Abstract
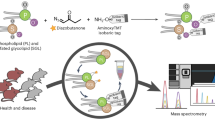
Protein lipidation is one of the most widespread post-translational modifications (PTMs) found in nature, regulating protein function, structure and subcellular localization. Lipid transferases and their substrate proteins are also attracting increasing interest as drug targets because of their dysregulation in many disease states. However, the inherent hydrophobicity and potential dynamic nature of lipid modifications makes them notoriously challenging to detect by many analytical methods. Chemical proteomics provides a powerful approach to identify and quantify these diverse protein modifications by combining bespoke chemical tools for lipidated protein enrichment with quantitative mass spectrometry–based proteomics. Here, we report a robust and proteome-wide approach for the exploration of five major classes of protein lipidation in living cells, through the use of specific chemical probes for each lipid PTM. In-cell labeling of lipidated proteins is achieved by the metabolic incorporation of a lipid probe that mimics the specific natural lipid, concomitantly wielding an alkyne as a bio-orthogonal labeling tag. After incorporation, the chemically tagged proteins can be coupled to multifunctional ‘capture reagents’ by using click chemistry, allowing in-gel fluorescence visualization or enrichment via affinity handles for quantitative chemical proteomics based on label-free quantification (LFQ) or tandem mass-tag (TMT) approaches. In this protocol, we describe the application of lipid probes for N-myristoylation, N- and S-acylation, O-cholesterylation, S-farnesylation and S-geranylgeranylation in multiple cell lines to illustrate both the workflow and data obtained in these experiments. We provide detailed workflows for method optimization, sample preparation for chemical proteomics and data processing. A properly trained researcher (e.g., technician, graduate student or postdoc) can complete all steps from optimizing metabolic labeling to data processing within 3 weeks. This protocol enables sensitive and quantitative analysis of lipidated proteins at a proteome-wide scale at native expression levels, which is critical to understanding the role of lipid PTMs in health and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Code availability
MaxQuant and Perseus are freely available from https://www.maxquant.org.
References
Chen, B., Sun, Y., Niu, J., Jarugumilli, G. K. & Wu, X. Protein lipidation in cell signaling and diseases: function, regulation, and therapeutic opportunities. Cell Chem. Biol. 25, 817–831 (2018).
Lanyon-Hogg, T., Faronato, M., Serwa, R. A. & Tate, E. W. Dynamic protein acylation: new substrates, mechanisms, and drug targets. Trends Biochem. Sci. 42, 566–581 (2017).
Hentschel, A., Zahedi, R. P. & Ahrends, R. Protein lipid modifications—more than just a greasy ballast. Proteomics 16, 759–782 (2016).
Garner, A. L. & Janda, K. D. cat-ELCCA: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT). Angew. Chem. Int. Ed. Engl. 49, 9630–9634 (2010).
Goncalves, V. et al. A fluorescence-based assay for N-myristoyltransferase activity. Anal. Biochem. 421, 342–344 (2012).
Lanyon-Hogg, T. et al. Click chemistry armed enzyme-linked immunosorbent assay to measure palmitoylation by hedgehog acyltransferase. Anal. Biochem. 490, 66–72 (2015).
Lanyon-Hogg, T. et al. Microfluidic mobility shift assay for real-time analysis of peptide N-palmitoylation. SLAS Discov. 22, 418–424 (2017).
Lanyon-Hogg, T. et al. Acylation-coupled lipophilic induction of polarisation (Acyl-cLIP): a universal assay for lipid transferase and hydrolase enzymes. Chem. Sci. 10, 8995–9000 (2019).
Kakugawa, S. et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature 519, 187–192 (2015).
Won, S. J. et al. Molecular mechanism for isoform-selective inhibition of acyl protein thioesterases 1 and 2 (APT1 and APT2). ACS Chem. Biol. 11, 3374–3382 (2016).
Ma, D. et al. Crystal structure of a membrane-bound O-acyltransferase. Nature 562, 286–290 (2018).
Rana, M. S. et al. Fatty acyl recognition and transfer by an integral membrane S-acyltransferase. Science 359, eaao6326 (2018).
Kuchay, S. et al. GGTase3 is a newly identified geranylgeranyltransferase targeting a ubiquitin ligase. Nat. Struct. Mol. Biol. 26, 628–636 (2019).
