Abstract
Single-guide RNAs can target exogenous CRISPR–Cas proteins to unique DNA locations, enabling genetic tools that are efficient, specific and scalable. Here we show that short synthetic guide Piwi-interacting RNAs (piRNAs) (21-nucleotide sg-piRNAs) expressed from extrachromosomal transgenes can, analogously, reprogram the endogenous piRNA pathway for gene-specific silencing in the hermaphrodite germline, sperm and embryos of Caenorhabditis elegans. piRNA-mediated interference (‘piRNAi’) is more efficient than RNAi and can be multiplexed, and auxin-mediated degradation of the piRNA-specific Argonaute PRG-1 allows conditional gene silencing. Target-specific silencing results in decreased messenger RNA levels, amplification of secondary small interfering RNAs and repressive chromatin modifications. Short (300 base pairs) piRNAi transgenes amplified from arrayed oligonucleotide pools also induce silencing, potentially making piRNAi highly scalable. We show that piRNAi can induce transgenerational epigenetic silencing of two endogenous genes (him-5 and him-8). Silencing is inherited for four to six generations after target-specific sg-piRNAs are lost, whereas depleting PRG-1 leads to essentially permanent epigenetic silencing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The sequencing data that support the findings of this study are available in NCBI Gene Expression Omnibus (GSE165210). Figures 1–4 and Extended Data Figs. 1–10 and Supplementary Figs. 1–5, 7–11 and 13 have associated raw data available in the source data files. The authors declare that all data supporting the findings of this study are available within the paper (and its Supplementary Information files). Genomes and annotations files for C. elegans and C. briggsae were downloaded from WormBase v.WS270 and WS190 (ftp.wormbase.org). All data are available with no restrictions. Source data are provided with this paper.
Code availability
The authors’ custom scripts are accessible at https://doi.org/10.5281/zenodo.5599659. Custom software apps are described at https://github.com/AmhedVargas/piRNAi_reprogramming.
All code is available with no restrictions.
References
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Shalem, O., Sanjana, N. E. & Zhang, F. High-throughput functional genomics using CRISPR–Cas9. Nat. Rev. Genet. 16, 299–311 (2015).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Kamath, R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003).
Long, L. et al. Regulation of transcriptionally active genes via the catalytically inactive Cas9 in C. elegans and D. rerio. Cell Res. 25, 638–641 (2015).
Ketting, R. F. & Plasterk, R. H. A genetic link between co-suppression and RNA interference in C. elegans. Nature 404, 296–298 (2000).
Dernburg, A. F., Zalevsky, J., Colaiácovo, M. P. & Villeneuve, A. M. Transgene-mediated cosuppression in the C. elegans germ line. Genes Dev. 14, 1578–1583 (2000).
Timmons, L. & Fire, A. Specific interference by ingested dsRNA. Nature 395, 854–854 (1998).
Tavernarakis, N., Wang, S. L., Dorovkov, M., Ryazanov, A. & Driscoll, M. Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nat. Genet. 24, 180–183 (2000).
Bezler, A. et al. Tissue- and sex-specific small RNAomes reveal sex differences in response to the environment. PLoS Genet. 15, e1007905 (2019).
Kamath, R. S., Martinez-Campos, M., Zipperlen, P., Fraser, A. G. & Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2, research0002.1 (2000).
Batista, P. J. et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol. Cell 31, 67–78 (2008).
Ashe, A. et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 150, 88–99 (2012).
Lee, H.-C. et al. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell 150, 78–87 (2012).
Luteijn, M. J. et al. Extremely stable Piwi-induced gene silencing in Caenorhabditis elegans. EMBO J. 31, 3422–3430 (2012).
Bagijn, M. P. et al. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science 337, 574–578 (2012).
Zhang, D. et al. The piRNA targeting rules and the resistance to piRNA silencing in endogenous genes. Science 359, 587–592 (2018).
Shen, E.-Z. et al. Identification of piRNA binding sites reveals the argonaute regulatory landscape of the C. elegans germline. Cell 172, 937–951.e18 (2018).
Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. & Zamore, P. D. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. (2018).
