Abstract
The discovery of multi-species synchronous spawning of scleractinian corals on the Great Barrier Reef in the 1980s stimulated an extraordinary effort to document spawning times in other parts of the globe. Unfortunately, most of these data remain unpublished which limits our understanding of regional and global reproductive patterns. The Coral Spawning Database (CSD) collates much of these disparate data into a single place. The CSD includes 6178 observations (3085 of which were unpublished) of the time or day of spawning for over 300 scleractinian species in 61 genera from 101 sites in the Indo-Pacific. The goal of the CSD is to provide open access to coral spawning data to accelerate our understanding of coral reproductive biology and to provide a baseline against which to evaluate any future changes in reproductive phenology.
Measurement(s) | Point in Time Date and Time Data Type • Spawning |
Technology Type(s) | Observation • digital curation |
Sample Characteristic - Organism | Scleractinia |
Sample Characteristic - Environment | ocean |
Sample Characteristic - Location | Pacific Ocean • Indian Ocean region |
Machine-accessible metadata file describing the reported data: https://doi.org/10.6084/m9.figshare.13100552
Similar content being viewed by others
Background & Summary
Scleractinian corals are the ecosystem engineers of coral reefs, the most species-rich marine ecosystems. Scleractinian corals have a bipartite life history, with a sessile adult stage and a planktonic larval stage that allows dispersal among reefs. Corals produce larvae in one of two ways: gametes are broadcast-spawned for external fertilization or the eggs are retained for internal fertilization, followed by the release of planula larvae from the polyp. The discovery of multi-species synchronous spawning on the Great Barrier Reef1 stimulated a large effort to document coral spawning times in other regions of the world. Similar multi-species spawning events sensu2 have now been documented in over 25 locations throughout the Indo-Pacific3,4,5. However, much additional data on coral sexual reproductive patterns remain unpublished. Even when spawning data are published, there is often insufficient detail, such as the precise time and duration of spawning, to address many important questions. Consequently, predicting the month of spawning has been the focus of many studies to date6.
Coral spawning times can be used to address many significant and fundamental questions in coral reef ecology. Most coral species are notoriously difficult to identify and spawning times have been used to infer pre-zygotic barriers to fertilization and thus assist decisions about species boundaries7,8. While proximate cues associated with the month of spawning are reasonably well understood in some taxa6,9, the relationship between cues for the date and time of spawning are poorly understood. Similarly, potential phylogenetic patterns and geographical variation in spawning times are only beginning to be explored10. Knowing when corals spawn is also important for managing coastal development. For example, in Western Australia, legislation requires dredging operations to cease during mass spawning events11,12. Coral spawning is also an economic boon for tourist operators in many parts of the world, such as the Great Barrier Reef. Furthermore, population level records of spawning times provide a baseline against which to evaluate potential changes in spawning synchrony or seasonality associated with anthropogenic disruptions to environmental cues, in particular, sea surface temperature13. Knowledge of the timing of spawning is also essential for accurately estimating levels of connectivity among populations, given season differences in current flow14. The value of long-term species level data on coral spawning has recently been demonstrated in a test of the influence of temperature and wind on the night of coral spawning15.
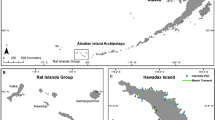
In this data descriptor, we present the Coral Spawning Database (CSD). The CSD includes spawning observations for reef building coral species from the Indo-Pacific. The CSD includes 6178 observations (3085 of which were unpublished) of the time or day of spawning for 300+ scleractinian species in 61 genera (Online-only Table 1) from 101 sites (Fig. 1) in the Indo-Pacific. The goals of the CSD are: (i) to assemble the scattered and mostly unpublished observations of scleractinian coral spawning times and (ii) to make these data readily available to the research community. Our vision is to help advance many aspects of coral reef science and conservation at a time of unprecedented environmental and societal change.
Methods
The CSD includes spawning times for broadcast spawning scleractinian coral species in the Indo-Pacific. There are two sources for these data: the literature and unpublished observations. Published literature was selected based on the authors’ knowledge of the subject area and a literature search using the terms “coral AND spawn*”. Over 50 researchers known by the authors to have extensive data on coral spawning times were approached to contribute unpublished data. This initial invitation led to a subsequent round of invitations to additional contributors. Of course, we encourage any researchers with data we have missed to contribute their observations in the annual update of the database. The database focusses on spawning times. Many other biological variables related to coral reproduction, such as fecundity, are available in the Coral Traits Database16.
The database is available as a Microsoft Access relational database or an Excel spreadsheet. To minimise repetition in data entry, spawning observation information is entered in three primary tables (Fig. 2). The first (“tblSitesForSpawningObservations”) is used to enter geographic information on each study site; the second (“tblSpawningObervations”) contains details of the spawning activity recorded at each site; the third (“tblReferencesForSpawningObervations”) contains either full bibliographic details for published studies or details of the source of unpublished data. To assist with data analysis, three accessory tables are also linked. The first (“tblEcoregionsVeron2015”) allows sites to be grouped into the biogeographical Ecoregions proposed by17 or by broader region (e.g. Indian Ocean, Western and Central Pacific, Eastern Pacific). The remaining two tables allow the coral species to be grouped systematically for analysis. The first (“tblCoralSpecies”) has a list of over 1600 coral species with genus and species names (primarily from18 or subsequent descriptions of new species) mapped to currently accepted names (primarily from19) where the taxonomy has changed. The second (“tblSystematics”) allows species to be grouped into major clades or currently accepted families19 as revealed by molecular studies20,21,22.