Dian, C. et al. High-resolution snapshots of human N-myristoyltransferase in action illuminate a mechanism promoting N-terminal Lys and Gly myristoylation. Nat. Commun. 11, 1132 (2020).
Jiang, Y., Benz, T. L. & Long, S. B. Substrate and product complexes reveal mechanisms of Hedgehog acylation by HHAT. Science 372, 1215–1219 (2021).
Tate, E. W., Kalesh, K. A., Lanyon-Hogg, T., Storck, E. M. & Thinon, E. Global profiling of protein lipidation using chemical proteomic technologies. Curr. Opin. Chem. Biol. 24, 48–57 (2015).
Thinon, E. et al. Global profiling of co- and post-translationally N-myristoylated proteomes in human cells. Nat. Commun. 5, 4919 (2014).
Daf Duluc, L. et al. Tipifarnib prevents development of hypoxia-induced pulmonary hypertension. Cardiovasc. Res. 113, 276–287 (2017).
Mousnier, A. et al. Fragment-derived inhibitors of human N-myristoyltransferase block capsid assembly and replication of the common cold virus. Nat. Chem. 10, 599–606 (2018).
Heal, W. P., Wickramasinghe, S. R., Leatherbarrow, R. J. & Tate, E. W. N-Myristoyl transferase-mediated protein labeling in vivo. Org. Biomol. Chem. 6, 2308–2315 (2008).
Wright, M. H., Heal, W. P., Mann, D. J. & Tate, E. W. Protein myristoylation in health and disease. J. Chem. Biol. 3, 19–35 (2010).
Chamberlain, L. H. & Shipston, M. J. The physiology of protein S-acylation. Physiol. Rev. 95, 341–376 (2015).
Rodgers, U. R. et al. Characterization of hedgehog acyltransferase inhibitors identifies a small molecule probe for hedgehog signaling by cancer cells. ACS Chem. Biol. 11, 3256–3262 (2016).
Storck, E. M. et al. Dual chemical probes enable quantitative system-wide analysis of protein prenylation and prenylation dynamics. Nat. Chem. 11, 552–561 (2019).
Ciepla, P. et al. New chemical probes targeting cholesterylation of Sonic Hedgehog in human cells and zebrafish. Chem. Sci. 5, 4249–4259 (2014).
Rioux, V. et al. Exogenous myristic acid acylates proteins in cultured rat hepatocytes. J. Nutr. Biochem. 13, 66–74 (2002).
Taguchi, Y. & Schätzl, H. M. Small-scale Triton X-114 extraction of hydrophobic proteins. Bio Protoc. 4, e1139 (2014).
Drisdel, R. C. & Green, W. N. Labeling and quantifying sites of protein palmitoylation. Biotechniques 36, 276–285 (2004).
Forrester, M. T. et al. Site-specific analysis of protein S-acylation by resin-assisted capture. J. Lipid Res. 52, 393–398 (2011).
Blanc, M. et al. SwissPalm: protein palmitoylation database. F1000Res 4, 261 (2015).
Devabhaktuni, A. et al. TagGraph reveals vast protein modification landscapes from large tandem mass spectrometry datasets. Nat. Biotechnol. 37, 469–479 (2019).
Heal, W. P., Wright, M. H., Thinon, E. & Tate, E. W. Multifunctional protein labeling via enzymatic N-terminal tagging and elaboration by click chemistry. Nat. Protoc. 7, 105–117 (2011).
Charron, G. et al. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J. Am. Chem. Soc. 131, 4967–4975 (2009).
Thinon, E., Morales-Sanfrutos, J., Mann, D. J. & Tate, E. W. N-Myristoyltransferase inhibition induces ER-stress, cell cycle arrest, and apoptosis in cancer cells. ACS Chem. Biol. 11, 2165–2176 (2016).
Kallemeijn, W. W. et al. Validation and invalidation of chemical probes for the human N-myristoyltransferases. Cell Chem. Biol. 26, 892–900.e4 (2019).
Demetriadou, A. et al. Mouse Stbd1 is N-myristoylated and affects ER-mitochondria association and mitochondrial morphology. J. Cell Sci. 130, 903–915 (2017).
Schlott, A. C. et al. Structure-guided identification of resistance breaking antimalarial N-myristoyltransferase inhibitors. Cell Chem. Biol. 26, 991–1000.e7 (2019).