Cecere, G., Zheng, G. X. Y., Mansisidor, A. R., Klymko, K. E. & Grishok, A. Promoters recognized by forkhead proteins exist for individual 21U-RNAs. Mol. Cell 47, 734–745 (2012).
Billi, A. C. et al. A conserved upstream motif orchestrates autonomous, germline-enriched expression of Caenorhabditis elegans piRNAs. PLoS Genet. 9, e1003392 (2013).
Meneely, P. M., McGovern, O. L., Heinis, F. I. & Yanowitz, J. L. Crossover distribution and frequency are regulated by him-5 in Caenorhabditis elegans. Genetics 190, 1251–1266 (2012).
Phillips, C. M. et al. HIM-8 binds to the X Chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell 123, 1051–1063 (2005).
Zhang, L., Ward, J. D., Cheng, Z. & Dernburg, A. F. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development 142, 4374–4384 (2015).
Simon, M. et al. Reduced insulin/IGF-1 signaling restores germ cell immortality to Caenorhabditis elegans Piwi mutants. Cell Rep. 7, 762–773 (2014).
Wahba, L., Hansen, L. & Fire, A. Z. An essential role for the piRNA pathway in regulating the ribosomal RNA pool in C. elegans. Dev. Cell 56, 2295–2312.e6 (2021).
Kosuri, S. et al. Scalable gene synthesis by selective amplification of DNA pools from high-fidelity microchips. Nat. Biotechnol. 28, 1295–1299 (2010).
Alcazar, R. M., Lin, R. & Fire, A. Z. Transmission dynamics of heritable silencing induced by double-stranded RNA in Caenorhabditis elegans. Genetics 180, 1275–1288 (2008).
Gu, S. G. et al. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat. Genet. 44, 157–164 (2012).
Shirayama, M. et al. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell 150, 65–77 (2012).
Weick, E.-M. et al. PRDE-1 is a nuclear factor essential for the biogenesis of Ruby motif-dependent piRNAs in C. elegans. Genes Dev. 28, 783–796 (2014).
Phillips, C. M., Montgomery, T. A., Breen, P. C. & Ruvkun, G. MUT-16 promotes formation of perinuclear Mutator foci required for RNA silencing in the C. elegans germline. Genes Dev. 26, 1433–1444 (2012).
Shukla, A. et al. poly(UG)-tailed RNAs in genome protection and epigenetic inheritance. Nature (2020).
Kalinava, N., Ni, J. Z., Peterman, K., Chen, E. & Gu, S. G. Decoupling the downstream effects of germline nuclear RNAi reveals that H3K9me3 is dispensable for heritable RNAi and the maintenance of endogenous siRNA-mediated transcriptional silencing in Caenorhabditis elegans. Epigenetics Chromatin 10, 6 (2017).
Buckley, B. A. et al. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489, 447–451 (2012).
Perez, M. F. & Lehner, B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 21, 143–151 (2019).
Vastenhouw, N. L. et al. Long-term gene silencing by RNAi. Nature 442, 882 (2006).
Lev, I., Gingold, H. & Rechavi, O. H3K9me3 is required for inheritance of small RNAs that target a unique subset of newly evolved genes. eLife 8, e40448 (2019).
Shukla, A., Perales, R. & Kennedy, S. piRNAs coordinate poly(UG) tailing to prevent aberrant and perpetual gene silencing. Curr. Biol. 31, 4473–4485.e3 (2021).
Kelly, W. G., Xu, S., Montgomery, M. K. & Fire, A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146, 227–238 (1997).
Fire, A., Albertson, D., Harrison, S. W. & Moerman, D. G. Production of antisense RNA leads to effective and specific inhibition of gene expression in C. elegans muscle. Development 113, 503–514 (1991).
Gönczy, P. et al. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature 408, 331–336 (2000).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Mello, C. C., Kramer, J. M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Frøkjær-Jensen, C. et al. An abundant class of non-coding DNA can prevent stochastic gene silencing in the C. elegans germline. Cell 166, 343–357 (2016).
Sturm, Á., Saskői, É., Tibor, K., Weinhardt, N. & Vellai, T. Highly efficient RNAi and Cas9-based auto-cloning systems for C. elegans research. Nucleic Acids Res. 46, e105 (2018).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Ni, J. Z., Chen, E. & Gu, S. G. Complex coding of endogenous siRNA, transcriptional silencing and H3K9 methylation on native targets of germline nuclear RNAi in C. elegans. BMC Genomics 15, 1157 (2014).