Data entry
Coral Spawning Database fields
-
1)
Site information (in tblSitesForSpawningObservations):
Ecoregion_ID link to Ecoregions (150) as defined by17
Country the country, territory (e.g. Guam) or island group (e.g. Hawaiian Islands) where spawning observation was made
Site accepted name for broad geographical location (e.g. archipelago, island, offshore reef, bay, etc.) of the observation
Subsite more precise site name within location (where applicable; na entered where no subsite)
Latitude in decimal degrees (-ve values for sites South of the Equator).
Longitude in decimal degrees (-ve values for sites West of the Greenwich Meridian).
-
2)
Spawning observations (in tblSpawningObservations):
Depth_m the approximate depth at which the colony was collected (for ex situ observations) or observed (for in situ observations). If not recorded then −99 entered.
Genus currently accepted genus name19
O_n open nomenclature qualifier: see explanation below under “Species identifications”.
Species the species name used by the observer
Date date of spawning observation in the format day/month/year (e.g. 24/11/1983)
N number of colonies or individuals observed spawning. Used −99 if not known. If exact number of colonies not counted but more than a specific number were observed to spawn (e.g. > 25), then minimum number counted was entered (e.g. 25).
Start_time time of first observation of spawning for colony(ies) of species: time (hh:mm) on a 24 hour clock e.g. 18:30. See “recording the time of spawning” below for ways to use the time fields to capture the various ways spawning is usually observed. No threshold applied to the intensity of spawning.
No_start no information on time that spawning started: True or False.
Quality_start if No_start is False, Exact or Approx.
End_time time of last observation of spawning for colony(ies) of species (if later than start time, normally): time (hh:mm) on a 24 h clock e.g. 18:30
No_end no information on time that spawning ended: True or False
Quality_end if No_end is False, Exact or Approx
Gamete_release (five character states as follows)
-
Bundles – eggs and sperm released together packaged in bundles
-
Eggs – only eggs released
-
Sperm – only sperm released
-
Both separately – eggs and sperm released separately from the same colony. Examples include Lobophyllia hemprichii and Goniastrea favulus
-
Not recorded – release of gametes not observed or not reported
Situation In situ = spawning observed underwater or Ex situ = spawning observed in tanks of colony(ies) recently removed from the reef.
Timezone local time zone on the date of the spawning observation. This allows local time of spawning to be related to local time of sunset (or occasionally sunrise, for daytime spawners). This field is not an integer to accommodate 30 minute time differences (e.g. India and Sri Lanka are on UTC + 5.5). Enter -ve values for sites west of the Greenwich Meridian: e.g. −11 for Hawaii. (Note: Daylight Saving Times mean that time zones at some sites vary with date, e.g. Fiji goes from UTC + 12 to UTC + 13 from early November to early January).
The next four fields contain benchmarks for comparing spawning among sites for different species or groups of species23. The first is the date of the nearest full moon (DoNFM) to the date of spawning (with 75% of spawning recorded in the week after the full moon). This allows all spawning dates to be calculated in terms of days before or after the full moon (DoSRtNFM). Sunset provides a benchmark for comparing the times of spawning for most spawners (over 90% of spawning started within 4 hours of sunset) and sunrise for a few daytime spawners such as Pocillopora verrucosa. Dates of full moon and times of sunrise and sunset are available for given locations from the web (e.g. www.timeanddate.com) and can be entered manually. However, they can also be calculated automatically in the database based on the date, time zone and, for sunrise and sunset, the latitude and longitude. Excel spreadsheets are also available on request from the corresponding authors to calculate dates of full moon and times of sunrise and sunset in addition to a data entry template.
DoNFM Date of Nearest Full Moon. Calculated automatically and corrected for longitude based on the local time zone.
DoSRtNFM Date of Spawning Relative to Nearest Full Moon. Calculated automatically using time zone and date of observation in days before (-ve) or after ( + ve) the nearest full moon (ranges from −15 days to + 14 days).
Sunset local time of sunset using a 24 h clock e.g. 18:30. Sunset and sunrise times were calculated for each observation based on latitude, longitude and time zone of the site and the date, using the method in the NOAA solar calculations day spreadsheet at https://www.esrl.noaa.gov/gmd/grad/solcalc/calcdetails.html. An Excel spreadsheet (Sunrise_Sunset_DoNFM_Calculations.xlsx) is provided for anyone wishing to use the Excel version of the dataset.
Sunrise local time of sunrise using a 24 h clock e.g. 05:30. See above.