Goya Grocin, A., Serwa, R. A., Morales Sanfrutos, J., Ritzefeld, M. & Tate, E. W. Whole proteome profiling of N-myristoyltransferase activity and inhibition using sortase A. Mol. Cell Proteomics 18, 115–126 (2019).
Tapodi, A. et al. BFSP1 C-terminal domains released by post-translational processing events can alter significantly the calcium regulation of AQP0 water permeability. Exp. Eye Res. 185, 107585 (2019).
Wright, M. H. et al. Validation of N-myristoyltransferase as an antimalarial drug target using an integrated chemical biology approach. Nat. Chem. 6, 112–121 (2014).
Wright, M. H. et al. Global analysis of protein N-myristoylation and exploration of N-myristoyltransferase as a drug target in the neglected human pathogen Leishmania donovani. Chem. Biol. 22, 342–354 (2015).
Wright, M. H., Paape, D., Price, H. P., Smith, D. F. & Tate, E. W. Global profiling and inhibition of protein lipidation in vector and host stages of the sleeping sickness parasite Trypanosoma brucei. ACS Infect. Dis. 2, 427–441 (2016).
Serwa, R. A., Abaitua, F., Krause, E., Tate, E. W. & O'Hare, P. Systems analysis of protein fatty acylation in herpes simplex virus-infected cells using chemical proteomics. Chem. Biol. 22, 1008–1017 (2015).
Schlott, A. C., Holder, A. A. & Tate, E. W. N-Myristoylation as a drug target in malaria: exploring the role of N-myristoyltransferase substrates in the inhibitor mode of action. ACS Infect. Dis. 4, 449–457 (2018).
Broncel, M. et al. Myristoylation profiling in human cells and zebrafish. Data Brief 4, 379–383 (2015).
Broncel, M. et al. Multifunctional reagents for quantitative proteome-wide analysis of protein modification in human cells and dynamic profiling of protein lipidation during vertebrate development. Angew. Chem. Int. Ed. Engl. 54, 5948–5951 (2015).
Rangan, K. J., Yang, Y. Y., Charron, G. & Hang, H. C. Rapid visualization and large-scale profiling of bacterial lipoproteins with chemical reporters. J. Am. Chem. Soc. 132, 10628–10629 (2010).
Charlton, T. M., Kovacs-Simon, A., Michell, S. L., Fairweather, N. F. & Tate, E. W. Quantitative lipoproteomics in Clostridium difficile reveals a role for lipoproteins in sporulation. Chem. Biol. 22, 1562–1573 (2015).
Martin, B. R., Wang, C., Adibekian, A., Tully, S. E. & Cravatt, B. F. Global profiling of dynamic protein palmitoylation. Nat. Methods 9, 84–89 (2011).
Won, S. J. & Martin, B. R. Temporal profiling establishes a dynamic S-palmitoylation cycle. ACS Chem. Biol. 13, 1560–1568 (2018).
Schroeder, G. N. et al. Legionella pneumophila effector LpdA is a palmitoylated phospholipase D virulence factor. Infect. Immun. 83, 3989–4002 (2015).
Kalinin, A. et al. Expression of mammalian geranylgeranyltransferase type-II in Escherichia coli and its application for in vitro prenylation of Rab proteins. Protein Expr. Purif. 22, 84–91 (2001).
Nguyen, U. T. et al. Analysis of the eukaryotic prenylome by isoprenoid affinity tagging. Nat. Chem. Biol. 5, 227–235 (2009).
Suazo, K. F. et al. Metabolic labeling of prenylated proteins using alkyne-modified isoprenoid analogues. Curr. Protoc. Chem. Biol. 10, e46 (2018).
Greaves, J. et al. Molecular basis of fatty acid selectivity in the zDHHC family of S-acyltransferases revealed by click chemistry. Proc. Natl Acad. Sci. USA 114, E1365–E1374 (2017).
Martin, B. R. Chemical approaches for profiling dynamic palmitoylation. Biochem. Soc. Trans. 41, 43–49 (2013).
Yang, Y., Yang, X. & Verhelst, S. H. Comparative analysis of click chemistry mediated activity-based protein profiling in cell lysates. Molecules 18, 12599–12608 (2013).
Werner, T. et al. Ion coalescence of neutron encoded TMT 10-plex reporter ions. Anal. Chem. 86, 3594–3601 (2014).
End, D. W. et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 61, 131–137 (2001).