Gent, J. I. et al. A Caenorhabditis elegans RNA-directed RNA polymerase in sperm development and endogenous RNA interference. Genetics 183, 1297–1314 (2009).
Schwartz-Orbach, L. et al. Caenorhabditis elegans nuclear RNAi factor SET-32 deposits the transgenerational histone modification, H3K23me3. eLife 9, e54309 (2020).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Frøkjær-Jensen, C. et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40, 1375–1383 (2008).
Aljohani, M. D., El Mouridi, S., Priyadarshini, M., Vargas-Velazquez, A. M. & Frøkjær-Jensen, C. Engineering rules that minimize germline silencing of transgenes in simple extrachromosomal arrays in C. elegans. Nat. Commun. 11, 6300 (2020).
Dickinson, D. J., Ward, J. D., Reiner, D. J. & Goldstein, B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10, 1028–1034 (2013).
Acknowledgements
We thank L. Wahba and A. Fire (Stanford University) for sharing reagents before publication. Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (grant no. P40 OD010440). The research was funded by KAUST Office of Sponsored Research grant no. OSR-CRG2019-4016 (C.F.-J.), a Rutgers University Busch Biomedical Grant (S.G.G.) and the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM111752 (S.G.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
M.P., J.Z.N. and C.F.-J. performed experiments. A.M.V.-V. and S.G.G. performed bioinformatic analysis. A.M.V.-V. developed the online piRNA app. C.F.-J. conceived and supervised the project. C.F.-J. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Donglei Zhang and the other, anonymous, reviewers for their contribution to the peer review of this work. Lei Tang was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 piRNAi can silence a variety of germline-expressed genes.
a. Quantification of the number of self-progeny in C. elegans strains with sg-piRNAs targeting the sperm specific genes spe-8 and spe-12. Fertility was assayed from unmated L4 hermaphrodites in a him-5(e1490) mutant background. Control (‘-’) = randomized sg-piRNAs in clusterA. Kruskal–Wallis ANOVA P = 0.0376, Dunn’s multiple comparison, * P = 0.0458, ns = P > 0.99. b. piRNAi against mes-4 and him-5 in wildtype (N2) animals. Transgenic animals were scored for sterile animals and for males (to identify ‘active arrays’). Two-tailed Mann-Whitney, * P = 0.0281 (sterility) and * P = 0.0455 (male frequency). c. piRNAi against dcr-1 and him-5 in wildtype animals (N2). Transgenic L4 stage animals were incubated at 20 °C and 25 °C and their progeny scored for lethality and males. dcr-1 genetic mutants are temperature-sensitive sterile at high temperatures. Two-tailed Mann-Whitney, ns P = 0.1320 and ** P = 0.0065. d. piRNAi against pie-1 and him-5 in the AID::PRG-1 strain maintained on 1 mM auxin (PRG-1 depleted). Larval stage animals from stable, independent transgenic lines were transferred to plates with or without auxin and scored for the number of progeny at 25 °C. Two-tailed Mann-Whitney, ** P = 0.0079. Data are presented as mean values + /- SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (a) n = 4 (‘-’), n = 4 (spe-8), n = 3 (spe-12), (b) n = 6 (him-5), n = 5 (him-5 + mes-4), (c) n = 6 (all conditions), (d) n = 5 biologically independent transgenic strains.
Extended Data Fig. 2 Rules for efficient piRNA silencing.
