093/bioinformatics/btp616 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 57, 289–300 (1995).
Fontanillo, C., Nogales-Cadenas, R., Pascual-Montano, A. & De las Rivas, J. Functional analysis beyond enrichment: non-redundant reciprocal linkage of genes and biological terms. PLoS One 6, e24289, doi:10.1371/journal.pone.0024289 (2011).
Griesinger, A. M. et al. Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol 191, 4880–4888, doi:10.4049/jimmunol.1301966 (2013).
Rao, R. D. et al. Disruption of parallel and converging signaling pathways contributes to the synergistic antitumor effects of simultaneous mTOR and EGFR inhibition in GBM cells. Neoplasia 7, 921–929, doi:10.1593/neo.05361 (2005).
Kanu, O. O. et al. Glioblastoma Multiforme Oncogenomics and Signaling Pathways. Clinical medicine. Oncology 3, 39–52 (2009).
Cui, J. et al. c-Jun NH(2)-terminal kinase 2alpha2 promotes the tumorigenicity of human glioblastoma cells. Cancer research 66, 10024–10031, doi:10.1158/0008-5472.CAN-06-0136 (2006).
De Benedetti, A. & Graff, J. R. eIF-4E expression and its role in malignancies and metastases. Oncogene 23, 3189–3199, doi:10.1038/sj.onc.1207545 (2004).
Heroux, M. S. et al. Comprehensive characterization of glioblastoma tumor tissues for biomarker identification using mass spectrometry-based label-free quantitative proteomics. Physiological genomics 46, 467–481, doi:10.1152/physiolgenomics.00034.2014 (2014).
Wallace, J. A. et al. Protein kinase C Beta in the tumor microenvironment promotes mammary tumorigenesis. Frontiers in oncology 4, 87, doi:10.3389/fonc.2014.00087 (2014).
Lambiv, W. L. et al. The Wnt inhibitory factor 1 (WIF1) is targeted in glioblastoma and has a tumor suppressing function potentially by induction of senescence. Neuro-oncology 13, 736–747, doi:10.1093/neuonc/nor036 (2011).
Zhang, L. et al. Rank-based predictors for response and prognosis of neoadjuvant taxane-anthracycline-based chemotherapy in breast cancer. Breast cancer research and treatment 139, 361–369, doi:10.1007/s10549-013-2566-2 (2013).
Hong, F. et al. RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics 22, 2825–2827, doi:10.1093/bioinformatics/btl476 (2006).
Qi, L. et al. Critical limitations of prognostic signatures based on risk scores summarized from gene expression levels: a case study for resected stage I non-small-cell lung cancer. Briefings in bioinformatics 17, 233–242, doi:10.1093/bib/bbv064 (2016).
Zhang, M. et al. Apparently low reproducibility of true differential expression discoveries in microarray studies. Bioinformatics 24, 2057–2063, doi:10.1093/bioinformatics/btn365 (2008).
Yang, D. et al. Gaining confidence in biological interpretation of the microarray data: the functional consistence of the significant GO categories. Bioinformatics 24, 265–271, doi:10.1093/bioinformatics/btm558 (2008).
Irizarry, R. A. et al. Multiple-laboratory comparison of microarray platforms. Nat Methods 2, 345–350, doi:nmeth756 (2005).
Puthiyedth, N., Riveros, C., Berretta, R. & Moscato, P. A New Combinatorial Optimization Approach for Integrated Feature Selection Using Different Datasets: A Prostate Cancer Transcriptomic Study. PLoS One 10, e0127702, doi:10.1371/journal.pone.0127702 (2015).
Pirone, J. R. et al. Age-associated gene expression in normal breast tissue mirrors qualitative age-at-incidence patterns for breast cancer. Cancer Epidemiol Biomarkers Prev 21, 1735–1744, doi:10.1158/1055-9965.EPI-12-0451 (2012).
Joseph, S. et al. Expression of drug transporters in human kidney: impact of sex, age, and ethnicity. Biol Sex Differ 6, 4, doi:10.1186/s13293-015-0020-3 (2015).
Acknowledgements
We would like to sincerely thank Dr. Andrew M. Donson for his kind assistance in providing the detailed cancer subtype information of the GBM samples. This work was supported in part by the National Natural Science Foundation of China (Grant Nos 81501829, 81501215, 81372213, 81572935), Natural Science Foundation of Fujian Province (Grant No. 2016J01706) and the Joint Fund for Program of Science and Technology innovation of Fujian Province (Grant No. 2016Y9102).
Author information
Authors and Affiliations
Contributions
All authors meet the authorship requirements. G.N.H. and H.D.L. contributed equally to this work. G.N.H., H.D.L. and Z.G. designed the study. J.H.Z. and Q.Z.G. analyzed the data. R.C. collected the gene expression datasets. G.N.H. and H.D.L. drafted the manuscript. Z.G. revised the manuscript critically for important intellectual content. G.N.H., H.D.L. and Z.G. agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, G., Li, H., Zhang, J. et al. Identifying disease-associated pathways in one-phenotype data based on reversal gene expression orderings. Sci Rep 7, 1348 (2017). https://doi.org/10.1038/s41598-017-01536-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01536-3
This article is cited by
-
Integrated analysis of diverse cancer types reveals a breast cancer-specific serum miRNA biomarker through relative expression orderings analysis
Breast Cancer Research and Treatment (2024)
-
Pollution assessment of heavy metal accumulation in the farmland soils of Beijing’s suburbs
Environmental Science and Pollution Research (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.