Ref_ID a link to reference information for the data if available. If not the names of the observers are listed (e.g. Baird, Connolly, Dornelas and Madin unpublished)
Comments any additional details provided
-
3)
Reference information (in tblReferencesForSpawningObservations):
Each set of observations is referenced to its published or unpublished source in this table via a Ref_ID. The table contains two main fields: “Short_ref” (e.g. Baird et al. 2015) and “Full_reference” (e.g. Baird AH, Cumbo VR, Gudge S, Keith SA, Maynard JA, Tan C-H, Woolsey ES (2015) Coral reproduction on the world’s southernmost reef at Lord Howe Island, Australia. Aquatic Biology 23:275–284). These can be filled in before or after entering spawning observations. An email address is provided for all unpublished contributions.
Notes to recording the time of spawning
For the quality of a start or end time to be ‘Exact’, a colony must be under continuous observation and the time of onset or end of spawning be observed and recorded. Most in situ observations would be expected to be approximate (‘Approx’).
The Quality_start, Quality_end, No_start and No_end fields are designed to accommodate the most common ways spawning is observed. A series of examples are given below.
-
1.
A colony is observed spawning but it is not known exactly when it started. No end time is recorded.
Here enter the time the colony was first observed spawning as the Start_time and the Quality_start as ‘Approx’. Leave the End_time blank and set No_end to True.
-
2.
A colony is followed closely until spawning is observed to begin but the precise time when spawning ends is not recorded. However, the colony is observed to be still dribbling spawn 30 minutes after spawning started.
Here enter the Quality_start_ as ‘Exact’ with the End_time set to 30 minutes after the Start_time and the Quality_end set to ‘Approx’.
-
3.
A colony is followed closely from the beginning until the end of spawning.
Here enter the times and note Quality_start and Quality_end as ‘Exact’.
-
4.
A colony is placed in a bucket and checked every 30 minutes. At the first observation there is no evidence of spawning, 30 min later the surface of the water is covered in bundles and the colony is no longer spawning.
Here enter the time of the first observation as the start time and the time of the second observation as the end time and set Quality_start and Quality_end to ‘Approx’.
-
5.
Only the night of spawning is known, for example, gametes are no longer apparent in a tagged and sequentially sampled colony.
Here don’t enter either a start time or an end time and leave Quality_start and Quality_end blank. Set No_start and No_end to True.
Species identifications
Species were generally identified following18,24 or by comparing skeletons to the type material or the original descriptions of nominal species. Specimens identified following18,24 were updated to the currently accepted names at the World Register of Marine Species19. The database also allows for uncertainties in species identifications to be indicated with the use of a series of open nomenclature qualifiers25,26 that allow the assignment of specimens to a nominal species with varying degrees of certainty. Specimens that closely resemble the type of a nominal species are given the qualifier cf. (e.g. Acropora cf. nasuta). Specimens that have morphological affinities to a nominal species but appear distinct are given the qualifier aff. (e.g. Acropora aff. pulchra): these specimens are either geographical variants of species with high morphological plasticity or potentially undescribed species. Species that could not be matched with the type material of any nominal species were labelled as sp. in addition to the location where they were collected (e.g. Acropora sp_1_Fiji). These specimens are most probably undescribed species. For 1% of records spawning colonies were only identified to genus (e.g. Montipora sp.). Contact the sources of these data for further information on the species identity.
Data Records
A snapshot of the data contained in this descriptor can be downloaded from figshare27. The data includes 6178 observations, 3085 of which were unpublished with the remainder gleaned from the literature28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128. These data have been through a rigorous quality control and editorial process. Annual updates of the dataset will be uploaded to figshare as new version and also made available at any time on request from the Editor (JRG). Contributions to the CSD are welcome at any time and should be sent to the Editor (JRG).
Technical Validation
The database is governed on a voluntary basis, by an Editor (JRG), Assistant Editors (JB & AGB), a Taxonomy Advisor (AHB) and a Database Administrator (AJE). Quality control of data and editorial procedures include:
-
1.
Contributor approval. Database users must request permission to become a database contributor.
-
2.
Editorial approval. Once a contributor sends data to the Editor, the data will be checked and if correctly formatted will be forward to the Database Administrator
-
3.
User feedback. Data issues can be reported for any observation by email to the Editor
References
Harrison, P. L. et al. Mass spawning in tropical reef corals. Science 223, 1186–1189 (1984).
Willis, B. L., Babcock, R. C., Harrison, P. L. & Oliver, J. K. Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proc 5th Int Coral Reef Symp 4, 343–348 (1985).
Baird, A. H., Guest, J. R. & Willis, B. L. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol., Evol. Syst. 40, 551–571 (2009).
Baird, A. H. et al. Coral reproduction on the world’s southernmost reef at Lord Howe Island, Australia. Aquat. Biol. 23, 275–284 (2015).
Harrison, P. L. in Coral Reefs: An Ecosystem in Transition (eds Z. Dubinsky & N. Stambler) 59-85 (Springer Science, 2011).
Keith, S. A. et al. Coral mass spawning predicted by rapid seasonal rise in ocean temperature. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 283, 20160011 (2016).