a. Schematic of sg-piRNA clusterE. b. Graph showing the effect of silencing him-5 with one, two, or three sg-piRNAs recoded in clusterE. c. Bar graph showing the effect of silencing a codon-optimized gfp expressed in the germline (Pmex-5::gfp) with zero, one, two, three, or six sg-piRNAs from clusterE. Independent biological strains (at least 11 animals per strain) were scored qualitatively on a dissection microscope blinded to genotype. d. Top. Schematic of the him-5 (D1086.4a.1) gene structure and the location of sg-piRNAs. Bottom. piRNAi using clusterE targeting him-5 exons or 5’ and 3’ untranslated regions (UTRs) or introns (‘non-coding’). Control = randomized sg-piRNAs. e. Three versions of piRNA clusterE using different sg-piRNAs were tested for him-5 silencing. Set 1 corresponds to Fig. 1b and the piRNAi transgenes in set 2 and set 3 target the same exons as set 1 but use different sg-piRNAs. f. The six sg-piRNAs in ‘set 1’ were shuffled (‘shuffle 1’ and ‘shuffle 2’), so each sg-piRNA was expressed by a different promoter in the piRNA cluster. The guide piRNA target locations in the him-5 transcript are shown as colored ovals. The strains were cultured at 25 °C. g. Transgenic animals were tested for him-5 silencing by piRNAi (piRNA clusterE) over 12 generations at various temperatures (15 °C, 20 °C, 25 °C). h. Propagation of two transgenic animals with sg-piRNAs targeting him-5 (left) and him-8 (right) that initially had a low frequency of males in the population. The two strains were propagated for 12 generations, and the male frequency was quantified every six generations. i. C. elegans piRNA clusterE targeting the gene cbr-him-8 in C. briggsae (AF16). Control (‘-’) = un-injected C. briggsae. Two-tailed t-test with Welch’s correction. ** P = 0.0236. Data are presented as mean values+/− SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (b) n = 5 (‘1’), n = 2 (‘2’), n = 4 (‘3’), n = 6 (‘4’), n = 4 (‘5’), n = 6 (‘6’), n = 4 (‘1 + 5’), n = 5 (‘2 + 4’), n = 4 (‘3 + 6’), n = 6 (‘1 + 4 + 5’), n = 4 (‘2 + 3 + 6’), (c) n = 4 (Neg. control), n = 6 (‘1’), n = 8 (‘2’), n = 10 (‘3’), n = 9 (‘4’), n = 7 (‘5’), n = 10 (‘6’), n = 8 (‘1 + 5’), n = 7 (‘3 + 4’), n = 5 (‘5 + 6’), n = 2 (‘2 + 3 + 6’), (d) n = 3 (control), n = 6 (Exons 2-4), n = 6 (exon 4), n = 5 (exons 5-6), n = 3 (spanning exons), n = 6 (5’ UTR), n = 6 (introns), n = 4 (3’ UTR), (e) n = 3 (set 1), n = 3 (set 2), n = 2 (set 3), (f) n = 3 (shuffle 1), n = 1 (shuffle 2), (g) n = 3 (all conditions), (h) n = 1 (all conditions), n = 3 (control), n = 3 (cbr-him-8) biologically independent transgenic strains.
Extended Data Fig. 3 piRNAi depends on plasmid structure and copy number.
a. Transgenic lines generated by injecting a piRNA cluster either as linear dsDNA (1.5 kb) or the same DNA cloned into a plasmid backbone (high copy ampicillin pTwist vector). The DNA transgenes were generated from clusterE targeting him-5 and him-8 with six sg-piRNAs. Two-tailed Mann-Whitney tests. *** P = 0.0006, ns = not significant (0.0659). b. him-5 piRNAi plasmid (from panel a) digested with restriction enzymes that cuts in the bacterial vector backbone (left) or undigested (right) was used to induce silencing. The plasmid samples were treated identically (incubated in restriction enzyme buffer with or without restriction enzymes and purified over spin column) and injected at the same concentration. Two-tailed Mann-Whitney test. *** P = < 0.0001. c. Comparison of transgene copy number by whole genome sequencing of transgenic lines with extrachromosomal arrays formed from linear (green) or circular (purple) plasmids. d. Comparison of sg-piRNA expression targeting him-5 from transgenic strains carrying linearized and circular piRNAi transgenes. e. One ‘inactive’ multiplexed piRNAi strain (blue circle in Fig. 3h) was propagated for six generations and scored for males in the population and silencing of germline GFP fluorescence. ‘Generation 0’ corresponds to 2-3 generations after the initial injection. f. Whole-genome sequencing on the strain shown in panel e to determine the copy number of all plasmids in the extra-chromosomal array at the early (generation 1) and late (generation 6) timepoints. Mitochondrial DNA (mtDNA) copy number was used as a control. The copy number was calculated relative to the average sequencing coverage across the entire C. elegans genome. Data are presented as mean values + /- SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (a) him-5: n = 8 (linear), n = 8 (circular), him-8: n = 7 (linear), n = 7 (circular), (b) n = 6 (digested), n = 6 (undigested), (c) n = 3 (all conditions), (d) n = 2 (linear), n = 2 (circular), (e) n = 1, (f) n = 1 biologically independent transgenic strains.