Lanyon-Hogg, T. et al. Photochemical probe identification of a small-molecule inhibitor binding site in hedgehog acyltransferase (HHAT)*. Angew. Chem. Int. Ed. Engl. 60, 13542–13547 (2021).
Rafiee, M. R. et al. Protease-resistant streptavidin for interaction proteomics. Mol. Syst. Biol. 16, e9370 (2020).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Percher, A., Thinon, E. & Hang, H. Mass-tag labeling using acyl-PEG exchange for the determination of endogenous protein S-fatty acylation. Curr. Protoc. Protein Sci. 89, 14.17.1–14.17.11 (2017).
McMichael, T. M. et al. The palmitoyltransferase ZDHHC20 enhances interferon-induced transmembrane protein 3 (IFITM3) palmitoylation and antiviral activity. J. Biol. Chem. 292, 21517–21526 (2017).
Lynes, E. M. et al. Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J. Cell Sci. 126, 3893–3903 (2013).
Acknowledgements
The authors thank L. Haig for assistance with MS, R. Serwa for aiding in setup of the proteomics protocols and R. Singh for proofreading. The Francis Crick Institute Cell Services are acknowledged for providing cell lines and STR verification. This work was supported by grants from The Royal Society (Newton International Fellowship grant NF161582 to W.W.K.), the European Commission (Marie Sklodowska Curie Individual Fellowship grant 752165 to W.W.K. and Marie Sklodowska Curie Intra European Fellowship to J.M.-S. and E.W.T.), the Royal Thai Government scholarship (PhD studentship to N.P.), the Imperial College London Institute of Chemical Biology EPSRC Centre for Doctoral Training (grant EP/F500416/1 to P.C.), EPSRC (Impact Acceleration Account grant PS1042 to W.W.K. and E.W.T.) and Cancer Research UK (C29637/A21451 and C29637/A20183 to E.W.T.). Work in the E.W.T. laboratories is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001057 and FC001097), the UK Medical Research Council (FC001057 and FC001097) and the Wellcome Trust (FC001057 and FC001097).
Author information
Authors and Affiliations
Contributions
W.W.K., N.P., T.L.-H. and E.W.T. were responsible for conceptualization. W.W.K., N.P., A.G.G. and J.M.-S. were responsible for methodology. W.W.K., T.L.H., N.P., A.G.G., J.M.-S. and P.C. were responsible for investigation. E.W.T. was responsible for resources. W.W.K. and T.L.H. wrote the original draft. All authors reviewed and edited the manuscript. W.W.K. was responsible for visualization. E.W.T. provided supervision.
Corresponding author
Ethics declarations
Competing interests
E.W.T. is a director and shareholder of Myricx Pharma Ltd. and an inventor on a patent application describing NMT inhibitors including IMP-1088 (Bell, A. S., Tate, E. W., Leatherbarrow, R. J., Hutton, J. A., Brannigan, J. A. Compounds and their use as inhibitors of N-myristoyl transferase. PCT In Appl. (2017) WO 2017001812).
Additional information
Peer review information Nature Protocols thanks Matthew Pratt, Tamara Kinzer-Ursem and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Kallemeijn, W. W. et al. Cell Chem. Biol. 26, 892–900.e4 (2019): https://doi.org/10.1016/j.chembiol.2019.03.006
Storck, E. M. et al. Nat. Chem. 11, 552–561 (2019): https://doi.org/10.1038/s41557-019-0237-6
Mousnier, A. et al. Nat. Chem. 10, 599–606 (2018): https://doi.org/10.1038/s41557-018-0039-2
Broncel, M. et al. Angew. Chem. Int. Ed. Engl. 54, 5948–5951 (2015): https://doi.org/10.1002/anie.201500342
Thinon, E. et al. Nat. Commun. 5, 4919 (2014): https://doi.org/10.1038/ncomms5919
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Rights and permissions
About this article
Cite this article
Kallemeijn, W.W., Lanyon-Hogg, T., Panyain, N. et al. Proteome-wide analysis of protein lipidation using chemical probes: in-gel fluorescence visualization, identification and quantification of N-myristoylation, N- and S-acylation, O-cholesterylation, S-farnesylation and S-geranylgeranylation. Nat Protoc 16, 5083–5122 (2021). https://doi.org/10.1038/s41596-021-00601-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00601-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.