Wolstenholme, J. K. Temporal reproductive isolation and gametic compatibility are evolutionary mechanisms in the Acropora humilis species group (Cnidaria; Scleractinia). Mar. Biol. 144, 567–582 (2004).
Morita, M. et al. Reproductive strategies in the intercrossing corals Acropora donei and A. tenuis to prevent hybridization. Coral Reefs 38, 1211–1223 (2019).
Randall, C. J. et al. Sexual production of corals for reef restoration in the Anthropocene. Mar. Ecol. Prog. Ser. 635, 203–232 (2020).
Bouwmeester, J. et al. Multi-species spawning synchrony within scleractinian coral assemblages in the Red Sea. Coral Reefs 34, 65–77 (2015).
Baird, A. H., Blakeway, D. R., Hurley, T. J. & Stoddart, J. A. Seasonality of coral reproduction in the Dampier Archipelago, northern Western Australia. Mar. Biol. 158, 275–285 (2011).
Styan, C. A. & Rosser, N. L. Is monitoring for mass spawning events in coral assemblages in north Western Australia likely to detect spawning? Mar. Pollut. Bull. 64, 2523–2527 (2012).
Visser, M. E. & Both, C. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 272, 2561–2569 (2005).
Hock, K., Doropoulos, C., Gorton, R., Condie, S. A. & Mumby, P. J. Split spawning increases robustness of coral larval supply and inter-reef connectivity. Nat. Comm. 10 (2019).
Sakai, Y. et al. Environmental factors explain spawning day deviation from full moon in the scleractinian coral Acropora. Biol. Lett. 16, 20190760 (2020).
Madin, J. S. et al. The Coral Trait Database, a curated database of trait information for coral species from the global oceans. Scientific Data 3, 160017 (2016).
Veron, J., Stafford-Smith, M., DeVantier, L. & Turak, E. Overview of distribution patterns of zooxanthellate Scleractinia. Front. Mar. Sci. 1 (2015).
Veron, J. E. N. Corals of the world. (AIMS, 2000).
Hoeksema, B. W. & Cairns, S. D. World List of Scleractinia. Accessed through: World Register of Marine Species at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=1363 (2020).
Fukami, H. et al. Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (order Scleractinia, class Anthozoa, phylum Cnidaria). PLoS ONE 3 (2008).
Arrigoni, R., Terraneo, T. I., Galli, P. & Benzoni, F. Lobophylliidae (Cnidaria, Scleractinia) reshuffled: Pervasive non-monophyly at genus level. Mol. Phylogen. Evol. 73, 60–64 (2014).
Huang, D. et al. Towards a phylogenetic classification of reef corals: the Indo-Pacific genera Merulina, Goniastrea and Scapophyllia (Scleractinia, Merulinidae). Zool. Scr. 43, 531–548 (2014).
Babcock, R. C. et al. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394 (1986).
Veron, J. E. N. Corals of Australia and the Indo-Pacific. (Angus & Robertson, 1986).
Bengtson, P. Open Nomenclature. Palaeontology 31, 223–227 (1988).
Sigovini, M., Keppel, E. & Tagliapietra, D. Open Nomenclature in the biodiversity era. Methods Ecol. Evol. 7, 1217–1225 (2016).
Baird, A. H. et al. Coral Spawning Database. Newcastle University, https://doi.org/10.25405/data.ncl.13082333 (2020).
Babcock, R., Mundy, C., Keesing, J. & Oliver, J. Predictable and unpredictable spawning events: in situ behavioural data from free-spawning coral reef invertebrates. Invertebr. Reprod. Dev. 22, 213–227 (1992).
Babcock, R. C. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs 2, 187–195 (1984).
Babcock, R. C., Willis, B. L. & Simpson, C. J. Mass spawning of corals on a high-latitude coral-reef. Coral Reefs 13, 161–169 (1994).
Boch, C. A., Ananthasubramaniam, B., Sweeney, A. M., Francis, J. D. III & Morse, D. E. Effects of Light Dynamics on Coral Spawning Synchrony. Biol. Bull. 220, 161–173 (2011).
Boch, C. A. & Morse, A. N. C. Testing the effectiveness of direct propagation techniques for coral restoration of Acropora spp. Ecol. Eng. 40, 11–17 (2012).
Bouwmeester, J., Gatins, R., Giles, E. C., Sinclair-Taylor, T. H. & Berumen, M. L. Spawning of coral reef invertebrates and a second spawning season for scleractinian corals in the central Red Sea. Invertebr. Biol. 135, 273–284 (2016).
Bronstein, O. & Loya, Y. Daytime spawning of Porites rus on the coral reefs of Chumbe Island in Zanzibar, Western Indian Ocean (WIO). Coral Reefs 30, 441–441 (2011).
Carroll, A., Harrison, P. L. & Adjeroud, M. Sexual reproduction of Acropora reef corals at Moorea, French Polynesia. Coral Reefs 25, 93–97 (2006).
Chelliah, A. et al. First record of multi-species synchronous coral spawning from Malaysia. Peerj 3, e777 (2015).
