Abstract
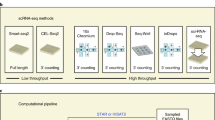
Existing protocols for full-length single-cell RNA sequencing produce libraries of high complexity (thousands of distinct genes) with outstanding sensitivity and specificity of transcript quantification. These full-length libraries have the advantage of allowing probing of transcript isoforms, are informative regarding single-nucleotide polymorphisms and allow assembly of the VDJ region of the T- and B-cell-receptor sequences. Since full-length protocols are mostly plate-based at present, they are also suited to profiling cell types where cell numbers are limiting, such as rare cell types during development. A disadvantage of these methods has been the scalability and cost of the experiments, which has limited their popularity as compared with droplet-based and nanowell approaches. Here, we describe an automated protocol for full-length single-cell RNA sequencing, including both an in-house automated Smart-seq2 protocol and a commercial kit–based workflow. The protocols take 3–5 d to complete, depending on the number of plates processed in a batch. We discuss these two protocols in terms of ease of use, equipment requirements, running time, cost per sample and sequencing quality. By benchmarking the lysis buffers, reverse transcription enzymes and their combinations, we have optimized the in-house automated protocol to dramatically reduce its cost. An automated setup can be adopted easily by a competent researcher with basic laboratory skills and no prior automation experience. These pipelines have been employed successfully for several research projects allied with the Human Cell Atlas initiative (www.humancellatlas.org).
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The raw sequencing data generated in this study have been deposited in ArrayExpress under the accession code E-MTAB-9345.
References
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Picelli, S. Single-cell RNA-sequencing: the future of genome biology is now. RNA Biol. 14, 637–650 (2017).
Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet 20, 257–272 (2019).
James, K. R. et al. Distinct microbial and immune niches of the human colon. Nat. Immunol. https://doi.org/10.1038/s41590-020-0602-z (2020).
Vieira Braga, F. A. et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 25, 1153–1163 (2019).
Haque, A., Engel, J., Teichmann, S. A. & Lönnberg, T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 9, 75 (2017).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353 (2018).
Popescu, D.-M. et al. Decoding human fetal liver haematopoiesis. Nature 574, 365–371 (2019).
Park, J.-E. et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 367, eaay3224 (2020).
Jiang, L. et al. Synthetic spike-in standards for RNA-seq experiments. Genome Res 21, 1543–1551 (2011).
Svensson, V. et al. Power analysis of single-cell RNA-sequencing experiments. Nat. Methods 14, 381–387 (2017).
Natarajan, K. N. et al. Comparative analysis of sequencing technologies for single-cell transcriptomics. Genome Biol. 20, 1–8 (2019).
Bagnoli, J. W. et al. Sensitive and powerful single-cell RNA sequencing using mcSCRB-seq. Nat. Commun. 9, 2937 (2018).
Svec, D. et al. Direct cell lysis for single-cell gene expression profiling. Front. Oncol. 3, 274 (2013).
Ilicic, T. et al. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 17, 29 (2016).
Ziegenhain, C. et al. Comparative analysis of single-cell RNA sequencing methods. Mol. Cell 65, 631–643.e4 (2017).
Wang, Y. J. et al. Comparative analysis of commercially available single-cell RNA sequencing platforms for their performance in complex human tissues. Preprint at bioRxiv https://doi.org/10.1101/541433 (2019).
Cao, J. et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357, 661–667 (2017).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Datlinger, P. et al. Ultra-high throughput single-cell RNA sequencing by combinatorial fluidic indexing. Preprint at bioRxiv https://doi.org/10.1101/2019.12.17.879304 (2019).
Lareau, C. A. et al. Droplet-based combinatorial indexing for massive-scale single-cell chromatin accessibility. Nat. Biotechnol. 37, 916–924 (2019).
Ramani, V. et al. Massively multiplex single-cell Hi-C. Nat. Methods 14, 263–266 (2017).