Extended Data Fig. 4 Time-course of conditional cdk-1 silencing using auxin.
a. Brightfield image of animals with piRNAi targeting cdk-1 in the AID::PRG-1 strain on auxin (left panel) or off auxin (right panel). The dotted yellow line outlines fertilized embryos in the hermaphrodite uterus. Arrows indicate the vulva and embryos. cdk-1 encodes a cyclin-dependent kinase that is required for cell division and embryos arrest at the single-cell stage in cdk-1 mutants. Representative images from more than ten embryos imaged. Scale bar = 25 µm. b. piRNAi against cdk-1 in the AID:PRG-1 strain. Injected animals were maintained on 1 mM auxin plates (to deplete PRG-1). L4 stage animals were transferred to plates with or without auxin and surviving progeny was scored by first counting eggs and three days later counting the total number of adult progeny. Negative control = wildtype animals (N2). Kruskal-Wallis ANOVA P = 0.0036, Dunn’s multiple comparison * P = 0.0146, ns P = 0.3594. c. piRNAi activation after removal from auxin. To determine how long it takes to ‘turn on’ piRNA-mediated silencing after auxin removal, animals were transferred to non-auxin plates at each larval stage and at 3-hour intervals as young adults. The uteruses of adult animals (at least 11 animals) were scored for the presence of single-cell arrested embryos. Data are presented as mean values+/− SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (b) n = 3 (N2), n = 3 (auxin, both conditions), (c) n = 1 (all conditions) biologically independent transgenic strains.
Extended Data Fig. 5 piRNAi silencing tolerates up to three mismatches.
a. sg-piRNA mismatch tolerance using piRNA clusterE. The six sg-piRNAs targeting him-5 each contained from zero to five mismatches. The schematics show the location of mismatches in the piRNA seed sequence (red boxes, nucleotides in positions 2 to 7) or in the remainder of the piRNA (white boxes, positions 8 to 21). For all sg-piRNAs (including controls), the leading ‘U’ was not modified as this base is required for piRNA transcription. In some transgenes the overall number of mismatches was constant but the location in each sg-piRNA was randomized (indicated by gray shading). The negative control contains inverted him-5 sg-piRNAs. b. Relationship between male frequency and the aggregate piRNA score calculated based on Wu et al. (2018), which takes the location of mismatch and wobble base pairing into account. Silencing data from panel a. Left: all piRNAs. Right: four highest expressed guide piRNA in clusterE (the two remaining sg-piRNAs were rarely detectable by small RNA sequencing). Simple linear regression. R2 = goodness of fit. c. Relationship between male frequency and the number of mismatches in each guide piRNA. Silencing data from panel a. Left: all piRNAs. Right: four highest expressed guide piRNA in clusterE. Simple linear regression. R2 = goodness of fit. Data are presented as mean values + /- SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (a) Controls: n = 6, n = 6, n = 7, n = 8, n = 8, n = 5 (negative), n = 5, n = 9, n = 7, n = 7, n = 6, n = 6, n = 9 (positive), 1 mismatch: n = 9, n = 7, n = 8, n = 7, n = 6, n = 10 (technical and biological replicate), n = 7, n = 8, 2 mismatches: n = 5, n = 10, n = 8, n = 7, n = 6, n = 8, n = 6, n = 6, 3 mismatches: n = 7, n = 5, n = 8, n = 9, n = 5, 4 mismatches: n = 9, n = 6, n = 8, n = 8, n = 5, 5 mismatches: n = 7, n = 6, n = 7, n = 5, n = 7, n = 9, n = 8, n = 6, n = 9, n = 7, n = 5, n = 5, all values top to bottom, (b) n = 382, (c) n = 343 biologically independent transgenic strains.