Chua, C. M., Leggat, W., Moya, A. & Baird, A. H. Temperature affects the early life history stages of corals more than near future ocean acidification. Mar. Ecol. Prog. Ser. 475, 85–92 (2013).
Chua, C. M., Leggat, W., Moya, A. & Baird, A. H. Near-future reductions in pH will have no consistent ecological effects on the early life-history stages of reef corals. Mar. Ecol. Prog. Ser. 486, 143–151 (2013).
Dai, C. F., Soong, K. & Fan, T. Y. Sexual reproduction of corals in northern and southern Taiwan. Proceeding of the 7th International Coral Reef Symposium 1, 448–455 (1992).
Doropoulos, C. & Diaz-Pulido, G. High CO2 reduces the settlement of a spawning coral on three common species of crustose coralline algae. Mar. Ecol. Prog. Ser. 475, 93–99 (2013).
Doropoulos, C. et al. Testing industrial-scale coral restoration techniques: Harvesting and culturing wild coral-spawn slicks. Front. Mar. Sci. 6 (2019).
Doropoulos, C., Ward, S., Diaz-Pulido, G., Hoegh-Guldberg, O. & Mumby, P. J. Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol. Lett., no-no, (2012).
Doropoulos, C., Ward, S., Marshell, A., Diaz-Pulido, G. & Mumby, P. J. Interactions among chronic and acute impacts on coral recruits: the importance of size-escape thresholds. Ecology 93, 2131–2138 (2012).
Eyal-Shaham, L. et al. Repetitive sex change in the stony coral Herpolitha limax across a wide geographic range. Scientific Reports 9, 2936 (2019).
Eyre, B. D., Glud, R. N. & Patten, N. Mass coral spawning: A natural large-scale nutrient addition experiment. Limnol. Oceanogr. 53, 997–1013 (2008).
Fadlallah, Y. H. Synchronous spawning of Acropora clathrata coral colonies from the western Arabian Gulf (Saudi Arabia). Bull. Mar. Sci. 59, 209–216 (1996).
Field, S. in Reproduction in Reef Corals (eds E. F. Cox, D. A. Krupp, & P. L. Jokiel) 111–119 (Hawaii Institute of Marine Biology, 1998).
Fiebig, S. M. & Vuki, V. C. Mass spawning of scleractinian corals on Fijian reefs and in particular, Suva Reef. The South Pacific Journal of Natural Sciences 15 (1997).
Fiene-Severns, P. in Reproduction in Reef Corals (eds E. F. Cox, D. A. Krupp, & P. L. Jokiel) 22–24 (Hawaii Institute of Marine Biology, 1998).
Fujiwara, S., Kezuka, D., Ishimizu, H., Tabata, S. & Nojima, S. Condition for mass spawning of scleractinian coral Acropora in the Sekisei Lagoon, Ryukyu Islands. Bull. Jap. Soc. Fish. Oceanogr. 79, 130–140 (2015).
Fukami, H., Omori, M., Shimoike, K., Hayashibara, T. & Hatta, M. Ecological and genetic aspects of reproductive isolation by different spawning times in Acropora corals. Mar. Biol. 142, 679–684 (2003).
Gilmour, J. Experimental investigation into the effects of suspended sediment on fertilisation, larval survival and settlement in a scleractinian coral. Mar. Biol. 135, 451–462 (1999).
Gilmour, J. P., Smith, L. D. & Brinkman, R. M. Biannual spawning, rapid larval development and evidence of self-seeding for scleractinian corals at an isolated system of reefs. Mar. Biol. 156, 1297–1309 (2009).
Glynn, P. W. et al. Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). 3. Agariciidae (Pavona gigantea and Gardineroseris planulata). Mar. Biol. 125, 579–601 (1996).
Glynn, P. W. et al. Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and the Galápagos Islands (Ecuador). VI. Agariciidae, Pavona clavus. Mar. Biol. 158, 1601–1617 (2011).
Glynn, P. W. et al. Reproductive ecology of the azooxanthellate coral Tubastraea coccinea in the equatorial eastern pacific: Part V. Dendrophylliidae. Mar. Biol. 153, 529–544 (2008).
Glynn, P. W., Colley, S. B., Ting, J. H., Mate, J. L. & Guzman, H. M. Reef coral reproduction in the eastern Pacific: Costa Rica, Panama, and Galapagos Islands (Ecuador). 4. Agariciidae, recruitment and recovery of Pavona varians and Pavona sp. A. Mar. Biol. 136, 785–805 (2000).
Gomez, E. J. et al. Gametogenesis and reproductive pattern of the reef-building coral Acropora millepora in northwestern Philippines. Invertebr. Reprod. Dev. 62, 202–208 (2018).
Graham, E. M., Baird, A. H. & Connolly, S. R. Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27, 529–539 (2008).
Gress, E. & Paige, N. & Bollard, S. Observations of Acropora spawning in the Mozambique Channel. West. Indian Ocean J. Mar. Sci. 13, 107 (2014).