Mulqueen, R. M. et al. Highly scalable generation of DNA methylation profiles in single cells. Nat. Biotechnol. 36, 428–431 (2018).
Gupta, I. et al. Single-cell isoform RNA sequencing characterizes isoforms in thousands of cerebellar cells. Nat. Biotechnol. 36, 1197–1202 (2018).
Volden, R. & Vollmers, C. Highly multiplexed single-cell full-length cDNA sequencing of human immune cells with 10X Genomics and R2C2. Preprint at bioRxiv https://doi.org/10.1101/2020.01.10.902361 (2020).
Lebrigand, K., Magnone, V., Barbry, P. & Waldmann, R. High throughput error corrected Nanopore single cell transcriptome sequencing. Nat. Commun. 11, 4025 (2020).
Zheng, Y.-F. et al. HIT-scISOseq: High-throughput and high-accuracy single-cell full-length isoform sequencing for corneal epithelium. Preprint at bioRxiv https://doi.org/10.1101/2020.07.27.222349 (2020).
Hagemann-Jensen, M. et al. Single-cell RNA counting at allele and isoform resolution using Smart-seq3. Nat. Biotechnol. 38, 708–714 (2020).
Isakova, A., Neff, N. & Quake, S. R. Single cell profiling of total RNA using Smart-seq-total. Preprint at bioRxiv https://doi.org/10.1101/2020.06.02.131060 (2020).
Proserpio, V. Single Cell Methods: Sequencing and Proteomics (Humana Press, 2019).
Madissoon, E. et al. scRNA-seq assessment of the human lung, spleen, and esophagus tissue stability after cold preservation. Genome Biol. 21, 1 (2019).
Vieth, B. et al. A systematic evaluation of single cell RNA-seq analysis pipelines. Nat. Commun. 10, 4667 (2019).
Zeisel, A. et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Mahata, B. et al. Tumors induce de novo steroid biosynthesis in T cells to evade immunity. Nat. Commun. 11, 3588 (2020).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Patro, R. et al. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Okonechnikov, K., Conesa, A. & García-Alcalde, F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 32, 292–294 (2016).
Wang, L., Wang, S. & Li, W. RSeQC: quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 (2012).
Macaulay, I. C. et al. Separation and parallel sequencing of the genomes and transcriptomes of single cells using G&T-seq. Nat. Protoc. 11, 2081–2103 (2016).
Yates, A. D. et al. Ensembl 2020. Nucleic Acids Res. 48, D682–D688 (2020).
The External RNA Controls Consortium. The External RNA Controls Consortium: a progress report. Nat. Methods 2, 731–734 (2005).
Hunt, S. E. et al. Ensembl variation resources. Database (Oxford) https://doi.org/10.1093/database/bay119 (2018).
Kent, W. J. et al. The Human Genome Browser at UCSC. Genome Res. 12, 996–1006 (2002).
Lee, H., Pine, P. S., McDaniel, J., Salit, M. & Oliver, B. External RNA Controls Consortium beta version update. J. Genomics 4, 19–22 (2016).
Paul, L. et al. SIRVs: spike-in RNA variants as external isoform controls in RNA-sequencing. Preprint at bioRxiv https://doi.org/10.1101/080747 (2016).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Lun, A. T. L., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with bioconductor. F1000Res 5, 2122 (2016).
Acknowledgements
This work was supported by the Wellcome Trust grant (206194) and ERC Consolidator Grant ThDEFINE, and H2020 FET MRG-GRAMMAR to SAT. ZM was supported by a Wellcome BioResource for a ‘Single Cell Gene Expression Atlas’ (WT 108437/Z/15/Z) and the Open Targets grant (OTAR2067). We thank X. Chen for expert technical assistance, and K. N. Natarajan for providing the mESC cell line. We thank the Sanger Flow Cytometry (especially B. L. Ng) and Sequencing facilities for cell sorting and sequencing, and R. Elmentaite, J. Park, C. Suo and F. A. Vieira Braga for proofreading the manuscript. Finally, we thank G. Bennett from PerkinElmer for his application support, and L. Apone, K. McKay, V. Panchapakesa and E. Dimalanta from NEB for their assistance in optimizing the library preparation process.