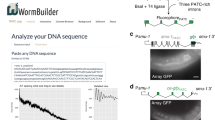
Extended Data Fig. 6 Web application allows easy piRNAi transgene design.
a. Screenshot from www.wormbuilder.org/piRNAi. Simple mode to target a single gene with pre-defined criteria. b. Advanced mode. In the advanced mode, several different piRNA clusters can be re-coded with either custom 20-mers (for example, targeting gfp) or by selecting all 20-mers at a given edit distance mapping to a selected gene isoform. c. Output file with the piRNAi transgene annotated in GenBank format. The sequence is displayed in ‘A plasmid Editor’ (ApE).
Extended Data Fig. 7 Silencing endogenously gfp-tagged his-72 with piRNAi.
a. Brightfield and fluorescence images of a strain with an endogenously gfp-tagged his-72 locus. Images show a non-targeting control (mCherry), or sg-piRNAs targeting his-72, gfp, or his-72 + gfp. Images were acquired at 40x magnification using an oil immersion objective. Representative image from more than 50 adult animals imaged. White scale bar = 25 µm. b. We quantified GFP fluorescence in the nucleus of the ‘last’ three oocytes before the spermatheca using ImageJ (NIH). Data are presented as mean values + /- SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (b) n = 62 (N2), n = 59 (non-targeting), n = 64 (his-72), n = 62 (gfp), n = 62 (his-72 + gfp) fluorescent images from biologically identical transgenic strains across three technical replicates for each condition.
Extended Data Fig. 8 Amplification of short piRNAi transgenes from Matrix Oligo Pools.
a. Schematic of two different 300 bp piRNAi transgenes, each encoding three sg-piRNAs. 300 bp oligos synthesized at large scale on oligo chips are delivered as arrayed sub-pools with 121 unique oligos in each of 384 wells. The piRNA transgenes are flanked by orthogonal forward and reverse primer sites that allow ‘dial-out’ PCR of transgenes from complex oligo pools (Kosuri et al., 2010). Each oligo pool contains 121 different oligos with a maximum length of 300 base pairs (Matrix Oligo Pools, Twist Bioscience). Two piRNAi transgenes (encoding six sg-piRNAs) are required for silencing. Each pool can, therefore, target 60 genes and individual piRNAi transgenes can be ‘dialed out’ using 16 orthogonal primers (8 forward * 8 reverse = 64 unique combinations). Restriction sites (DraI or EcoRV) allow Sanger sequencing of pair-wise amplified piRNA transgenes. b. 96-well amplification of 60 different piRNA transgene pairs targeting him-5 using two rounds of PCR. The first PCR was a bulk amplification of all oligos in the pool using all 16 amplification primers concurrently. 60 specific piRNA transgenes were amplified in a second round of PCR performed with pair-wise orthogonal primers listed above wells (expected size = 300 bp, indicated by arrow). Control reactions contained no template to assess background amplification of contaminants. With no optimization, we were able to amplify 51 of 60 piRNA transgenes (nine wells with weak or no band at 300 bp). Ladder = 100-10,000 bp VersaLadder (GoldBio). For a large-scale library (for example, a whole-genome library) a subcloning step after the first bulk amplification and transformation of the amplified oligo pool into bacteria would maintain long-term integrity and facilitate distribution of the oligo-pool as lyophilized plasmid pools. c. Representative example of Sanger sequencing of a PCR-amplified piRNA transgene from the oligo pool. 12 of 12 sequenced PCR products contained the expected three unique sg-piRNAs. From the sequencing trace, a low level of cross-talk is visible (minor peaks below the three sg-piRNAs), which can likely be minimized by reducing the number of PCR cycles and using a sub-cloned library as a PCR template.
Extended Data Fig. 9 Repressive chromatin modifications spreads in response to piRNAi.
a. ChIP-seq with antibodies against Pol II, H3K9me3, and H3K23me3 in two strains carrying piRNAi transgenes targeting him-5 (red trace) and him-8 (blue trace). The piRNAi target genes are indicated by dotted lines. zim-1, zim-2, zim-3, and him-8 are part of a single operon (CEOP4384, green bar). b. Control loci not targeted by piRNAi. c. mCherry and gfp were co-expressed in an operon under the mex-5 promoter (Pmex-5::mCherry::H2B - gpd-2 operon – gfp::h2b::cye-1 3’UTR) as a single copy insertion at ttTi5605 (Frøkjær-Jensen et al., 2008). The fluorophores were targeted individually or together (control) for silencing with piRNA transgenes using piRNA clusterE. Data are presented as mean values+/− SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (c) mCherry: n = 3 (-/-), n = 5 (+/-), n = 7 (−/+), n = 5 (+/+), gfp: n = 3 (-/-), n = 5 (+/-), n = 7 (−/+), n = 5 (+/+) biologically independent transgenic strains.