Guest, J. R., Baird, A. H., Goh, B. P. L. & Chou, L. M. Reproductive seasonality in an equatorial assemblage of scleractinian corals. Coral Reefs 24, 112–116 (2005).
Hayashibara, T. & Shimoike, K. Cryptic species of Acropora digitifera. Coral Reefs 21, 224–225 (2002).
Hayashibara, T. et al. Patterns of coral spawning at Akajima Island, Okinawa, Japan. Mar. Ecol. Prog. Ser. 101, 253–262 (1993).
Heyward, A., Yamazato, K., Yeemin, T. & Minei, M. Sexual reproduction of coral in Okinawa. Galaxea 6, 331–343 (1987).
Heyward, A. J. in Coral Reef Population Biology (eds P. L. Jokiel, R. H. Richmond, & R. A. Rogers) 170–178 (Sea Grant Coop, 1986).
Heyward, A. J. & Babcock, R. C. Self- and cross-fertilization in scleractinian corals. Mar. Biol. 90, 191–195 (1986).
Heyward, A. J. & Negri, A. P. Natural inducers for coral larval metamorphosis. Coral Reefs 18, 273–279 (1999).
Hirose, M. & Hidaka, M. Early development of zooxanthella-containing eggs of the corals Porites cylindrica and Montipora digitata: The endodermal localization of zooxanthellae. Zool. Sci. 23, 873–881 (2006).
Hirose, M., Kinzie, R. A. & Hidaka, M. Timing and process of entry of zooxanthellae into oocytes of hermatypic corals. Coral Reefs 20, 273–280 (2001).
Hodgson, G. Potential gamete wastage in synchronously spawning corals due to hybrid inviability. Proceedings of the 6th International Coral Reef Symposium 2, 707–714 (1988).
Howells, E. J., Abrego, D., Vaughan, G. O. & Burt, J. A. Coral spawning in the Gulf of Oman and relationship to latitudinal variation in spawning season in the northwest Indian Ocean. Scientific Reports 4, 7484 (2014).
Howells, E. J., Berkelmans, R., van Oppen, M. J. H., Willis, B. L. & Bay, L. K. Historical thermal regimes define limits to coral acclimatization. Ecology 94, 1078–1088 (2013).
Hunter, C. L. Environmental cues controlling spawning in two Hawaiian corals, Montipora verrucosa and M. dilitata. Proceedings of the 6th International Coral Reef Symposium 2, 727–732 (1988).
Itano, D. & Buckley, T. Observations of the mass spawning of corals and palolo (Eunice viridis) in American Samoa. (Department of Marine and Wildlife Resources, American Samoa, American Samoa, 1988).
Jamodiong, E. A. et al. Coral spawning and spawn-slick observation in the Philippines. Mar. Biodivers. 48, 2187–2192 (2018).
Kenyon, J. C. Hybridization and polyploidy in the coral genus. Acropora. Pac. Sci. 48, 203–204 (1994).
Kenyon, J. C. Latitudinal differences between Palau and Yap in coral reproductive synchrony. Pac. Sci. 49, 156–164 (1995).
Kinzie, R. A. Spawning in the reef corals Pocillopora verrucosa and P. eydouxi at Sesoko Island, Okinawa. Galaxea 11, 93–105 (1993).
Kojis, B. L. & Quinn, N. J. Aspects of sexual reproduction and larval development in the shallow water hermatypic coral, Goniastrea australensis (Edwards and Haime, 1857). Bull. Mar. Sci. 31, 558–573 (1981).
Kojis, B. L. & Quinn, N. J. Reproductive ecology of two faviid corals (Coelenterata: Scleractinia). Mar. Ecol. Prog. Ser. 8, 251–255 (1982).
Kojis, B. L. & Quinn, N. J. Reproductive strategies in four species of Porites (Scleractinia). Proceedings of the 4th International Coral Reef Symposium 2, 145–151 (1982).
Kongjandtre, N., Ridgway, T., Ward, S. & Hoegh-Guldberg, O. Broadcast spawning patterns of Favia species on the inshore reefs of Thailand. Coral Reefs 29, 227–234 (2010).
Krupp, D. A. Sexual reproduction and early development of the solitary coral Fungia scutaria (Anthozoa: Scleractinia). Coral Reefs 2, 159–164 (1983).
Ligson, C. A., Tabalanza, T. D., Villanueva, R. D. & Cabaitan, P. C. Feasibility of early outplanting of sexually propagated Acropora verweyi for coral reef restoration demonstrated in the Philippines. Restor. Ecol. 28, 244–251 (2020).
Lin, C. H. & Nozawa, Y. Variability of spawning time (lunar day) in Acropora versus merulinid corals: a 7-yr record of in situ coral spawning in Taiwan. Coral Reefs 36, 1269–1278 (2017).
Loya, Y., Heyward, A. & Sakai, K. Reproductive patterns of fungiid corals in Okinawa, Japan. Galaxea 11, 119–129 (2009).
Loya, Y. & Sakai, K. Bidirectional sex change in mushroom stony corals. Proceedings of the Royal Society B: Biological Sciences 275, 2335–2343 (2008).