Author information
Authors and Affiliations
Contributions
L.M. designed and supervised the project. Z.M. performed the bioinformatics analysis. L.M., A.J., P.E. and L.S. performed the experiments. L.M., Z.M., L.S. and S.A.T. wrote the manuscript. All authors reviewed and approved the manuscript. L.M. and Z.M. contributed equally to this paper.
Corresponding authors
Ethics declarations
Competing interests
In the past 3 years, S.A.T. has consulted for Genentech and Roche, and is a member of Scientific Advisory Boards of Foresite Labs, Biogen and GlaxoSmithKline. The other authors declare no competing financial interests.
Additional information
Peer review information Nature Protocols thanks Wolfgang Enard and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Vieira Braga, F. A. et al. Nat. Med. 25, 1153–1163 (2019): https://doi.org/10.1038/s41591-019-0468-5
Vento-Tormo, R. et al. Nature 563, 347–353 (2018): https://doi.org/10.1038/s41586-018-0698-6
James, K. R. et al. Nat. Immun. 21, 343–353 (2020): https://doi.org/10.1038/s41590-020-0602-z
Extended data
Extended Data Fig. 1 The comparison of enzymes and lysis buffers in the Zephyr 96-well method (continued from Fig. 2).
a–h,This figure extends the comparison in Fig. 2 and shows the distributions of the percentage of reads mapped to exonic regions (a), the percentage of reads mapped to intronic regions (b), the percentage of reads mapped to intergenic regions (c), the total number of processed reads (d), the distribution of detection limits (e) and accuracy (f) as violin plots and jitter plots. (g) and (h) show the relative gene body coverage distributions for the SMT enzyme and Maxima enzyme, respectively. Appropriate institutional regulatory board permission was obtained for the animal experiments.
Extended Data Fig. 2 The assessment of NEBNext UltraTM II protocol on CD45+ cells and mESCs.
a, Schematic overview of the NEBNext UltraTM II scRNA-seq experiment of mESC and mouse splenocytes. Mouse spleen was dissociated into single cells, and the splenocytes were sorted by FACS after CD45 enrichment. Next, the dissociated splenocytes and the mES cells were treated with NEB cell lysis buffers and then with the reverse transcription enzyme. All the cells were converted into cDNA sequencing libraries by a conventional library construction protocol (fragmentation, end-repair, ligation). Finally, the cDNA libraries were sequenced on an Illumina HiSeq4000 platform. b, Detection limits (sensitivities) of single-cell RNA-seq were assessed by downsampling reads across two orders of magnitude (104 to 106 reads). c, Single-cell RNA-seq accuracies for mouse splenocytes were also measured across two orders of magnitude. The grey dotted lines in b and c indicate downsampled single cells at different read depths, while the red line indicates the limit for sequencing saturation. d–m, The distributions of the total number of mapped counts (d), the total number of genes (e), the percentages of rRNA mapped reads (f) and percentages of mitochondrial contents (g), detection limit (h), accuracy (i), percentage of reads mapped to the exonic regions (j), percentage of reads mapped to intronic regions (k), percentage of reads mapped to intergenic regions (l) are shown as violin plots and jitter plots. (m) shows the relative gene body coverage distributions in the two cell types. Appropriate institutional regulatory board permission was obtained for the animal experiments.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Supplementary Tables 1–3, Supplementary Methods and Supplementary Protocol.
Rights and permissions
About this article
Cite this article
Mamanova, L., Miao, Z., Jinat, A. et al. High-throughput full-length single-cell RNA-seq automation. Nat Protoc 16, 2886–2915 (2021). https://doi.org/10.1038/s41596-021-00523-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00523-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.