Extended Data Fig. 10 piRNAi-induced transgenerational silencing.
a. Schematic showing assay to determine him-5 and him-8 inherited silencing. To quantify the proportion of males generated from self-fertilization (as opposed to male cross progeny generated by mating), we picked four to six unmated L4 animals in every generation to new plates and scored their progeny for males (out of 100 animals). The assay has a ‘transition generation’ (G0) where a subset of animals are allowed to lose the extra-chromosomal piRNAi-array. Due to the shared germline cytoplasm, non-transgenic animals in the following G1 generation were exposed to sg-piRNAs in early embryonic development. The strains were not exposed to de novo sg-piRNAs generated during the L4 molt when sperm are made and female oocyte production is initiated. b. Duration of him-5 and him-8 transgenerational inheritance. We quantified transgenerational inheritance by counting the number of generations after losing the piRNA array before the fraction of males in the population was <= 1%. Data aggregated from panels Fig. 4bc and Supplementary Fig. 13. Kruskal-Wallis ANOVA, P=<0.0001, Dunn’s post hoc test *** P = 0.0007. c. Duration of transgenerational silencing in hrde-1 mutants. Two-tailed Mann-Whitney test, * P = 0.0286, ** P = 0.0079. d. Inherited silencing of piRNAi targeting spe-8 and spe-12 in a him-5(e1490) mutant background. Unmated L4 hermaphrodites were scored for viable progeny in the presence (solid black bar) or absence (solid white bar) of sg-piRNAs targeting spe-8 and spe-12, respectively. Control = him-5(e1490) animals with a randomized piRNAi cluster. Hygromycin was used to select transgenic animals, including controls, causing the increase in brood size of controls after the animals were grown on non-selective plates. Statistics: One-way ANOVA at each generation. * P = 0.0057 (generation -1), * P = 0.0127 (generation 0), ns P = 0.8312 (generation +1). Data are presented as mean values + /- SEM with each data point corresponding to an independently derived transgenic strain. Sample sizes (b) n = 6 (control), n = 14 (him-5), n = 8 (him-8), (c) him-5: n = 5 (N2), n = 5 (hrde-1), him-8: n = 4 (N2), n = 4 (hrde-1), (d) n = 2 (control), n = 2 (spe-8), n = 2 (spe-12) biologically independent transgenic strains.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Discussion, Methods, Protocols and References.
Supplementary Table 1
Source data for supplementary figures and oligo sequences.
Supplementary Data 1
Annotated GenBank files for all transgenes.
Source data
Source Data Fig. 1
Data for graphs in an Excel spreadsheet.
Source Data Fig. 2
Data for graphs in an Excel spreadsheet.
Source Data Fig. 3
Data for graphs in an Excel spreadsheet.
Source Data Fig. 4
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 1
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 2
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 3
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 4
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 5
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 7
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 9
Data for graphs in an Excel spreadsheet.
Source Data Extended Data Fig. 10
Data for graphs in an Excel spreadsheet.
Rights and permissions
About this article
Cite this article
Priyadarshini, M., Ni, J.Z., Vargas-Velazquez, A.M. et al. Reprogramming the piRNA pathway for multiplexed and transgenerational gene silencing in C. elegans. Nat Methods 19, 187–194 (2022). https://doi.org/10.1038/s41592-021-01369-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01369-z
This article is cited by
-
Cisplatin exposure alters tRNA-derived small RNAs but does not affect epimutations in C. elegans
BMC Biology (2023)
-
piRNAs regulate a Hedgehog germline-to-soma pro-aging signal
Nature Aging (2023)
-
Christian Frøkjær-Jensen
Nature Methods (2022)