Maboloc, E. A., Jamodiong, E. A. & Villanueva, R. D. Reproductive biology and larval development of the scleractinian corals Favites colemani and F. abdita (Faviidae) in northwestern Philippines. Invertebr. Reprod. Dev. 60, 1–11 (2016).
Mangubhai, S. Reproductive ecology of the scleractinian corals Echinopora gemmacea and Leptoria phrygia (Faviidae) on equatorial reefs in Kenya. Invertebr. Reprod. Dev. 53, 67–79 (2009).
Mangubhai, S., Harris, A. & Graham, N. A. J. Synchronous daytime spawning of the solitary coral Fungia danai (Fungiidae) in the Chagos Archipelago, central Indian Ocean. Coral Reefs 26, 15–15 (2007).
Markey, K. L., Baird, A. H., Humphrey, C. & Negri, A. Insecticides and a fungicide affect multiple coral life stages. Mar. Ecol. Prog. Ser. 330, 127–137 (2007).
Mate, J. F. in Reproduction in Reef Corals (eds E. F. Cox, D. A. Krupp, & P. L. Jokiel) 25-37 (Hawaii Institute of Marine Biology, 1998).
Mate, J. F., Wilson, J., Field, S. & Neves, E. G. in Reproduction in Reef Corals (eds E. F. Cox, D. A. Krupp, & P. L. Jokiel) 25–37 (Hawaii Institute of Marine Biology, 1998).
Mezaki, T. et al. Spawning patterns of high latitude scleractinian corals from 2002 to 2006 at Nishidomari, Otsuki, Kochi, Japan. Kuroshio Biosphere 3, 33–47 (2007).
Mohamed, A. R. et al. The transcriptomic response of the coral Acropora digitifera to a competent Symbiodinium strain: the symbiosome as an arrested early phagosome. Mol. Ecol. 25, 3127–3141 (2016).
Mundy, C. N. & Green, A. Spawning observations of corals and other invertebrates in American Samoa. (Department of Marine and Wildlife Resources, American Samoa Governement, Samoa, 1999).
Nitschke, M. R., Davy, S. K. & Ward, S. Horizontal transmission of Symbiodinium cells between adult and juvenile corals is aided by benthic sediment. Coral Reefs 35, 335–344 (2016).
Nozawa, Y. & Harrison, P. L. Temporal settlement patterns of larvae of the broadcast spawning reef coral Favites chinensis and the broadcast spawning and brooding reef coral Goniastrea aspera from Okinawa, Japan. Coral Reefs 24, 274–282 (2005).
Nozawa, Y., Tokeshi, M. & Nojima, S. Reproduction and recruitment of scleractinian corals in a high-latitude coral community, Amakusa, southwestern Japan. Mar. Biol. 149, 1047–1058 (2006).
Okamoto, M., Nojima, S., Furushima, Y. & Phoel, W. C. A basic experiment of coral culture using sexual reproduction in the open sea. Fish. Sci. 71, 263–270 (2005).
Omori, M., Fukami, H., Kobinata, H. & Hatta, M. Significant drop of fertilization of Acropora corals in 1999. An after-effect of heavy coral bleaching? Limnol. Oceanogr. 46, 704–706 (2001).
Penland, L., Kloulechad, J., Idip, D. & van Woesik, R. Coral spawning in the western Pacific Ocean is related to solar insolation: evidence of multiple spawning events in Palau. Coral Reefs 23, 133–140 (2004).
Plathong, S. et al. Daytime gamete release from the reef-building coral, Pavona sp., in the Gulf of Thailand. Coral Reefs 25, 72–72 (2006).
Rapuano, H. et al. Reproductive strategies of the coral Turbinaria reniformis in the northern Gulf of Aqaba (Red Sea). Scientific Reports 7, 42670 (2017).
Raj, K. D. & Edward, J. K. P. Observations on the reproduction of Acropora corals along the Tuticorin coast of the Gulf of Mannar, Southeastern India. Indian. J. Mar. Sci. 39, 219–226 (2010).
Richmond, R. H. Competency and dispersal potential of planula larvae of a spawning versus a brooding coral. Proceedings of the 6th International Coral Reef Symposium 2, 827–831 (1988).
Sakai, K. Gametogenesis, spawning, and planula brooding by the reef coral Goniastrea aspera (Scleractinia) in Okinawa, Japan. Mar. Ecol. Prog. Ser. 151, 67–72 (1997).
Schmidt-Roach, S., Miller, K. J., Woolsey, E., Gerlach, G. & Baird, A. H. Broadcast spawning by Pocillopora species on the Great Barrier Reef. PLoS ONE 7, e50847 (2012).
Shlesinger, T., Grinblat, M., Rapuano, H., Amit, T. & Loya, Y. Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology 99, 421–437 (2018).
Shlesinger, T. & Loya, Y. Breakdown in spawning synchrony: A silent threat to coral persistence. Science 365, 1002–1007 (2019).
Shlesinger, Y. & Loya, Y. Coral community reproductive patterns: Red Sea versus the Great Barrier Reef. Science 228, 1333–1335 (1985).
Siboni, N. et al. Using bacterial extract along with differential gene expression in Acropora millepora larvae to decouple the processes of attachment and metamorphosis. PLoS ONE 7, e37774–e37774 (2012).
Simpson, C. J. Mass spawning of scleractinian corals in the Dampier Archipelago and the implications for management of coral reefs in Western Australia. Report No. 244, (Dept. Conservation and Environment Western Australia Bulletin, Perth, 1985).
Stanton, F. G. Spatio-temporal patterns of spawning in the coral, Montipora verrucosa in Hawaii. Proceeding of the 7th International Coral Reef Symposium 1, 489–493 (1992).
Tan, C. H. et al. Multispecific synchronous coral spawning on Pulau Bidong, Malaysia, South China Sea. Bull. Mar. Sci. 96, 193–194 (2020).
Tomascik, T., Mah, A. J., Nontij, A. & Moosa, M. K. The Ecology of the Indonesian Seas. Vol. One (Periplus, 1997).
Twan, W. H., Hwang, J. S. & Chang, C. F. Sex steroids in scleractinian coral, Euphyllia ancora: Implication in mass spawning. Biol. Reprod. 68, 2255–2260 (2003).
Van Oppen, M. J. H., Willis, B. L., Van Rheede, T. & Miller, D. J. Spawning times, reproductive compatibilities and genetic structuring in the Acropora aspera group: evidence for natural hybridization and semi-permeable species boundaries in corals. Mol. Ecol. 11, 1363–1376 (2002).
van Woesik, R. Coral communities at high latitude are not pseudopopulations: evidence of spawning at 32°N, Japan. Coral Reefs 14, 119–120 (1995).
Wallace, C. C. Reproduction, recruitment and fragmentation in nine sympatric species of the coral genus. Acropora. Mar. Biol. 88, 217–233 (1985).
Wei, N. W. V. et al. Reproductive Isolation among Acropora species (Scleractinia: Acroporidae) in a marginal coral assemblage. Zool. Stud. 51, 85–92 (2012).
Wild, C., Tollrian, R. & Huettel, M. Rapid recycling of coral mass-spawning products in permeable reef sediments. Mar. Ecol. Prog. Ser. 271, 159–166 (2004).
Wilson, J. R. & Harrison, P. L. Sexual reproduction in high latitude coral communities at the Solitary Islands, eastern Australia. Proceedings of the 8th International Coral Reef Symposium, 533–540 (1997).
Wilson, J. R. & Harrison, P. L. Spawning patterns of scleractinian corals at the Solitary Islands - a high latitude coral community in eastern Australia. Mar. Ecol. Prog. Ser. 260, 115–123 (2003).
Wolstenholme, J., Nozawa, Y., Byrne, M. & Burke, W. Timing of mass spawning in corals: potential influence of the coincidence of lunar factors and associated changes in atmospheric pressure from northern and southern hemisphere case studies. Invertebr. Reprod. Dev. 62, 98–108 (2018).
Woolsey, E. S., Byrne, M. & Baird, A. H. The effects of temperature on embryonic development and larval survival in two scleractinian corals. Mar. Ecol. Prog. Ser. 493, 179–184 (2013).
Woolsey, E. S., Keith, S. A., Byrne, M., Schmidt-Roach, S. & Baird, A. H. Latitudinal variation in thermal tolerance thresholds of early life stages of corals. Coral Reefs 34, 471–478 (2015).
Yeemin, T. Ecological studies of scleractinian coral communities above the northern limit of coral reef development in the western Pacific PhD thesis, Kyushu University, (1991).
Acknowledgements
The authors would like to thank the ARC Centre of Excellence for Coral Reef Studies for funding the Coral Spawning Workshop in Singapore in 2017 where the database was initially developed.
Author information
Authors and Affiliations
Contributions
A.H.B. and J.R.G. conceived the idea. A.H.B., J.R.G., A.J.E., J.B., A.G.B., S.-H.N. & H.M. compiled the data and jointly wrote the data descriptor. A.J.E. designed the database. All other authors contributed unpublished data and commented on the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Online-only Table
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files associated with this article.
About this article
Cite this article
Baird, A.H., Guest, J.R., Edwards, A.J. et al. An Indo-Pacific coral spawning database. Sci Data 8, 35 (2021). https://doi.org/10.1038/s41597-020-00793-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-020-00793-8
This article is cited by
-
Larval precompetency and settlement behaviour in 25 Indo-Pacific coral species
Communications Biology (2024)
-
Integration of population genetics with oceanographic models reveals strong connectivity among coral reefs across Seychelles
Scientific Reports (2024)
-
Conservation of Protein Kinase A Substrates in the Cnidarian Coral Spermatozoa Among Animals and Their Molecular Evolution
Journal of Molecular Evolution (2024)
-
Assessing coral reef conservation planning in Wakatobi National Park (Indonesia) from larval connectivity networks
Coral Reefs (2024)
-
Global disruption of coral broadcast spawning associated with artificial light at night
Nature Communications (2023)